Photorespiration Enhances Acidification of the Thylakoid Lumen, Reduces the Plastoquinone Pool, and Contributes to the Oxidation of P700 at a Lower Partial Pressure of CO2 in Wheat Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Gas Exchange, Chlorophyll Fluorescence, P700+, Electrochromic Shift, and Spectroscopic Analyses
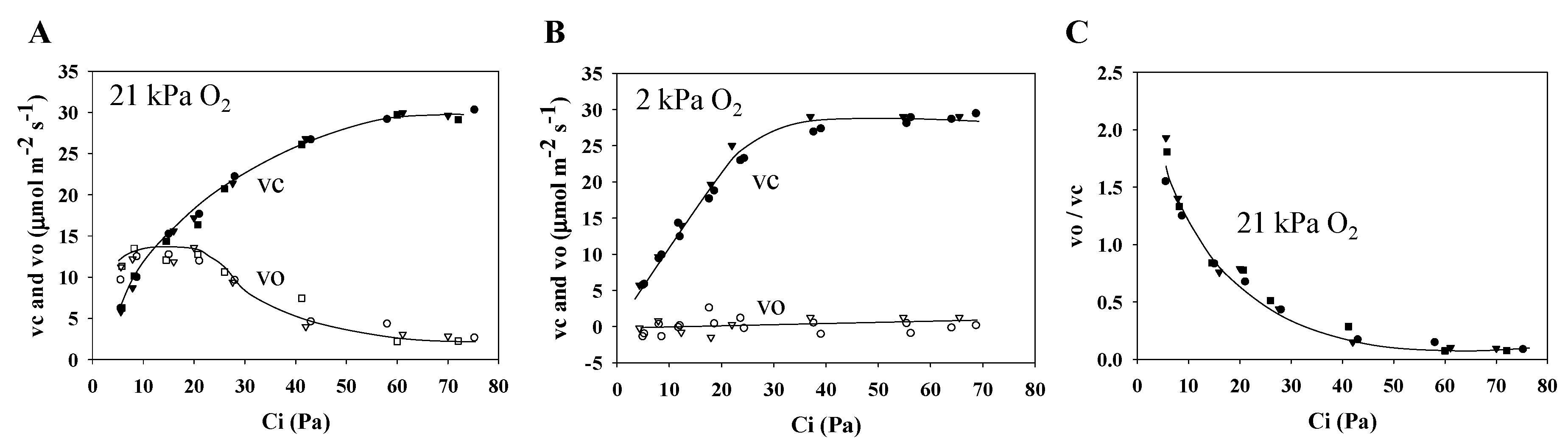
2.3. Ribulose 1,5-Bisphosphate (RuBP) Carboxylation Rate and RuBP Oxygenation Rate in Wheat Leaves
2.4. H+ Consumption Rate Estimated from the Stoichiometries of Photosynthesis and Photorespiration
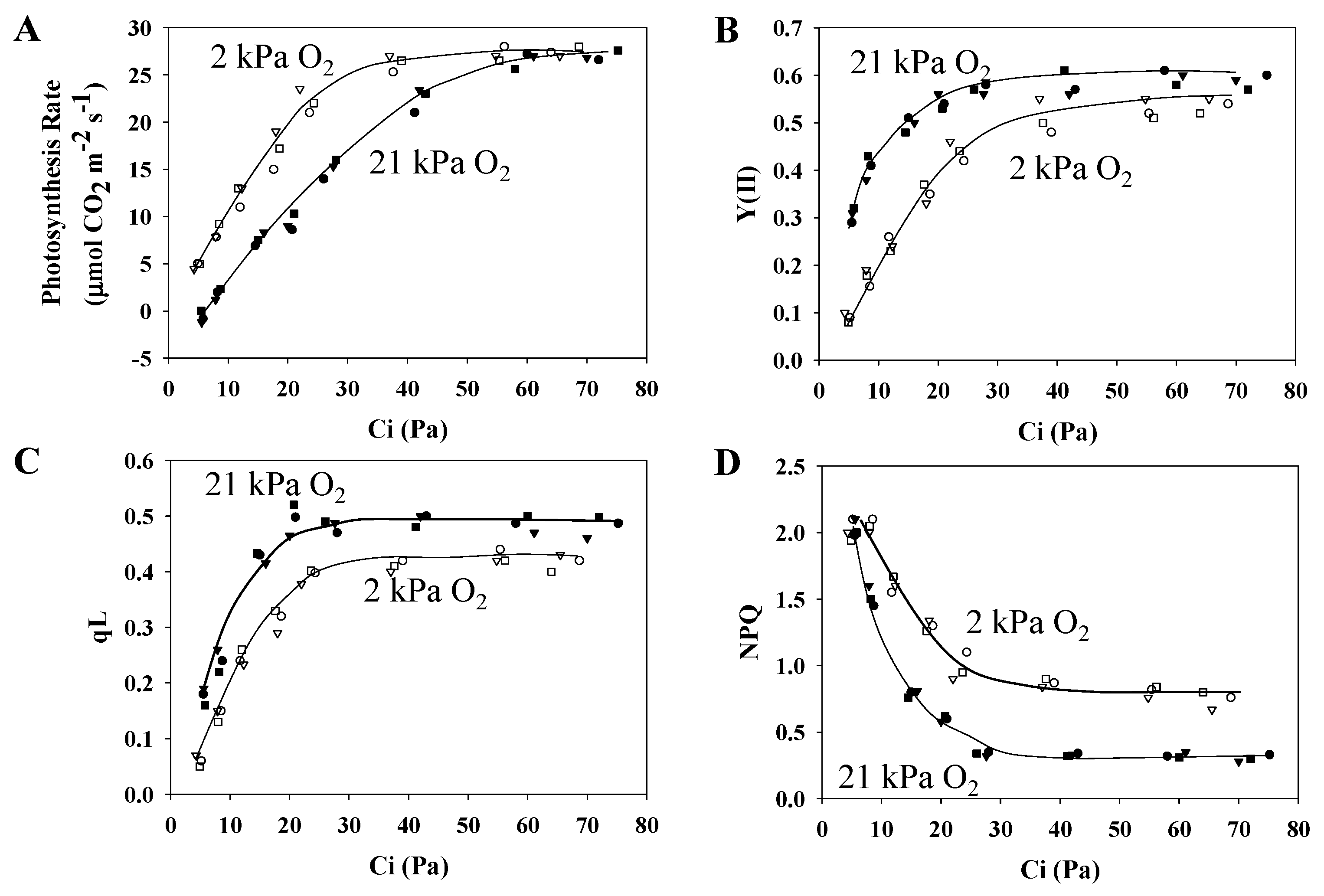
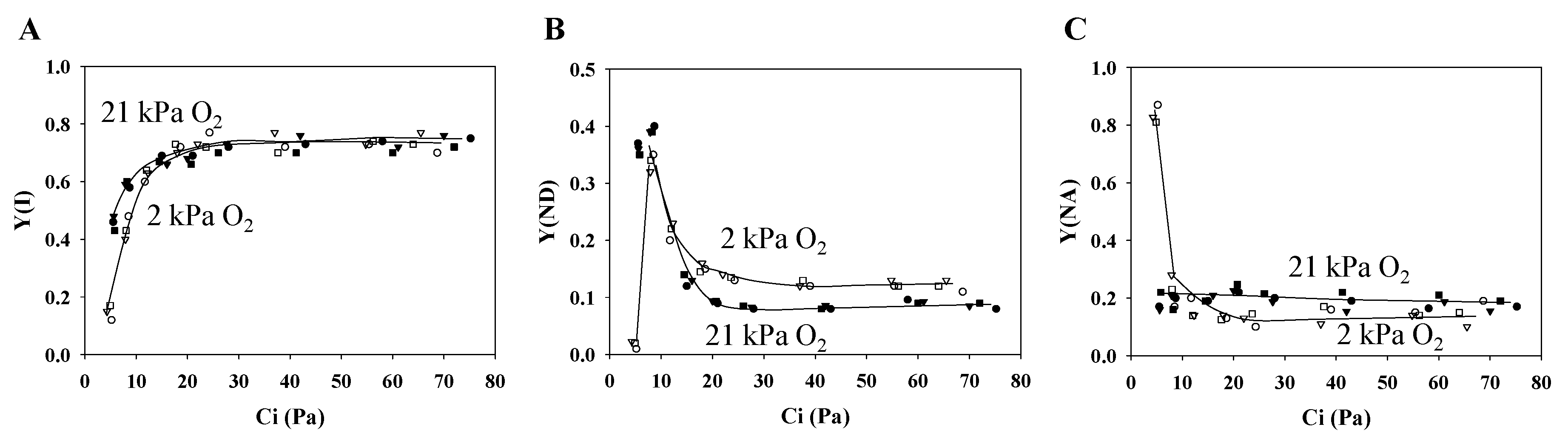
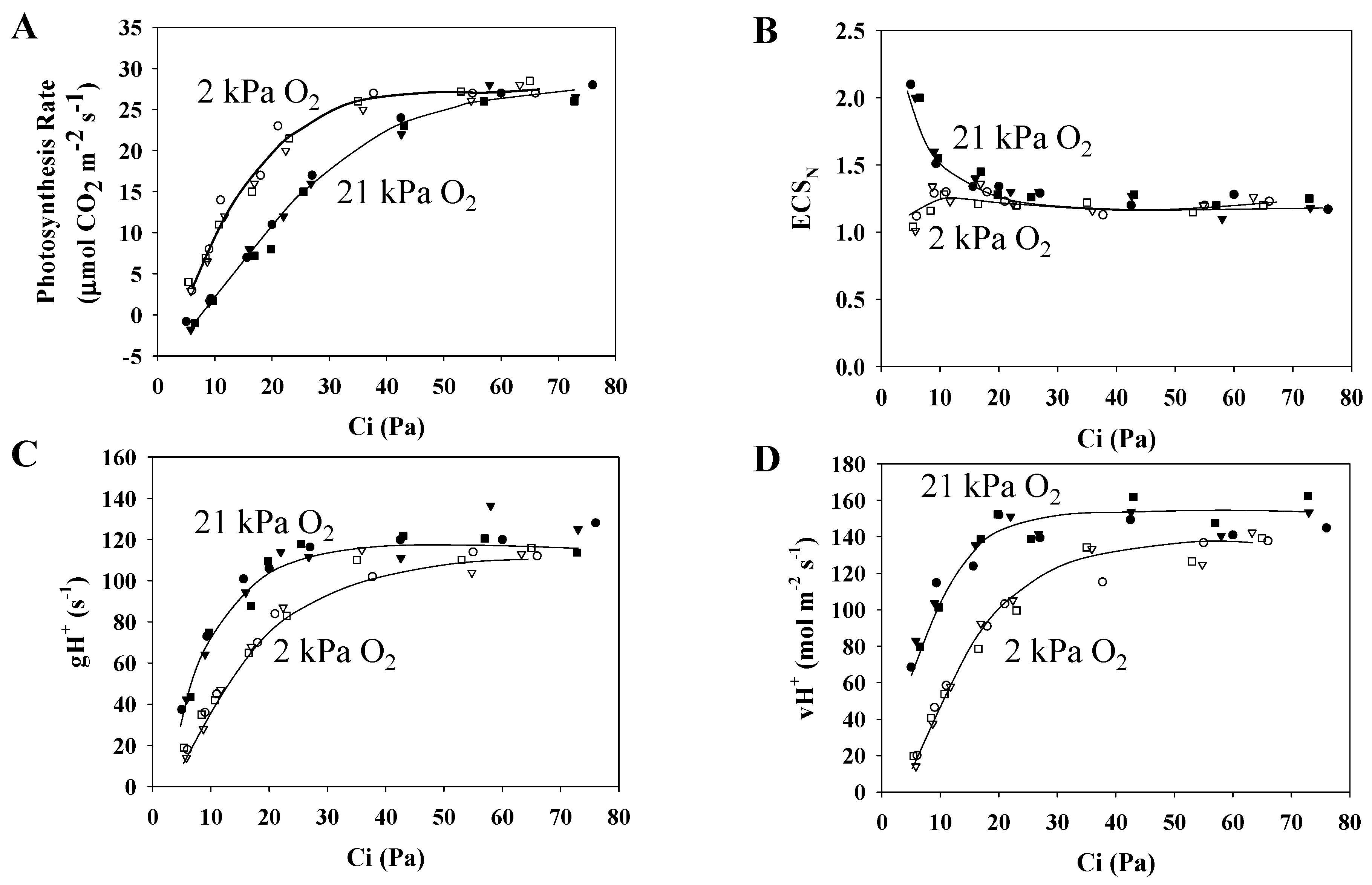
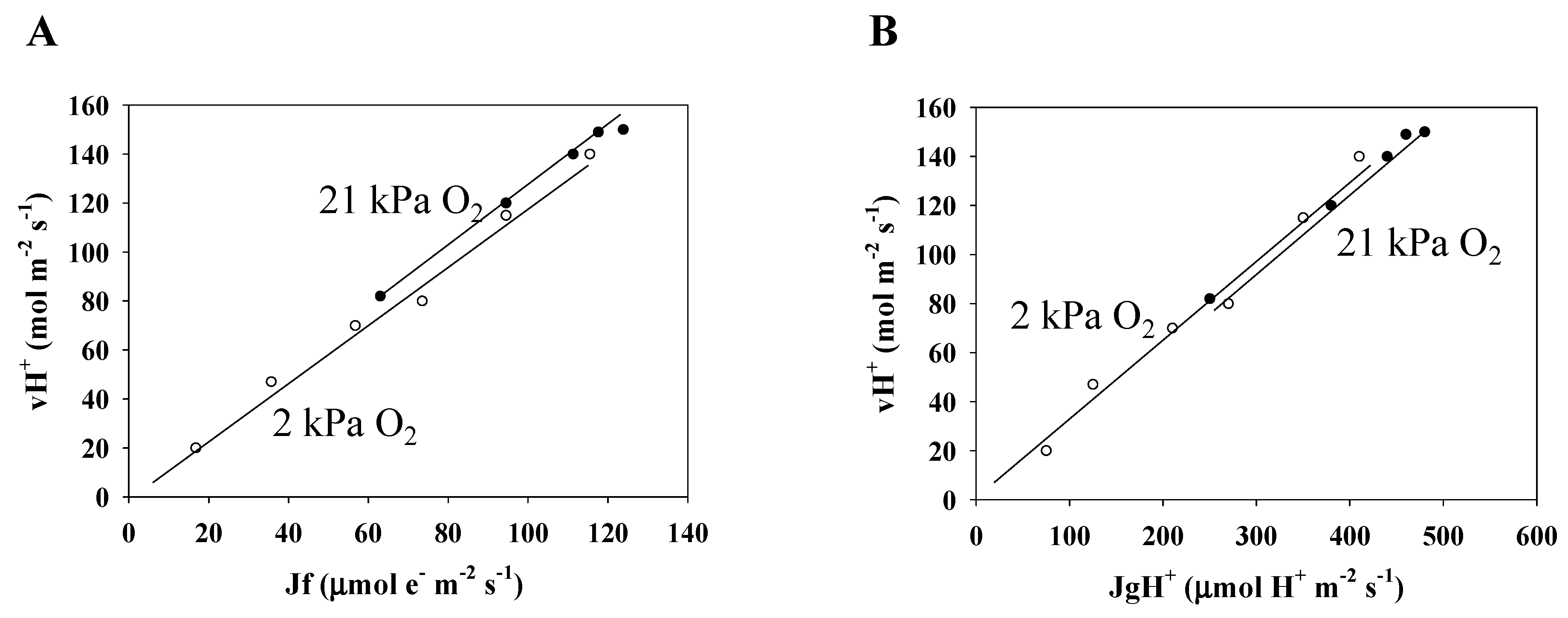
3. Results
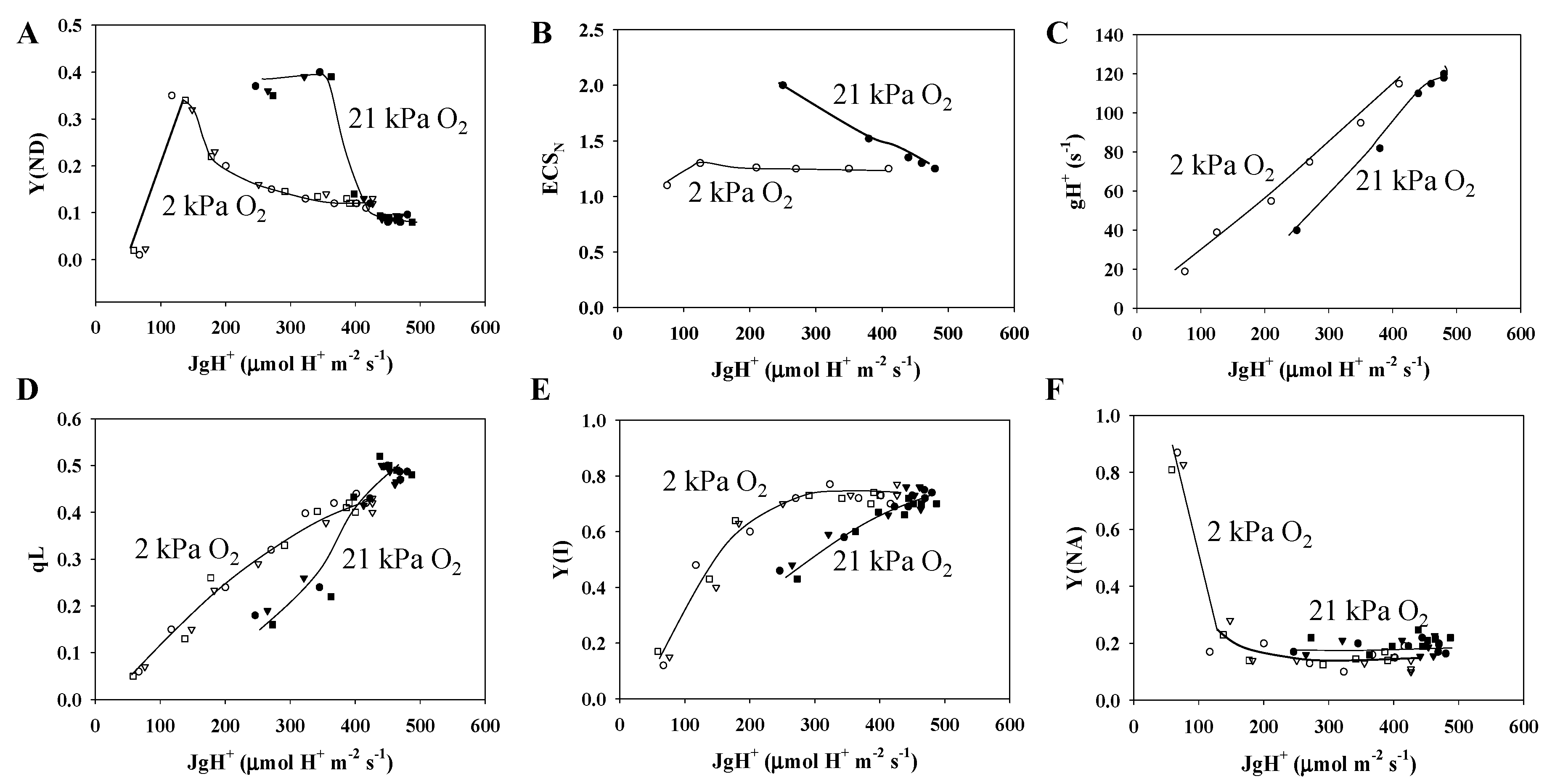
3.1. Characteristics of PSII and PSI Parameters in Response to Changes in the Partial Pressure of CO2
3.2. Characteristics of the Electrochromic Shift Signal and H+ Conductance in Response to Changes in pCO2
3.3. Electron Flux of Photosynthetic LEF Matches the Rate of ECS Deay Driven by Photosynthesis and Photorespiration
3.4. Contribution of Photorespiration to P700 Oxidation and ECSN in Response to Changes in the H+ Consumption Rate
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shimakawa, G.; Miyake, C. Oxidation of P700 ensures robust photosynthesis. Front. Plant Sci. 2018, 9, 1617. [Google Scholar] [CrossRef]
- Asada, K. THE WATER-WATER CYCLE IN CHLOROPLASTS: Scavenging of Active Oxygens and Dissipation of Excess Photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Sejima, T.; Takagi, D.; Fukayama, H.; Makino, A.; Miyake, C. Repetitive short-pulse light mainly inactivates photosystem I in sunflower leaves. Plant Cell Physiol. 2014, 55, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Shimakawa, G.; Shaku, K.; Miyake, C. Oxidation of P700 in Photosystem I Is Essential for the Growth of Cyanobacteria. Plant Physiol. 2016, 172, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Zivcak, M.; Brestic, M.; Kunderlikova, K.; Sytar, O.; Allakhverdiev, S.I. Repetitive light pulse-induced photoinhibition of photosystem I severely affects CO2 assimilation and photoprotection in wheat leaves. Photosynth Res. 2015, 126, 449–463. [Google Scholar] [CrossRef]
- Charepanov, D.A.; Milanovsky, G.E.; Petrova, A.A.; Tikhonov, A.N.; Semenov, A.Y. Electron transfer through the acceptor side of photosystem I: Interaction with exogenous acceptors and molecular oxygen. Biochemistry (Moscow) 2017, 82, 1249–1268. [Google Scholar] [CrossRef]
- Shimakawa, G.; Murakami, A.; Niwa, K.; Matsuda, Y.; Wada, A.; Miyake, C. Comparative analysis of strategies to prepare electron sinks in aquatic photoautotrophs. Photosynth Res. 2019, 139, 401–411. [Google Scholar] [CrossRef]
- Shaku, K.; Shimakawa, G.; Hashiguchi, M.; Miyake, C. Reduction-Induced Suppression of Electron Flow (RISE) in the Photosynthetic Electron Transport System of Synechococcus elongatus PCC 7942. Plant Cell Physiol. 2016, 57, 1443–1453. [Google Scholar] [CrossRef]
- Shimakawa, G.; Matsuda, Y.; Nakajima, K.; Tamoi, M.; Shigeoka, S.; Miyake, C. Diverse strategies of O2 usage for preventing photo-oxidative damage under CO2 limitation during algal photosynthesis. Sci. Rep. 2017, 7, 41022. [Google Scholar] [CrossRef]
- Shimakawa, G.; Shaku, K.; Miyake, C. Reduction-Induced Suppression of Electron Flow (RISE) Is Relieved by Non-ATP-Consuming Electron Flow in Synechococcus elongatus PCC 7942. Front. Microbiol. 2018, 9, 886. [Google Scholar] [CrossRef]
- Takagi, D.; Amako, K.; Hashiguchi, M.; Fukaki, H.; Ishizaki, K.; Goh, T.; Fukao, Y.; Sano, R.; Kurata, T.; Demura, T.; et al. Chloroplastic ATP synthase builds up a proton motive force preventing production of reactive oxygen species in photosystem I. Plant J. 2017, 91, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Takagi, D.; Ishizaki, K.; Hanawa, H.; Mabuchi, T.; Shimakawa, G.; Yamamoto, H.; Miyake, C. Diversity of strategies for escaping reactive oxygen species production within photosystem I among land plants: P700 oxidation system is prerequisite for alleviating photoinhibition in photosystem I. Physiol. Plant 2017, 161, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Golding, A.J.; Johnson, G.N. Down-regulation of linear and activation of cyclic electron transport during drought. Planta 2003, 218, 107–114. [Google Scholar] [CrossRef]
- Miyake, C.; Miyata, M.; Shinzaki, Y.; Tomizawa, K. CO2 response of cyclic electron flow around PSI (CEF-PSI) in tobacco leaves--relative electron fluxes through PSI and PSII determine the magnitude of non-photochemical quenching (NPQ) of Chl fluorescence. Plant Cell Physiol. 2005, 46, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Harbinson, J.; Foyer, C.H. Relationships between the Efficiencies of Photosystems I and II and Stromal Redox State in CO2-Free Air: Evidence for Cyclic Electron Flow in Vivo. Plant Physiol. 1991, 97, 41–49. [Google Scholar] [CrossRef]
- Harbinson, J.; Genty, B.; Baker, N.R. Relationship between the Quantum Efficiencies of Photosystems I and II in Pea Leaves. Plant Physiol. 1989, 90, 1029–1034. [Google Scholar] [CrossRef]
- Harbinson, J.; Genty, B.; Baker, N.R. The relationship between CO2 assimilation and electron transport in leaves. Photosynth Res. 1990, 25, 213–224. [Google Scholar] [CrossRef]
- Harbinson, J.; Genty, B.; Foyer, C.H. Relationship between Photosynthetic Electron Transport and Stromal Enzyme Activity in Pea Leaves: Toward an Understanding of the Nature of Photosynthetic Control. Plant Physiol. 1990, 94, 545–553. [Google Scholar] [CrossRef]
- Harbinson, J.; Hedley, C.L. Changes in P-700 Oxidation during the Early Stages of the Induction of Photosynthesis. Plant Physiol. 1993, 103, 649–660. [Google Scholar] [CrossRef]
- Wada, S.; Takagi, D.; Miyake, C.; Makino, A.; Suzuki, Y. Responses of the Photosynthetic Electron Transport Reactions Stimulate the Oxidation of the Reaction Center Chlorophyll of Photosystem I, P700, under Drought and High Temperatures in Rice. Int. J. Mol. Sci. 2019, 20, 2068. [Google Scholar] [CrossRef]
- von Caemmerer, S. Biochemical Models of Leaf Photosynthesis; CSIRO Publishing: Collingwood, Australia, 2000. [Google Scholar]
- Baker, N.R.; Harbinson, J.; Kramer, D.M. Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ. 2007, 30, 1107–1125. [Google Scholar] [CrossRef] [PubMed]
- Klughammer, C.; Schreiber, U. An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700+-absorbance changes at 830 nm. Planta 1994, 192, 261–268. [Google Scholar] [CrossRef]
- Tikhonov, A.N. The cytochrome b6f complex at the crossroad of photosynthetic electron transport pathways. Plant Physiol. Biochem. 2014, 81, 163–183. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.A.; Sacksteder, C.A.; Kanazawa, A.; Kramer, D.M. Contribution of electric field (Δψ) to steady-state transthylakoid proton motive force (pmf) in vitro and in vivo. control of pmf parsing into Δψ and ΔpH by ionic strength. Biochemistry 2001, 40, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Avenson, T.J.; Cruz, J.A.; Kramer, D.M. Modulation of energy-dependent quenching of excitons in antennae of higher plants. Proc. Natl. Acad. Sci. USA 2004, 101, 5530–5535. [Google Scholar] [CrossRef]
- Klughammer, C.; Siebke, K.; Schreiber, U. Continuous ECS-indicated recording of the proton-motive charge flux in leaves. Photosynth Res. 2013, 117, 471–487. [Google Scholar] [CrossRef]
- Cornic, G.; Ghashghaie, J. Effect of temperature on net CO2 assimilation and photosystem II quantum yield of electron transfer of French bean (Phaseolus vulgaris L.) leaves during drought stress. Planta 1991, 185, 255–260. [Google Scholar] [CrossRef]
- Cornic, G.; Briantais, J.M. Partitioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf (Phaseolus vulgaris L.) at different CO2 concentrations and during drought stress. Planta 1991, 183, 178–184. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Miyake, C.; Yokota, A. Determination of the rate of photoreduction of O2 in the water-water cycle in watermelon leaves and enhancement of the rate by limitation of photosynthesis. Plant Cell Physiol. 2000, 41, 335–343. [Google Scholar] [CrossRef]
- Sejima, T.; Hanawa, H.; Shimakawa, G.; Takagi, D.; Suzuki, Y.; Fukayama, H.; Makino, A.; Miyake, C. Post-illumination transient O2-uptake is driven by photorespiration in tobacco leaves. Physiol. Plant 2016, 156, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Seelert, H.; Poetsch, A.; Dencher, N.A.; Engel, A.; Stahlberg, H.; Muller, D.J. Structural biology. Proton-powered turbine of a plant motor. Nature 2000, 405, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D. Models describing the kinetics of ribulose biphosphate carboxylase-oxygenase. Arch. Biochem. Biophys. 1979, 193, 456–468. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. Models of photosynthesis. Plant Physiol. 2001, 125, 42–45. [Google Scholar] [CrossRef]
- Miyake, C.; Amako, K.; Shiraishi, N.; Sugimoto, T. Acclimation of tobacco leaves to high light intensity drives the plastoquinone oxidation system--relationship among the fraction of open PSII centers, non-photochemical quenching of Chl fluorescence and the maximum quantum yield of PSII in the dark. Plant Cell Physiol. 2009, 50, 730–743. [Google Scholar] [CrossRef]
- Kadota, K.; Furutani, R.; Makino, A.; Suzuki, Y.; Wada, S.; Miyake, C. Oxidation of P700 Induces Alternative Electron Flow in Photosystem I in Wheat Leaves. Plants 2019, 8, 152. [Google Scholar] [CrossRef]
- Ruuska, S.A.; Badger, M.R.; Andrews, T.J.; von Caemmerer, S. Photosynthetic electron sinks in transgenic tobacco with reduced amounts of Rubisco: Little evidence for significant Mehler reaction. J. Exp. Bot. 2000, 51, 357–368. [Google Scholar] [CrossRef]
- Munekage, Y.; Hojo, M.; Meurer, J.; Endo, T.; Tasaka, M.; Shikanai, T. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 2002, 110, 361–371. [Google Scholar] [CrossRef]
- Shikanai, T. Regulatory network of proton motive force: Contribution of cyclic electron transport around photosystem I. Photosynth Res. 2016, 129, 253–260. [Google Scholar] [CrossRef]
- Shikanai, T.; Yamamoto, H. Contribution of Cyclic and Pseudo-Cyclic Electron Transport to the Formation of Proton Motive Force in Chloroplasts. Mol. Plant 2017, 10, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Shikanai, T. PGR5-Dependent Cyclic Electron Flow Protects Photosystem I under Fluctuating Light at Donor and Acceptor Sides. Plant Physiol. 2019, 179, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Takahashi, S.; Badger, M.R.; Shikanai, T. Artificial remodelling of alternative electron flow by flavodiiron proteins in Arabidopsis. Nat. Plants 2016, 2, 16012. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Shikanai, T. Physiological Functions of Cyclic Electron Transport Around Photosystem I in Sustaining Photosynthesis and Plant Growth. Annu. Rev. Plant Biol. 2016, 67, 81–106. [Google Scholar] [CrossRef]
- Yamori, W.; Makino, A.; Shikanai, T. A physiological role of cyclic electron transport around photosystem I in sustaining photosynthesis under fluctuating light in rice. Sci. Rep. 2016, 6, 20147. [Google Scholar] [CrossRef]
- Kramer, D.M.; Avenson, T.J.; Edwards, G.E. Dynamic flexibility in the light reactions of photosynthesis governed by both electron and proton transfer reactions. Trends Plant Sci. 2004, 9, 349–357. [Google Scholar] [CrossRef]
- Shimakawa, G.; Miyake, C. Changing frequency of fluctuating light reveals the molecular mechanism for P700 oxidation in plant leaves. Plant Direct 2018, 2, e00073. [Google Scholar] [CrossRef]
- Zhang, M.M.; Fan, D.Y.; Murakami, K.; Badger, M.R.; Sun, G.Y.; Chow, W.S. Partially dissecting electron fluxes in both photosystems in spinach leaf discs during photosynthetic induction. Plant Cell Physiol. 2019. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, S.; Suzuki, Y.; Miyake, C. Photorespiration Enhances Acidification of the Thylakoid Lumen, Reduces the Plastoquinone Pool, and Contributes to the Oxidation of P700 at a Lower Partial Pressure of CO2 in Wheat Leaves. Plants 2020, 9, 319. https://doi.org/10.3390/plants9030319
Wada S, Suzuki Y, Miyake C. Photorespiration Enhances Acidification of the Thylakoid Lumen, Reduces the Plastoquinone Pool, and Contributes to the Oxidation of P700 at a Lower Partial Pressure of CO2 in Wheat Leaves. Plants. 2020; 9(3):319. https://doi.org/10.3390/plants9030319
Chicago/Turabian StyleWada, Shinya, Yuji Suzuki, and Chikahiro Miyake. 2020. "Photorespiration Enhances Acidification of the Thylakoid Lumen, Reduces the Plastoquinone Pool, and Contributes to the Oxidation of P700 at a Lower Partial Pressure of CO2 in Wheat Leaves" Plants 9, no. 3: 319. https://doi.org/10.3390/plants9030319
APA StyleWada, S., Suzuki, Y., & Miyake, C. (2020). Photorespiration Enhances Acidification of the Thylakoid Lumen, Reduces the Plastoquinone Pool, and Contributes to the Oxidation of P700 at a Lower Partial Pressure of CO2 in Wheat Leaves. Plants, 9(3), 319. https://doi.org/10.3390/plants9030319





