Homologous Proteins of the Manganese Transporter PAM71 Are Localized in the Golgi Apparatus and Endoplasmic Reticulum
Abstract
1. Introduction
2. Results
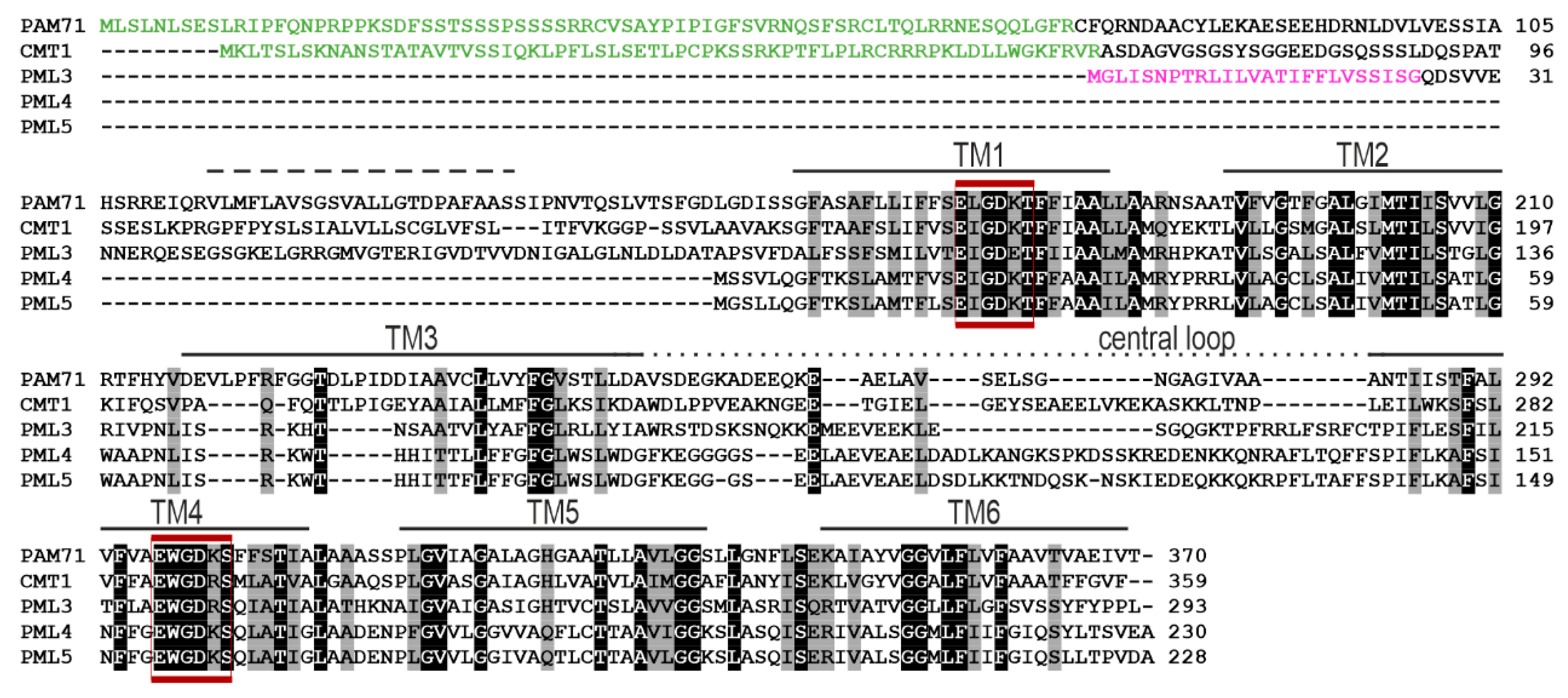
2.1. PML3, PML4 and PML5 Are Predicted Substrates of the Secretory Pathway
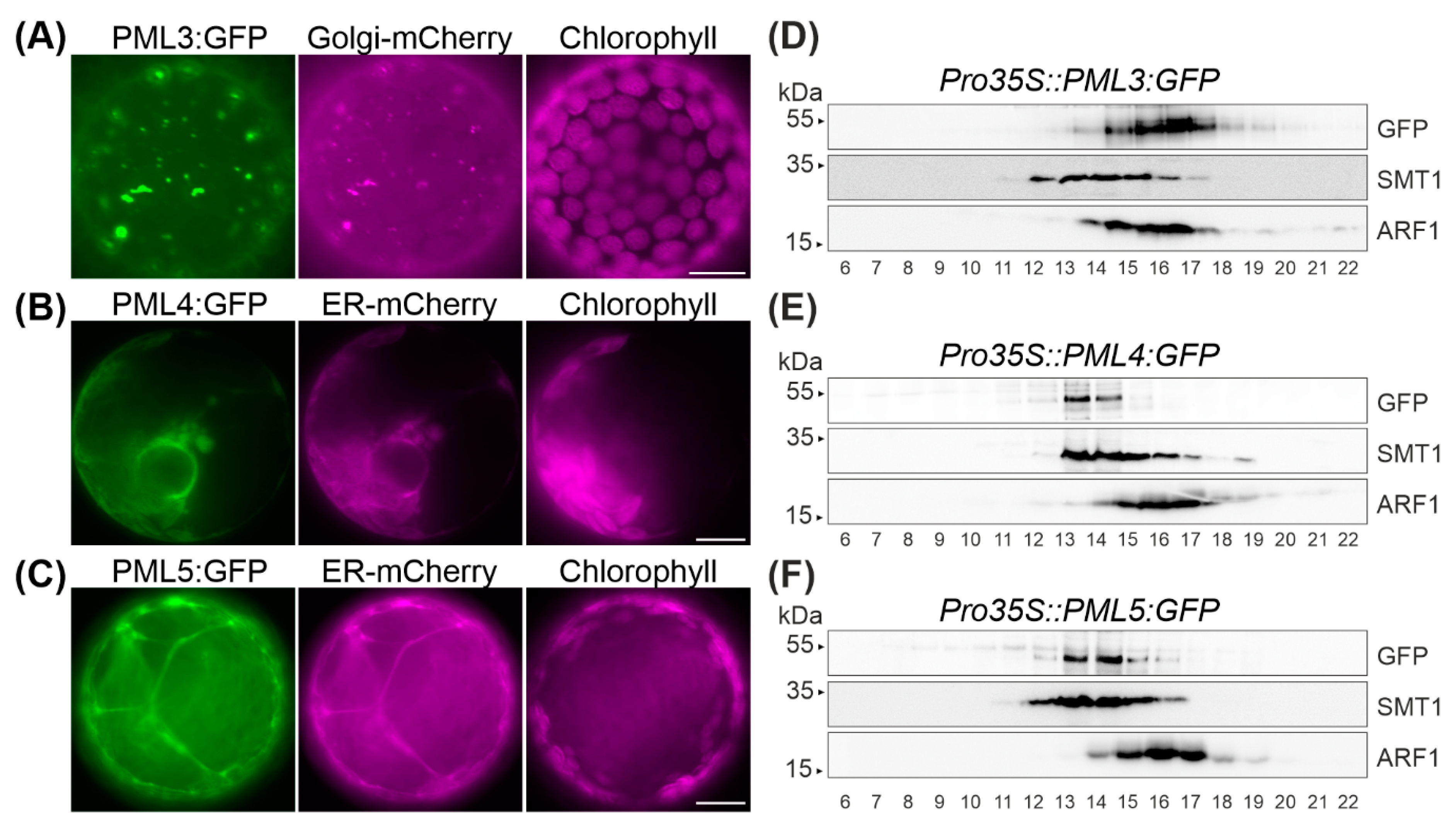
2.2. PML3 Localizes to the Golgi, and PML4 and PML5 Are Found in the Endoplasmic Reticulum
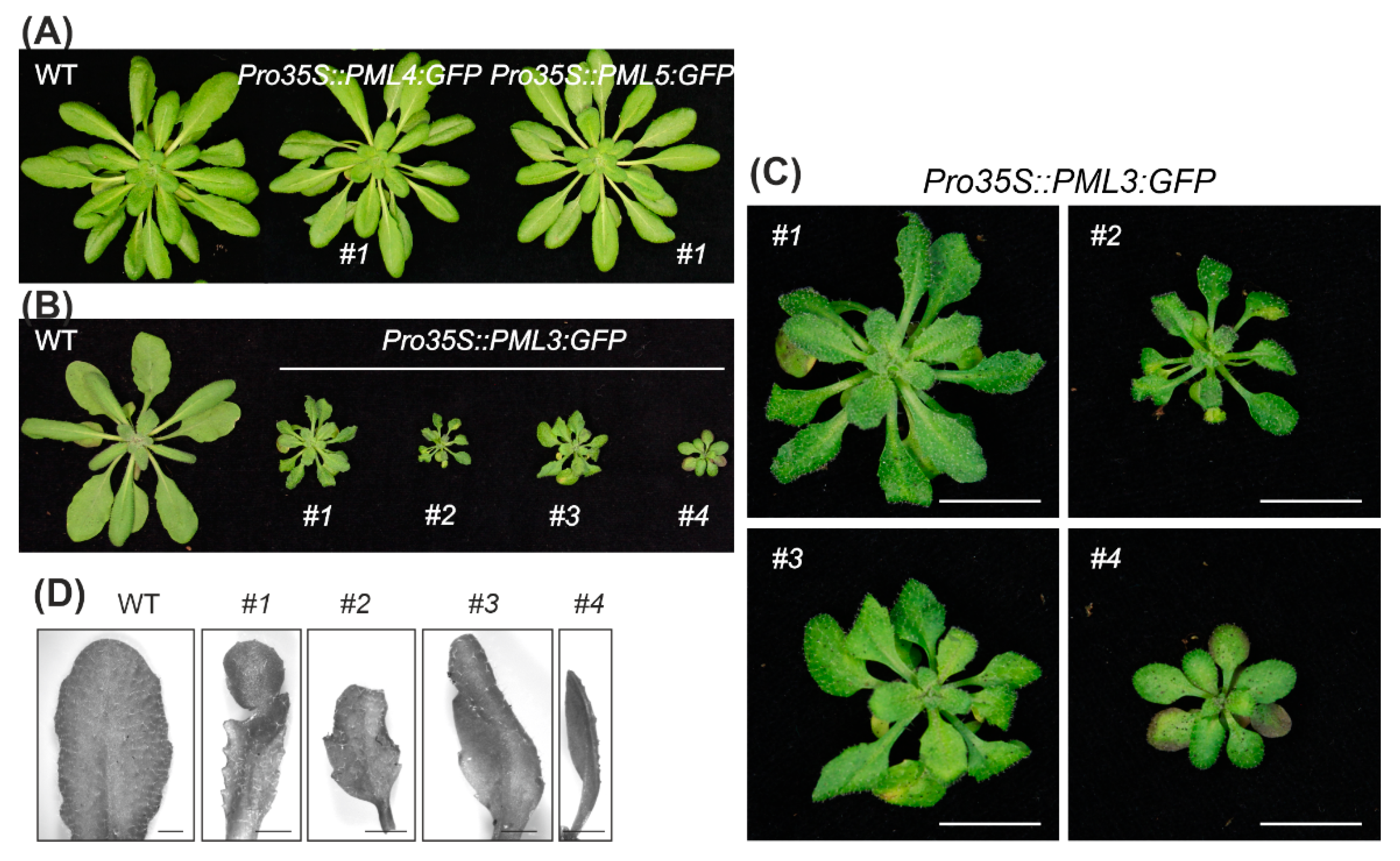
2.3. Transgenic Arabidopsis Lines Expressing PML3:GFP Show a Stunted Growth Phenotype
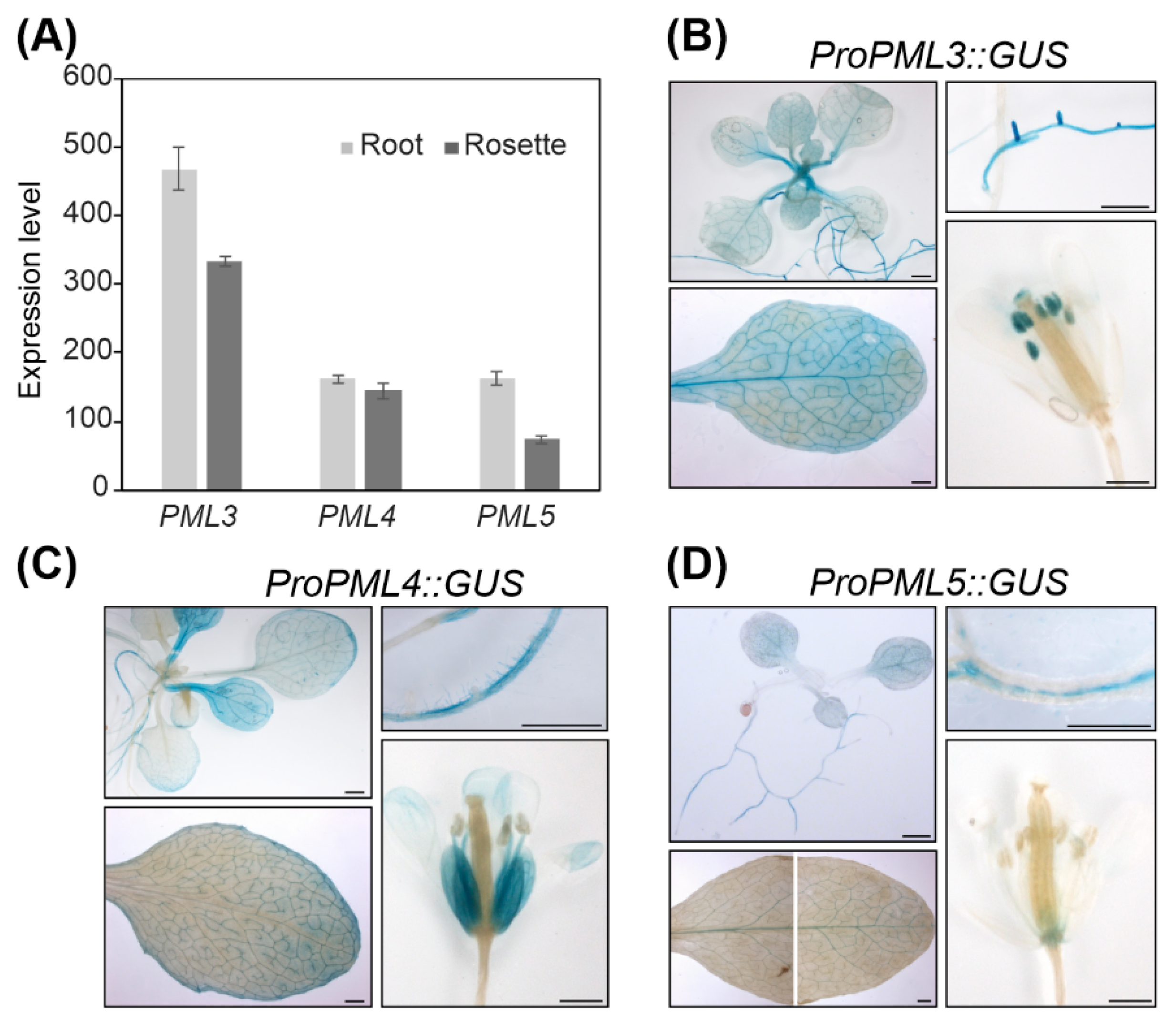
2.4. Expression Patterns of PML3, PML4 and PML5 in Arabidopsis Tissues
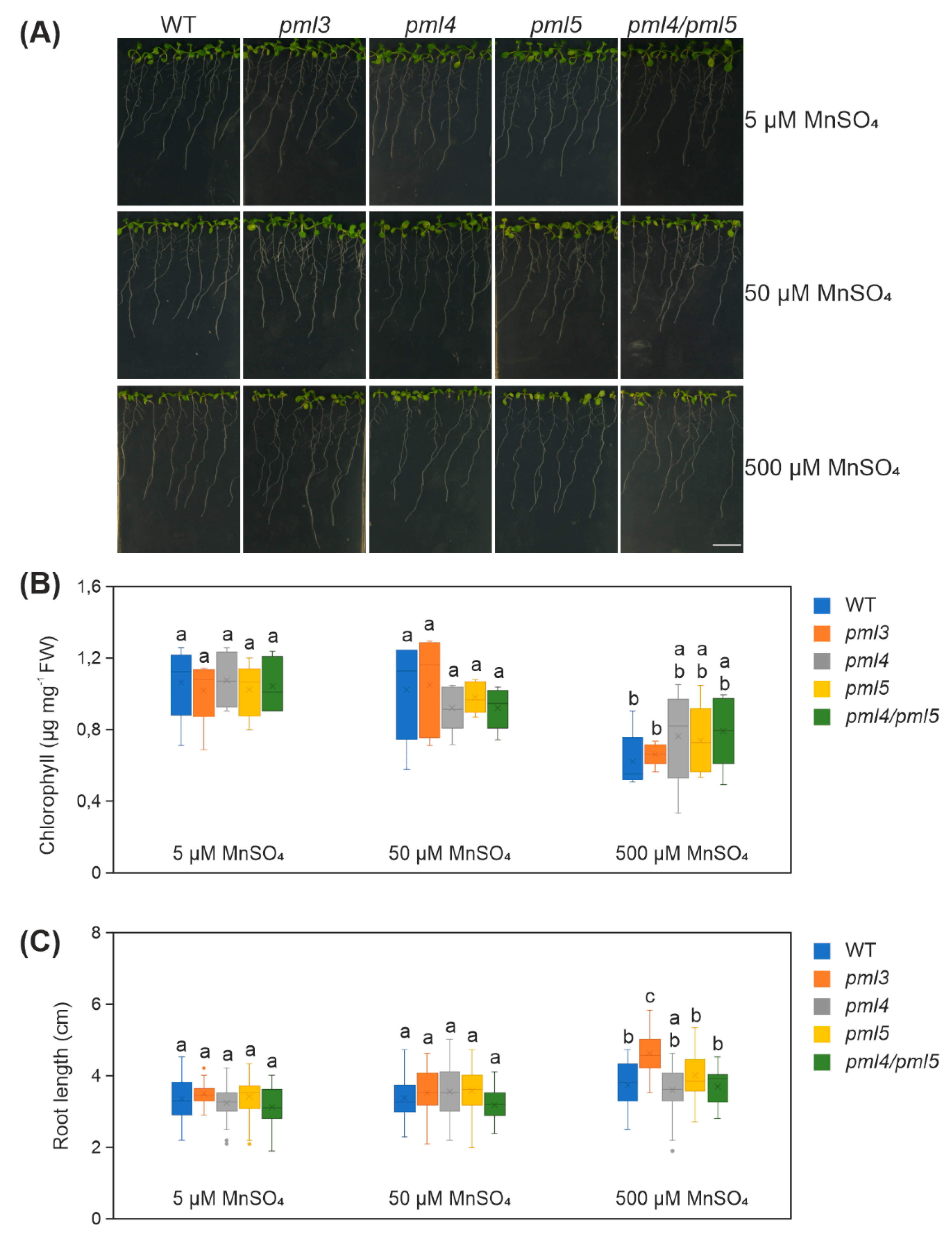
2.5. Root Elongation in pml3 Is Distinct from Other Genotypes in 500 µM MnSO4
3. Discussion
4. Materials and Methods
4.1. Plasmid Construction and Plant Material
4.2. Protoplast Isolation and Fluorescence Microscopy
4.3. Microsomal Preparation and Western Blot Analysis
4.4. Promotor Activity Analysis and Light Microscopy
4.5. Real-time PCR Analysis
4.6. Sequence Analysis and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Milner, M.J.; Seamon, J.; Craft, E.; Kochian, L.V. Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J. Exp. Bot. 2013, 64, 369–381. [Google Scholar] [CrossRef]
- Socha, A.L.; Guerinot, M.L. Mn-euvering manganese: The role of transporter gene family members in manganese uptake and mobilization in plants. Front. Plant Sci. 2014, 5, 106. [Google Scholar] [CrossRef] [PubMed]
- Pittman, J.K. Managing the manganese: Molecular mechanisms of manganese transport and homeostasis. New Phytol. 2005, 167, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Umena, Y.; Kawakami, K.; Shen, J.R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 A. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.R. The Structure of Photosystem II and the Mechanism of Water Oxidation in Photosynthesis. Annu. Rev. Plant Biol. 2015, 66, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Su, X.; Cao, P.; Liu, X.; Chang, W.; Li, M.; Zhang, X.; Liu, Z. Structure of spinach photosystem II-LHCII supercomplex at 3.2 A resolution. Nature 2016, 534, 69–74. [Google Scholar] [CrossRef]
- Su, X.; Ma, J.; Wei, X.; Cao, P.; Zhu, D.; Chang, W.; Liu, Z.; Zhang, X.; Li, M. Structure and assembly mechanism of plant C2S2M2-type PSII-LHCII supercomplex. Science 2017, 357, 815–820. [Google Scholar] [CrossRef]
- Eisenhut, M.; Hoecker, N.; Schmidt, S.B.; Basgaran, R.M.; Flachbart, S.; Jahns, P.; Eser, T.; Geimer, S.; Husted, S.; Weber, A.P.M.; et al. The Plastid Envelope CHLOROPLAST MANGANESE TRANSPORTER1 Is Essential for Manganese Homeostasis in Arabidopsis. Mol. Plant 2018, 11, 955–969. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, C.; Liu, C.; Jing, Y.; Wang, Y.; Jin, L.; Yang, L.; Fu, A.; Shi, J.; Zhao, F.; et al. Inner Envelope CHLOROPLAST MANGANESE TRANSPORTER 1 Supports Manganese Homeostasis and Phototrophic Growth in Arabidopsis. Mol. Plant 2018, 11, 943–954. [Google Scholar] [CrossRef]
- Schneider, A.; Steinberger, I.; Herdean, A.; Gandini, C.; Eisenhut, M.; Kurz, S.; Morper, A.; Hoecker, N.; Ruhle, T.; Labs, M.; et al. The Evolutionarily Conserved Protein PHOTOSYNTHESIS AFFECTED MUTANT71 Is Required for Efficient Manganese Uptake at the Thylakoid Membrane in Arabidopsis. Plant Cell 2016, 28, 892–910. [Google Scholar] [CrossRef]
- Brandenburg, F.; Schoffman, H.; Kurz, S.; Kramer, U.; Keren, N.; Weber, A.P.; Eisenhut, M. The Synechocystis Manganese Exporter Mnx Is Essential for Manganese Homeostasis in Cyanobacteria. Plant. Physiol. 2017, 173, 1798–1810. [Google Scholar] [CrossRef] [PubMed]
- Gandini, C.; Schmidt, S.B.; Husted, S.; Schneider, A.; Leister, D. The transporter SynPAM71 is located in the plasma membrane and thylakoids, and mediates manganese tolerance in Synechocystis PCC6803. New Phytol. 2017, 215, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Lanquar, V.; Ramos, M.S.; Lelievre, F.; Barbier-Brygoo, H.; Krieger-Liszkay, A.; Kramer, U.; Thomine, S. Export of vacuolar manganese by AtNRAMP3 and AtNRAMP4 is required for optimal photosynthesis and growth under manganese deficiency. Plant. Physiol. 2010, 152, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, S.; Meier, B.; von Wiren, N.; Peiter, E. The Vacuolar Manganese Transporter MTP8 Determines Tolerance to Iron Deficiency-Induced Chlorosis in Arabidopsis. Plant. Physiol. 2016, 170, 1030–1045. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, S.; Giehl, R.F.H.; Meier, B.; Takahashi, M.; Terada, Y.; Ignatyev, K.; Andresen, E.; Kupper, H.; Peiter, E.; von Wiren, N. Metal Tolerance Protein 8 Mediates Manganese Homeostasis and Iron Reallocation during Seed Development and Germination. Plant. Physiol. 2017, 174, 1633–1647. [Google Scholar] [CrossRef]
- Peiter, E.; Montanini, B.; Gobert, A.; Pedas, P.; Husted, S.; Maathuis, F.J.; Blaudez, D.; Chalot, M.; Sanders, D. A secretory pathway-localized cation diffusion facilitator confers plant manganese tolerance. Proc. Natl. Acad. Sci. USA 2007, 104, 8532–8537. [Google Scholar] [CrossRef]
- Delhaize, E.; Gruber, B.D.; Pittman, J.K.; White, R.G.; Leung, H.; Miao, Y.; Jiang, L.; Ryan, P.R.; Richardson, A.E. A role for the AtMTP11 gene of Arabidopsis in manganese transport and tolerance. Plant J. 2007, 51, 198–210. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, F.; Hong, B.; Young, J.C.; Sussman, M.R.; Harper, J.F.; Sze, H. An endoplasmic reticulum-bound Ca2+/Mn2+ pump, ECA1, supports plant growth and confers tolerance to Mn2+ stress. Plant. Physiol. 2002, 130, 128–137. [Google Scholar] [CrossRef]
- Mills, R.F.; Doherty, M.L.; Lopez-Marques, R.L.; Weimar, T.; Dupree, P.; Palmgren, M.G.; Pittman, J.K.; Williams, L.E. ECA3, a Golgi-localized P2A-type ATPase, plays a crucial role in manganese nutrition in Arabidopsis. Plant. Physiol. 2008, 146, 116–128. [Google Scholar] [CrossRef]
- Li, X.; Chanroj, S.; Wu, Z.; Romanowsky, S.M.; Harper, J.F.; Sze, H. A distinct endosomal Ca2+/Mn2+ pump affects root growth through the secretory process. Plant. Physiol. 2008, 147, 1675–1689. [Google Scholar] [CrossRef]
- Alejandro, S.; Cailliatte, R.; Alcon, C.; Dirick, L.; Domergue, F.; Correia, D.; Castaings, L.; Briat, J.F.; Mari, S.; Curie, C. Intracellular Distribution of Manganese by the Trans-Golgi Network Transporter NRAMP2 Is Critical for Photosynthesis and Cellular Redox Homeostasis. Plant Cell 2017, 29, 3068–3084. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Xie, W.; Yang, C.; Xu, J.; Li, J.; Wang, H.; Chen, X.; Huang, C.F. NRAMP2, a trans-Golgi network-localized manganese transporter, is required for Arabidopsis root growth under manganese deficiency. New Phytol. 2018, 217, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Hoecker, N.; Leister, D.; Schneider, A. Plants contain small families of UPF0016 proteins including the PHOTOSYNTHESIS AFFECTED MUTANT71 transporter. Plant Signal. Behav. 2017, 12, e1278101. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.tcdb.org/ (accessed on 12 February 2020).
- Demaegd, D.; Colinet, A.S.; Deschamps, A.; Morsomme, P. Molecular evolution of a novel family of putative calcium transporters. PLoS ONE 2014, 9, e100851. [Google Scholar] [CrossRef]
- Nelson, B.K.; Cai, X.; Nebenfuhr, A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 2007, 51, 1126–1136. [Google Scholar] [CrossRef]
- Boutte, Y.; Frescatada-Rosa, M.; Men, S.; Chow, C.M.; Ebine, K.; Gustavsson, A.; Johansson, L.; Ueda, T.; Moore, I.; Jurgens, G.; et al. Endocytosis restricts Arabidopsis KNOLLE syntaxin to the cell division plane during late cytokinesis. EMBO J. 2010, 29, 546–558. [Google Scholar] [CrossRef]
- Matheson, L.A.; Suri, S.S.; Hanton, S.L.; Chatre, L.; Brandizzi, F. Correct targeting of plant ARF GTPases relies on distinct protein domains. Traffic 2008, 9, 103–120. [Google Scholar] [CrossRef]
- Available online: http://bbc.botany.utoronto.ca (accessed on 12 February 2020).
- Leskova, A.; Giehl, R.F.H.; Hartmann, A.; Fargasova, A.; von Wiren, N. Heavy Metals Induce Iron Deficiency Responses at Different Hierarchic and Regulatory Levels. Plant. Physiol. 2017, 174, 1648–1668. [Google Scholar] [CrossRef]
- Gruber, B.D.; Giehl, R.F.; Friedel, S.; von Wiren, N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant. Physiol. 2013, 163, 161–179. [Google Scholar] [CrossRef]
- Demaegd, D.; Foulquier, F.; Colinet, A.S.; Gremillon, L.; Legrand, D.; Mariot, P.; Peiter, E.; Van Schaftingen, E.; Matthijs, G.; Morsomme, P. Newly characterized Golgi-localized family of proteins is involved in calcium and pH homeostasis in yeast and human cells. Proc. Natl. Acad. Sci. USA 2013, 110, 6859–6864. [Google Scholar] [CrossRef]
- Potelle, S.; Morelle, W.; Dulary, E.; Duvet, S.; Vicogne, D.; Spriet, C.; Krzewinski-Recchi, M.A.; Morsomme, P.; Jaeken, J.; Matthijs, G.; et al. Glycosylation abnormalities in Gdt1p/TMEM165 deficient cells result from a defect in Golgi manganese homeostasis. Hum. Mol. Genet. 2016, 25, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Foulquier, F.; Amyere, M.; Jaeken, J.; Zeevaert, R.; Schollen, E.; Race, V.; Bammens, R.; Morelle, W.; Rosnoblet, C.; Legrand, D.; et al. TMEM165 deficiency causes a congenital disorder of glycosylation. Am. J. Hum. Genet. 2012, 91, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Driouich, A.; Follet-Gueye, M.L.; Bernard, S.; Kousar, S.; Chevalier, L.; Vicre-Gibouin, M.; Lerouxel, O. Golgi-mediated synthesis and secretion of matrix polysaccharides of the primary cell wall of higher plants. Front. Plant Sci. 2012, 3, 79. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.F.; Harholt, J.; Oikawa, A.; Scheller, H.V. Plant Glycosyltransferases Beyond CAZy: A Perspective on DUF Families. Front. Plant Sci. 2012, 3, 59. [Google Scholar] [CrossRef]
- Nunan, K.J.; Scheller, H.V. Solubilization of an arabinan arabinosyltransferase activity from mung bean hypocotyls. Plant. Physiol. 2003, 132, 331–342. [Google Scholar] [CrossRef]
- Strasser, R. Plant protein glycosylation. Glycobiology 2016, 26, 926–939. [Google Scholar] [CrossRef]
- Amos, R.A.; Mohnen, D. Critical Review of Plant Cell Wall Matrix Polysaccharide Glycosyltransferase Activities Verified by Heterologous Protein Expression. Front. Plant Sci. 2019, 10, 915. [Google Scholar] [CrossRef]
- Saito, F.; Suyama, A.; Oka, T.; Yoko, O.T.; Matsuoka, K.; Jigami, Y.; Shimma, Y.I. Identification of Novel Peptidyl Serine alpha-Galactosyltransferase Gene Family in Plants. J. Biol. Chem. 2014, 289, 20405–20420. [Google Scholar] [CrossRef]
- Karimi, M.; Inze, D.; Depicker, A. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 2002, 7, 193–195. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Schweiger, R.; Schwenkert, S. Protein-protein interactions visualized by bimolecular fluorescence complementation in tobacco protoplasts and leaves. J. Vis. Exp. 2014, 85, e51327. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, R.; Muller, N.C.; Schmitt, M.J.; Soll, J.; Schwenkert, S. AtTPR7 is a chaperone-docking protein of the Sec translocon in Arabidopsis. J. Cell Sci. 2012, 125 Pt 21, 5196–5207. [Google Scholar] [CrossRef]
- Ruge, H.; Flosdorff, S.; Ebersberger, I.; Chigri, F.; Vothknecht, U.C. The calmodulin-like proteins AtCML4 and AtCML5 are single-pass membrane proteins targeted to the endomembrane system by an N-terminal signal anchor sequence. J. Exp. Bot. 2016, 67, 3985–3996. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ebi.ac.uk/Tools/msa/clustalo/ (accessed on 12 February 2020).
- Available online: http://www.cbs.dtu.dk/services/TargetP (accessed on 12 February 2020).
- Schwacke, R.; Schneider, A.; van der Graaff, E.; Fischer, K.; Catoni, E.; Desimone, M.; Frommer, W.B.; Flugge, U.I.; Kunze, R. ARAMEMNON, a novel database for Arabidopsis integral membrane proteins. Plant. Physiol. 2003, 131, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://aramemnon.uni-koeln.de/ (accessed on 12 February 2020).
- Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 12 February 2020).
- Available online: https://astatsa.com/OneWay_Anova_with_TukeyHSD/ (accessed on 12 February 2020).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoecker, N.; Honke, A.; Frey, K.; Leister, D.; Schneider, A. Homologous Proteins of the Manganese Transporter PAM71 Are Localized in the Golgi Apparatus and Endoplasmic Reticulum. Plants 2020, 9, 239. https://doi.org/10.3390/plants9020239
Hoecker N, Honke A, Frey K, Leister D, Schneider A. Homologous Proteins of the Manganese Transporter PAM71 Are Localized in the Golgi Apparatus and Endoplasmic Reticulum. Plants. 2020; 9(2):239. https://doi.org/10.3390/plants9020239
Chicago/Turabian StyleHoecker, Natalie, Anna Honke, Katharina Frey, Dario Leister, and Anja Schneider. 2020. "Homologous Proteins of the Manganese Transporter PAM71 Are Localized in the Golgi Apparatus and Endoplasmic Reticulum" Plants 9, no. 2: 239. https://doi.org/10.3390/plants9020239
APA StyleHoecker, N., Honke, A., Frey, K., Leister, D., & Schneider, A. (2020). Homologous Proteins of the Manganese Transporter PAM71 Are Localized in the Golgi Apparatus and Endoplasmic Reticulum. Plants, 9(2), 239. https://doi.org/10.3390/plants9020239






