Mitochondrial DNA Repair in an Arabidopsis thaliana Uracil N-Glycosylase Mutant
Abstract
1. Introduction
2. Results
2.1. Lack of UNG Activity in Mutants
2.2. Mutation Accumulation in the Absence of UNG
2.3. Nuclear Mutation Accumulation
2.4. Alternative Repair Pathway Genes
2.5. Increased Mitochondrial Genome Abandonment
2.6. Transmission of SNPs Across Generations
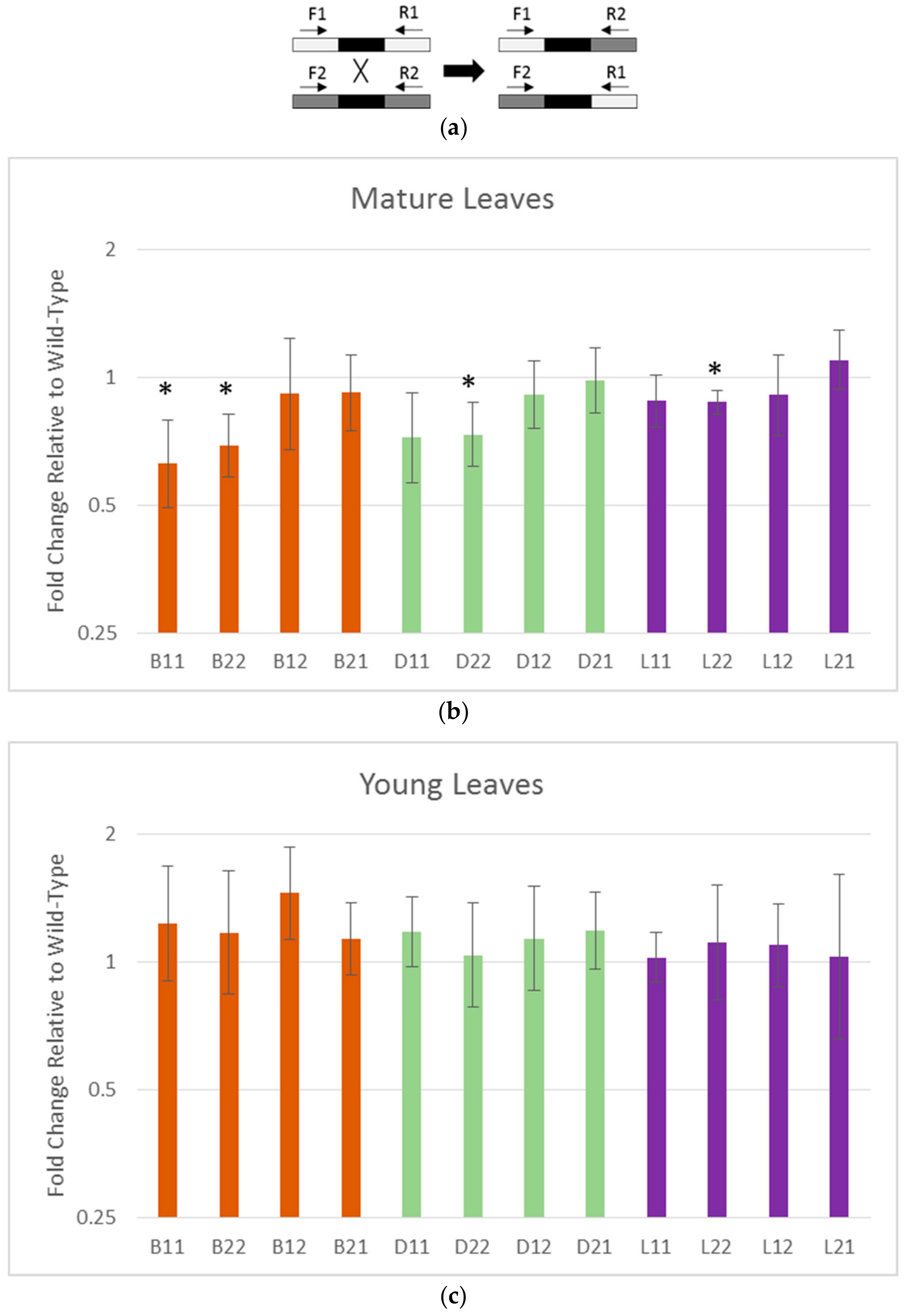
2.7. SNP Accumulation in Young vs. Mature Leaves
2.8. Quality Control of DNA Library Preparation
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions
4.2. Vector Construction
4.3. RT-PCR
4.4. Repeat Recombination qPCR
4.5. DNA Sequencing
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Drouin, G.; Daoud, H.; Xia, J. Relative rates of synonymous substitutions in the mitochondrial, chloroplast and nuclear genomes of seed plants. Mol. Phylogenet. Evol. 2008, 49, 827–831. [Google Scholar] [CrossRef]
- Palmer, J.D.; Herbon, L.A. Plant mitochondrial DNA evolves rapidly in structure, but slowly in sequence. J. Mol. Evol. 1988, 28, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.O.; Rice, D.W.; Young, G.J.; Alverson, A.J.; Palmer, J.D. The “fossilized” mitochondrial genome of Liriodendron tulipifera: Ancestral gene content and order, ancestral editing sites, and extraordinarily low mutation rate. BMC Biol. 2013, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Siekevitz, P. Powerhouse of the cell. Sci. Am. 1957, 197, 131–144. [Google Scholar] [CrossRef]
- Wolfe, K.; Li, W.; Sharp, P. Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast and nuclear DNAs. Proc. Natl. Acad. Sci. USA 1987, 84, 9054–9058. [Google Scholar] [CrossRef]
- Sloan, D.B.; Alverson, A.J.; Chuckalovcak, J.P.; Wu, M.; McCauley, D.E.; Palmer, J.D.; Taylor, D.R. Rapid evolution of enormous, multichromosomal genomes in flowering plant mitochondria with exceptionally high mutation rates. PLoS Biol. 2012, 10, e1001241. [Google Scholar] [CrossRef]
- Sloan, D.B.; Taylor, D.R. Testing for selection on synonymous sites in plant mitochondrial DNA: The role of codon bias and RNA editing. J. Mol. Evol. 2010, 70, 479–491. [Google Scholar] [CrossRef]
- Wynn, E.L.; Christensen, A.C. Are synonymous substitutions in flowering plant mitochondria neutral? J. Molec. Evol. 2015, 81, 131–135. [Google Scholar] [CrossRef]
- Kumar, R.A.; Oldenburg, D.J.; Bendich, A.J. Changes in DNA damage, molecular integrity, and copy number for plastid DNA and mitochondrial DNA during maize development. J. Exp. Bot. 2014, 65, 6425–6439. [Google Scholar] [CrossRef]
- Segui-Simarro, J.M.; Coronado, M.J.; Staehelin, L.A. The mitochondrial cycle of Arabidopsis shoot apical meristem and leaf primordium meristematic cells is defined by a perinuclear tentaculate/cage-like mitochondrion. Plant. Physiol. 2008, 148, 1380–1393. [Google Scholar] [CrossRef]
- Segui-Simarro, J.M.; Staehelin, L.A. Mitochondrial reticulation in shoot apical meristem cells of Arabidopsis provides a mechanism for homogenization of mtDNA prior to gamete formation. Plant. Signal. Behav. 2009, 4, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Boesch, P.; Ibrahim, N.; Paulus, F.; Cosset, A.; Tarasenko, V.; Dietrich, A. Plant mitochondria possess a short-patch base excision DNA repair pathway. Nucleic Acids Res. 2009, 37, 5690–5700. [Google Scholar] [CrossRef] [PubMed]
- Gualberto, J.M.; Newton, K.J. Plant mitochondrial genomes: Dynamics and mechanisms of mutation. Annu. Rev. Plant. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.C. Genes and junk in plant mitochondria—Repair mechanisms and selection. Genome Biol. Evol. 2014, 6, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.C. Mitochondrial DNA repair and genome evolution. In Annual Plant Reviews, 2nd ed.; Logan, D.C., Ed.; Wiley-Blackwell: New York, NY, USA, 2018; Volume 50, Plant Mitochondria; pp. 11–31. [Google Scholar]
- Cordoba-Cañero, D.; Dubois, E.; Ariza, R.R.; Doutriaux, M.P.; Roldan-Arjona, T. Arabidopsis uracil DNA glycosylase (UNG) is required for base excision repair of uracil and increases plant sensitivity to 5-fluorouracil. J. Biol. Chem. 2010, 285, 7475–7483. [Google Scholar] [CrossRef] [PubMed]
- Krokan, H.E.; Standal, R.; Slupphaug, G. DNA glycosylases in the base excision repair of DNA. Biochem. J. 1997, 325, 1–16. [Google Scholar] [CrossRef]
- Christensen, A.C. Plant mitochondrial genome evolution can be explained by DNA repair mechanisms. Genome Biol. Evol. 2013, 5, 1079–1086. [Google Scholar] [CrossRef]
- Wynn, E.L.; Christensen, A.C. Repeats of unusual size in plant mitochondrial genomes: Identification, incidence and evolution. G3 Genes Genomes Genet. 2019, 9, 549–559. [Google Scholar] [CrossRef]
- Klein, M.; Eckert-Ossenkopp, U.; Schmiedeberg, I.; Brandt, P.; Unseld, M.; Brennicke, A.; Schuster, W. Physical mapping of the mitochondrial genome of Arabidopsis thaliana by cosmid and YAC clones. Plant. J. 1994, 6, 447–455. [Google Scholar] [CrossRef]
- Palmer, J.D.; Shields, C.R. Tripartite structure of the Brassica campestris mitochondrial genome. Nature 1984, 307, 437. [Google Scholar] [CrossRef]
- Unseld, M.; Marienfeld, J.R.; Brandt, P.; Brennicke, A. The mitochondrial genome of Arabidopsis thaliana contains 57 genes in 366,924 nucleotides. Nat. Genet. 1997, 15, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Arrieta-Montiel, M.P.; Shedge, V.; Davila, J.; Christensen, A.C.; Mackenzie, S.A. Diversity of the arabidopsis mitochondrial genome occurs via nuclear-controlled recombination activity. Genetics 2009, 183, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Davila, J.I.; Arrieta-Montiel, M.P.; Wamboldt, Y.; Cao, J.; Hagmann, J.; Shedge, V.; Xu, Y.Z.; Weigel, D.; Mackenzie, S.A. Double-strand break repair processes drive evolution of the mitochondrial genome in Arabidopsis. BMC Biol. 2011, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Abdelnoor, R.V.; Yule, R.; Elo, A.; Christensen, A.C.; Meyer-Gauen, G.; Mackenzie, S.A. Substoichiometric shifting in the plant mitochondrial genome is influenced by a gene homologous to MutS. Proc. Natl. Acad. Sci. USA 2003, 100, 5968–5973. [Google Scholar] [CrossRef]
- Miller-Messmer, M.; Kuhn, K.; Bichara, M.; Le Ret, M.; Imbault, P.; Gualberto, J.M. RecA-dependent DNA repair results in increased heteroplasmy of the Arabidopsis mitochondrial genome. Plant. Physiol. 2012, 159, 211–226. [Google Scholar] [CrossRef]
- Shedge, V.; Arrieta-Montiel, M.; Christensen, A.C.; Mackenzie, S.A. Plant mitochondrial recombination surveillance requires unusual RecA and MutS homologs. Plant. Cell 2007, 19, 1251–1264. [Google Scholar] [CrossRef]
- Odahara, M.; Inouye, T.; Fujita, T.; Hasebe, M.; Sekine, Y. Involvement of mitochondrial-targeted RecA in the repair of mitochondrial DNA in the moss, Physcomitrella patens. Genes Genet. Syst. 2007, 82, 43–51. [Google Scholar] [CrossRef][Green Version]
- Abdelnoor, R.V.; Christensen, A.C.; Mohammed, S.; Munoz-Castillo, B.; Moriyama, H.; Mackenzie, S.A. Mitochondrial genome dynamics in plants and animals: Convergent gene fusions of a MutS homolog. J. Molec. Evol. 2006, 63, 165–173. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Wolfs, J.M.; Edgell, D.R. The monomeric GIY-YIG homing endonuclease I-BmoI uses a molecular anchor and a flexible tether to sequentially nick DNA. Nucleic Acids Res. 2013, 41, 5413–5427. [Google Scholar] [CrossRef]
- Fukui, K.; Harada, A.; Wakamatsu, T.; Minobe, A.; Ohshita, K.; Ashiuchi, M.; Yano, T. The GIY-YIG endonuclease domain of Arabidopsis MutS homolog 1 specifically binds to branched DNA structures. FEBS Lett. 2018, 592, 4066–4077. [Google Scholar] [CrossRef]
- Bendich, A.J. DNA abandonment and the mechanisms of uniparental inheritance of mitochondria and chloroplasts. Chromosome Res. Int. J. Mol. Supramol. Evol. Asp. Chromosome Biol. 2013, 21, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.M.; Martemyanova, N.; Lu, Y.; Shindo, K.; Matsuo, H.; Harris, R.S. Extensive mutagenesis experiments corroborate a structural model for the DNA deaminase domain of APOBEC3G. FEBS Lett. 2007, 581, 4761–4766. [Google Scholar] [CrossRef] [PubMed]
- Grefen, C.; Donald, N.; Hashimoto, K.; Kudla, J.; Schumacher, K.; Blatt, M.R. A ubiquitin-10 promoter-based vector set for fluorescent protein tagging facilitates temporal stability and native protein distribution in transient and stable expression studies. Plant. J. 2010, 64, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar]
- Lai, Z.; Markovets, A.; Ahdesmaki, M.; Chapman, B.; Hofmann, O.; McEwen, R.; Johnson, J.; Dougherty, B.; Barrett, J.C.; Dry, J.R. VarDict: A novel and versatile variant caller for next-generation sequencing in cancer research. Nucleic Acids Res. 2016, 44, e108. [Google Scholar] [CrossRef]
- Sandmann, S.; de Graaf, A.O.; Karimi, M.; van der Reijden, B.A.; Hellstrom-Lindberg, E.; Jansen, J.H.; Dugas, M. Evaluating variant calling tools for non-matched next-generation sequencing data. Sci. Rep. 2017, 7, 43169. [Google Scholar] [CrossRef]
- Michalovova, M.; Vyskot, B.; Kejnovsky, E. Analysis of plastid and mitochondrial DNA insertions in the nucleus (NUPTs and NUMTs) of six plant species: Size, relative age and chromosomal localization. Heredity 2013, 111, 314–320. [Google Scholar] [CrossRef]
- Richly, E.; Leister, D. NUMTs in sequenced eukaryotic genomes. Mol. Biol. Evol. 2004, 21, 1081–1084. [Google Scholar] [CrossRef]
- Stupar, R.M.; Lilly, J.W.; Town, C.D.; Cheng, Z.; Kaul, S.; Buell, C.R.; Jiang, J. Complex mtDNA constitutes an approximate 620-kb insertion on Arabidopsis thaliana chromosome 2: Implication of potential sequencing errors caused by large-unit repeats. Proc. Natl. Acad. Sci. USA 2001, 98, 5099–5103. [Google Scholar] [CrossRef]
- Halligan, D.L.; Keightley, P.D. Spontaneous mutation accumulation studies in evolutionary genetics. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 151–172. [Google Scholar] [CrossRef]
- Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 2011, 27, 2987–2993. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, A.C.; Song, D.; Alvarez, L.A.; Wall, M.K.; Almond, D.; McClellan, D.A.; Maxwell, A.; Nielsen, B.L. Characterization of a mitochondrially targeted single-stranded DNA-binding protein in Arabidopsis thaliana. Mol. Genet. Genom. 2005, 273, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Gualberto, J.M.; Mileshina, D.; Wallet, C.; Niazi, A.K.; Weber-Lotfi, F.; Dietrich, A. The plant mitochondrial genome: Dynamics and maintenance. Biochimie 2014, 100, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Khazi, F.R.; Edmondson, A.C.; Nielsen, B.L. An arabidopsis homologue of bacterial RecA that complements an E. coli recA deletion is targeted to plant mitochondria. Mol. Genet. Genom. 2003, 269, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Wallet, C.; Le Ret, M.; Bergdoll, M.; Bichara, M.; Dietrich, A.; Gualberto, J.M. The RECG1 DNA translocase is a key factor in recombination surveillance, repair, and segregation of the mitochondrial DNA in arabidopsis. Plant. Cell 2015, 27, 2907–2925. [Google Scholar] [CrossRef]
- Chen, L.; Liu, P.; Evans, T.C.; Ettwiller, L.M. DNA damage is a pervasive cause of sequencing errors, directly confounding variant identification. Science 2017, 355, 752–756. [Google Scholar] [CrossRef]
- Trasviña-Arenas, C.H.; Baruch-Torres, N.; Cordoba-Andrade, F.J.; Ayala-Garcia, V.M.; Garcia-Medel, P.L.; Diaz-Quezada, C.; Peralta-Castro, A.; Ordaz-Ortiz, J.J.; Brieba, L.G. Identification of a unique insertion in plant organellar DNA polymerases responsible for 5′-dRP lyase and strand-displacement activities: Implications for Base Excision Repair. DNA Repair. 2018, 65, 1–10. [Google Scholar] [CrossRef]
- Schaack, S.; Ho, E.K.H.; Macrae, F. Disentangling the intertwined roles of mutation, selection and drift in the mitochondrial genome. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190173. [Google Scholar] [CrossRef]
- McGrew, D.; Knight, K. Molecular design and functional organization of the RecA protein. Crit. Rev. Biochem. Mol. Biol. 2003, 38, 385–432. [Google Scholar] [CrossRef]
- Rowan, B.A.; Oldenburg, D.J.; Bendich, A.J. RecA maintains the integrity of chloroplast DNA molecules in Arabidopsis. J. Exp. Bot. 2010, 61, 2575–2588. [Google Scholar] [CrossRef]
- Xu, L.; Marians, K. A dynamic RecA filament permits DNA polymerase-catalyzed extension of the invading strand in recombination intermediates. J. Biol. Chem. 2002, 277, 14321–14328. [Google Scholar] [CrossRef] [PubMed]
- Eggler, A.L.; Lusetti, S.L.; Cox, M.M. The C terminus of the Escherichia coli RecA protein modulates the DNA binding competition with single-stranded DNA-binding protein. J. Biol. Chem. 2003, 278, 16389–16396. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Medel, P.L.; Baruch-Torres, N.; Peralta-Castro, A.; Trasvina-Arenas, C.H.; Torres-Larios, A.; Brieba, L.G. Plant organellar DNA polymerases repair double-stranded breaks by microhomology-mediated end-joining. Nucleic Acids Res. 2019, 47, 3028–3044. [Google Scholar] [CrossRef] [PubMed]
- Zaegel, V.; Guermann, B.; Le Ret, M.; Andres, C.; Meyer, D.; Erhardt, M.; Canaday, J.; Gualberto, J.M.; Imbault, P. The plant-specific ssDNA binding protein OSB1 is involved in the stoichiometric transmission of mitochondrial DNA in Arabidopsis. Plant. Cell 2006, 18, 3548–3563. [Google Scholar] [CrossRef]
- Backert, S.; Borner, T. Phage T4-like intermediates of DNA replication and recombination in the mitochondria of the higher plant Chenopodium album (L.). Curr. Genet. 2000, 37, 304–314. [Google Scholar] [CrossRef]
- Rose, R.J.; McCurdy, D.W. New beginnings: Mitochondrial renewal by massive mitochondrial fusion. Trends Plant. Sci. 2017, 22, 641–643. [Google Scholar] [CrossRef]
- Drake, J.W.; Baltz, R.H. The biochemistry of mutagenesis. Annu. Rev. Biochem. 1976, 45, 11–37. [Google Scholar] [CrossRef]
- Lewis, C.A., Jr.; Crayle, J.; Zhou, S.; Swanstrom, R.; Wolfenden, R. Cytosine deamination and the precipitous decline of spontaneous mutation during Earth’s history. Proc. Natl. Acad. Sci. USA 2016, 113, 8194–8199. [Google Scholar] [CrossRef]
- Kauppila, J.H.K.; Bonekamp, N.A.; Mourier, A.; Isokallio, M.A.; Just, A.; Kauppila, T.E.S.; Stewart, J.B.; Larsson, N.G. Base-excision repair deficiency alone or combined with increased oxidative stress does not increase mtDNA point mutations in mice. Nucleic Acids Res. 2018, 46, 6642–6669. [Google Scholar] [CrossRef]
- Carpenter, M.A.; Rajagurubandara, E.; Wijesinghe, P.; Bhagwat, A.S. Determinants of sequence-specificity within human AID and APOBEC3G. DNA Repair. 2010, 9, 579–587. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacteriummediated transformation of Arabidopsis thaliana. Plant. J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Karimi, M.; Inze, D.; Depicker, A. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant. Sci. 2002, 7, 193–195. [Google Scholar] [CrossRef]
- Onate-Sanchez, L.; Vicente-Carbajosa, J. DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Res. Notes 2008, 1, 93. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.C.; Flores-Vergara, M.A.; Krasynanski, S.; Kumar, S.; Thompson, W.F. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat. Protoc. 2006, 1, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Rowan, B.A.; Patel, V.; Weigel, D.; Schneeberger, K. Rapid and inexpensive whole-genome genotyping-by-sequencing for crossover localization and fine-scale genetic mapping. G3 Genes Genomes Genet. 2015, 5, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Rowan, B.A.; Seymour, D.K.; Chae, E.; Lundberg, D.S.; Weigel, D. Methods for Genotyping-by-Sequencing. In Genotyping: Methods and Protocols; White, S.J., Cantsilieris, S., Eds.; Springer: New York, NY, USA, 2017; pp. 221–242. [Google Scholar] [CrossRef]
- Caruccio, N. Preparation of next-generation sequencing libraries using Nextera technology: Simultaneous DNA fragmentation and adaptor tagging by in vitro transposition. Methods Mol. Biol. 2011, 733, 241–255. [Google Scholar] [CrossRef]
- Baym, M.; Kryazhimskiy, S.; Lieberman, T.D.; Chung, H.; Desai, M.M.; Kishony, R. Inexpensive multiplexed library preparation for megabase-sized genomes. PLoS ONE 2015, 10, e0128036. [Google Scholar] [CrossRef]
- Sloan, D.B.; Wu, Z.; Sharbrough, J. Correction of persistent errors in arabidopsis reference mitochondrial genomes. Plant. Cell 2018, 30, 525–527. [Google Scholar] [CrossRef]
- Berardini, T.Z.; Reiser, L.; Li, D.; Mezheritsky, Y.; Muller, R.; Strait, E.; Huala, E. The arabidopsis information resource: Making and mining the “gold standard” annotated reference plant genome. Genesis 2015, 53, 474–485. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and samtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]

| Sample | A-C | A-G | A-T | C-A | C-G | C-T | G-A | G-C | G-T | T-A | T-C | T-G | Total | GC-AT/Total Ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Col-0 | 0 | 17 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 29 | 0 |
| UNG10 115 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 7 | 0 | 15 | 0 |
| UNG10 159 | 0 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 21 | 0 | 36 | 0 |
| UNG10 163 | 1 | 305 | 10 | 3 | 1 | 3 | 7 | 0 | 1 | 21 | 281 | 0 | 633 | 0.016 |
| UNG10 176 | 0 | 97 | 3 | 0 | 0 | 0 | 1 | 0 | 2 | 4 | 76 | 0 | 183 | 0.0055 |
| UNG10 198 | 0 | 9 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 6 | 0 | 17 | 0.059 |
| UNG10 201 | 0 | 12 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 15 | 0 | 30 | 0.033 |
| UNG10 203 | 0 | 9 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 16 | 0 |
| Col-0 MTP A3G | 0 | 6 | 0 | 0 | 0 | 3 | 4 | 0 | 0 | 0 | 2 | 0 | 15 | 0.47 |
| UNG MTP A3G | 0 | 0 | 0 | 0 | 0 | 5 | 7 | 0 | 0 | 0 | 1 | 0 | 13 | 0.92 |
| Sample | GC-AT SNPs | Total SNPs | GC-AT/Total Ratio |
|---|---|---|---|
| Col-0 | 0 | 0 | 0 |
| UNG10 115 | 21 | 69 | 0.30 |
| UNG10 159 | 27 | 79 | 0.34 |
| UNG10 163 | 10 | 43 | 0.23 |
| UNG10 176 | 28 | 74 | 0.38 |
| UNG10 198 | 23 | 73 | 0.32 |
| UNG10 201 | 53 | 131 | 0.40 |
| UNG10 203 | 35 | 97 | 0.36 |
| Col-0 MTP-A3G | 0 | 0 | 0 |
| UNG MTP-A3G | 44 | 154 | 0.29 |
| Col-0 1 Young | 31 | 81 | 0.38 |
| Col-0 2 Young | 44 | 101 | 0.44 |
| UNG11 163 1 Young | 31 | 111 | 0.28 |
| UNG11 163 2 Young | 59 | 141 | 0.42 |
| UNG11 176 1 Young | 32 | 149 | 0.21 |
| UNG11 176 2 Young | 48 | 126 | 0.38 |
| UNG11 198 1 Young | 60 | 236 | 0.25 |
| UNG11 198 2 Young | 99 | 311 | 0.32 |
| Col-0 MTP-A3G 1 Young | 17 | 53 | 0.32 |
| Col-0 MTP-A3G 2 Young | 25 | 58 | 0.43 |
| UNG MTP-A3G 1 Young | 130 | 453 | 0.29 |
| UNG MTP-A3G 2 Young | 589 | 2711 | 0.22 |
| Col-0 1 Mature | 17 | 47 | 0.36 |
| Col-0 2 Mature | 15 | 43 | 0.35 |
| UNG11 163 1 Mature | 27 | 113 | 0.24 |
| UNG11 163 2 Mature | 23 | 77 | 0.30 |
| UNG11 176 1 Mature | 31 | 99 | 0.31 |
| UNG11 176 2 Mature | 27 | 93 | 0.29 |
| UNG11 198 1 Mature | 41 | 157 | 0.26 |
| UNG11 198 2 Mature | 66 | 195 | 0.34 |
| Col-0 MTP-A3G 1 Mature | 15 | 45 | 0.33 |
| Col-0 MTP-A3G 2 Mature | 13 | 37 | 0.35 |
| UNG MTP-A3G 1 Mature | 106 | 339 | 0.32 |
| UNG MTP-A3G 2 Mature | 175 | 684 | 0.26 |
| Sample | A-C | A-G | A-T | C-A | C-G | C-T | G-A | G-C | G-T | T-A | T-C | T-G | Total | GC-AT/Total Ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Col-0 1 Young | 0 | 224 | 3 | 0 | 0 | 4 | 6 | 0 | 0 | 3 | 227 | 0 | 467 | 0.0214 |
| Col-0 2 Young | 0 | 45 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 33 | 1 | 84 | 0.0238 |
| UNG11 163 1 Young | 0 | 205 | 4 | 0 | 0 | 7 | 7 | 0 | 0 | 4 | 216 | 0 | 443 | 0.0316 |
| UNG11 163 2 Young | 1 | 27 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 47 | 0 | 78 | 0.0128 |
| UNG11 176 1 Young | 0 | 65 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 3 | 79 | 0 | 149 | 0.0134 |
| UNG11 176 2 Young | 0 | 194 | 0 | 0 | 0 | 2 | 5 | 0 | 0 | 7 | 171 | 0 | 379 | 0.0185 |
| UNG11 198 1 Young | 1 | 62 | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 59 | 0 | 128 | 0.0234 |
| UNG11 198 2 Young | 0 | 63 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 4 | 68 | 0 | 138 | 0.00725 |
| Col-0 MTP A3G 1 Young | 0 | 151 | 5 | 2 | 0 | 11 | 15 | 0 | 1 | 2 | 158 | 0 | 345 | 0.0754 |
| Col-0 MTP A3G 2 Young | 0 | 154 | 2 | 0 | 0 | 7 | 3 | 0 | 1 | 6 | 136 | 0 | 309 | 0.0324 |
| UNG MTP A3G 1 Young | 0 | 84 | 0 | 0 | 0 | 3 | 4 | 0 | 0 | 2 | 82 | 0 | 175 | 0.04 |
| UNG MTP A3G 2 Young | 0 | 49 | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 46 | 2 | 103 | 0.0291 |
| Col-0 1 Mature | 0 | 50 | 0 | 0 | 0 | 3 | 3 | 0 | 0 | 1 | 54 | 0 | 111 | 0.0541 |
| Col-0 2 Mature | 0 | 65 | 2 | 1 | 0 | 2 | 1 | 0 | 0 | 4 | 60 | 0 | 135 | 0.0222 |
| UNG11 163 1 Mature | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 4 | 0 |
| UNG11 163 2 Mature | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 8 | 0 |
| UNG11 176 1 Mature | 0 | 35 | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 2 | 50 | 1 | 93 | 0.0323 |
| UNG11 176 2 Mature | 0 | 50 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 57 | 0 | 110 | 0.0273 |
| UNG11 198 1 Mature | 0 | 42 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 42 | 0 | 88 | 0.0227 |
| UNG11 198 2 Mature | 0 | 29 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 33 | 0 | 65 | 0.0154 |
| Col-0 MTP A3G 1 Mature | 0 | 4 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 8 | 0.125 |
| Col-0 MTP A3G 2 Mature | 0 | 14 | 0 | 0 | 0 | 9 | 12 | 0 | 0 | 0 | 10 | 0 | 45 | 0.467 |
| UNG MTP A3G 1 Mature | 0 | 13 | 0 | 0 | 0 | 33 | 41 | 0 | 0 | 0 | 13 | 0 | 100 | 0.74 |
| UNG MTP A3G 2 Mature | 0 | 78 | 1 | 0 | 0 | 25 | 36 | 0 | 1 | 0 | 69 | 0 | 210 | 0.290 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wynn, E.; Purfeerst, E.; Christensen, A. Mitochondrial DNA Repair in an Arabidopsis thaliana Uracil N-Glycosylase Mutant. Plants 2020, 9, 261. https://doi.org/10.3390/plants9020261
Wynn E, Purfeerst E, Christensen A. Mitochondrial DNA Repair in an Arabidopsis thaliana Uracil N-Glycosylase Mutant. Plants. 2020; 9(2):261. https://doi.org/10.3390/plants9020261
Chicago/Turabian StyleWynn, Emily, Emma Purfeerst, and Alan Christensen. 2020. "Mitochondrial DNA Repair in an Arabidopsis thaliana Uracil N-Glycosylase Mutant" Plants 9, no. 2: 261. https://doi.org/10.3390/plants9020261
APA StyleWynn, E., Purfeerst, E., & Christensen, A. (2020). Mitochondrial DNA Repair in an Arabidopsis thaliana Uracil N-Glycosylase Mutant. Plants, 9(2), 261. https://doi.org/10.3390/plants9020261





