Exogenous Isoprene Confers Physiological Benefits in a Negligible Isoprene Emitter (Acer monspessulanum L.) under Water Deficit
Abstract
:1. Introduction
2. Results
2.1. Verification of the Negligible Terpene Emissions from A. monspessulanum
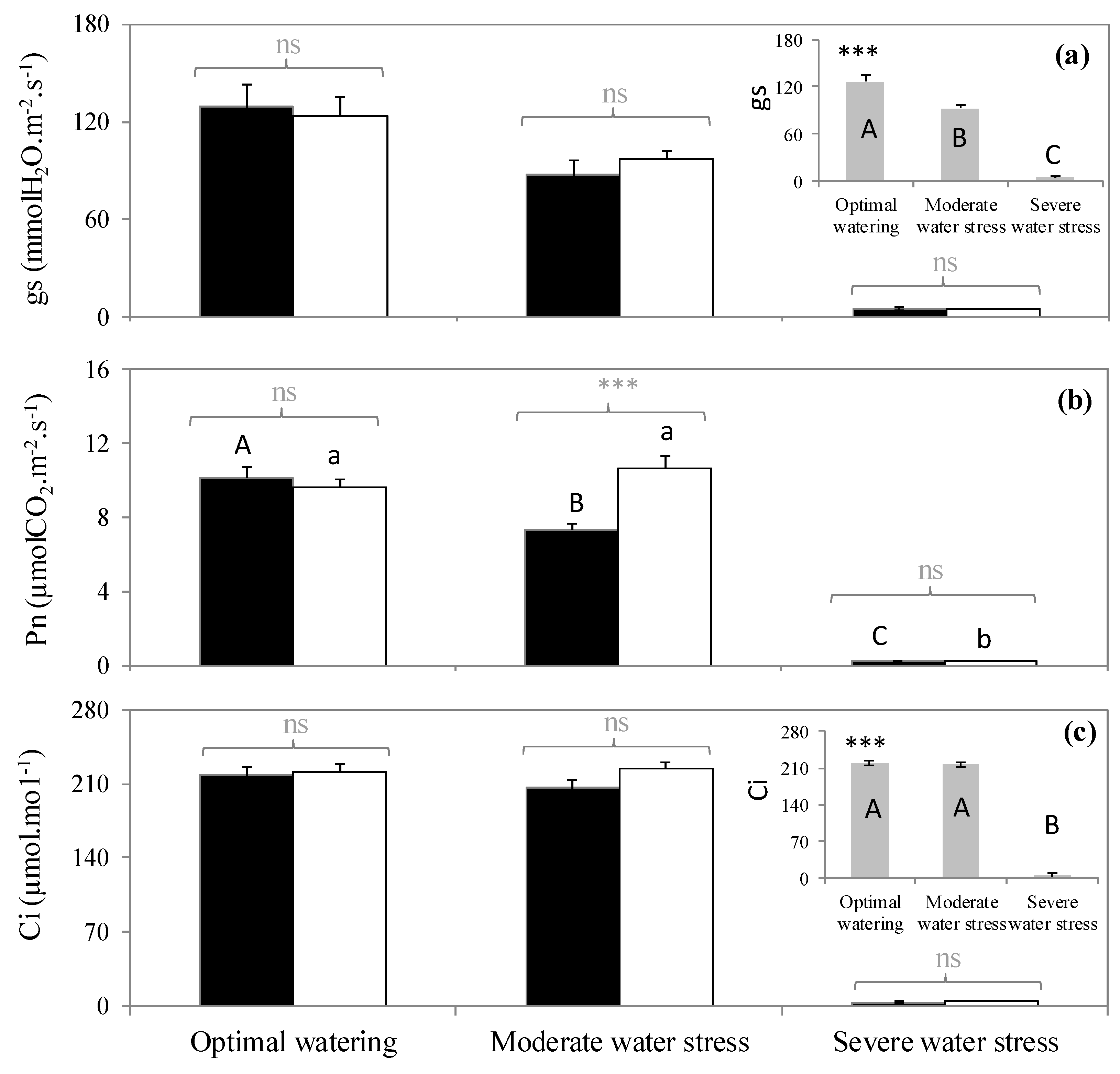
2.2. Variation of Physiological Traits According to Watering and Fumigation Treatments
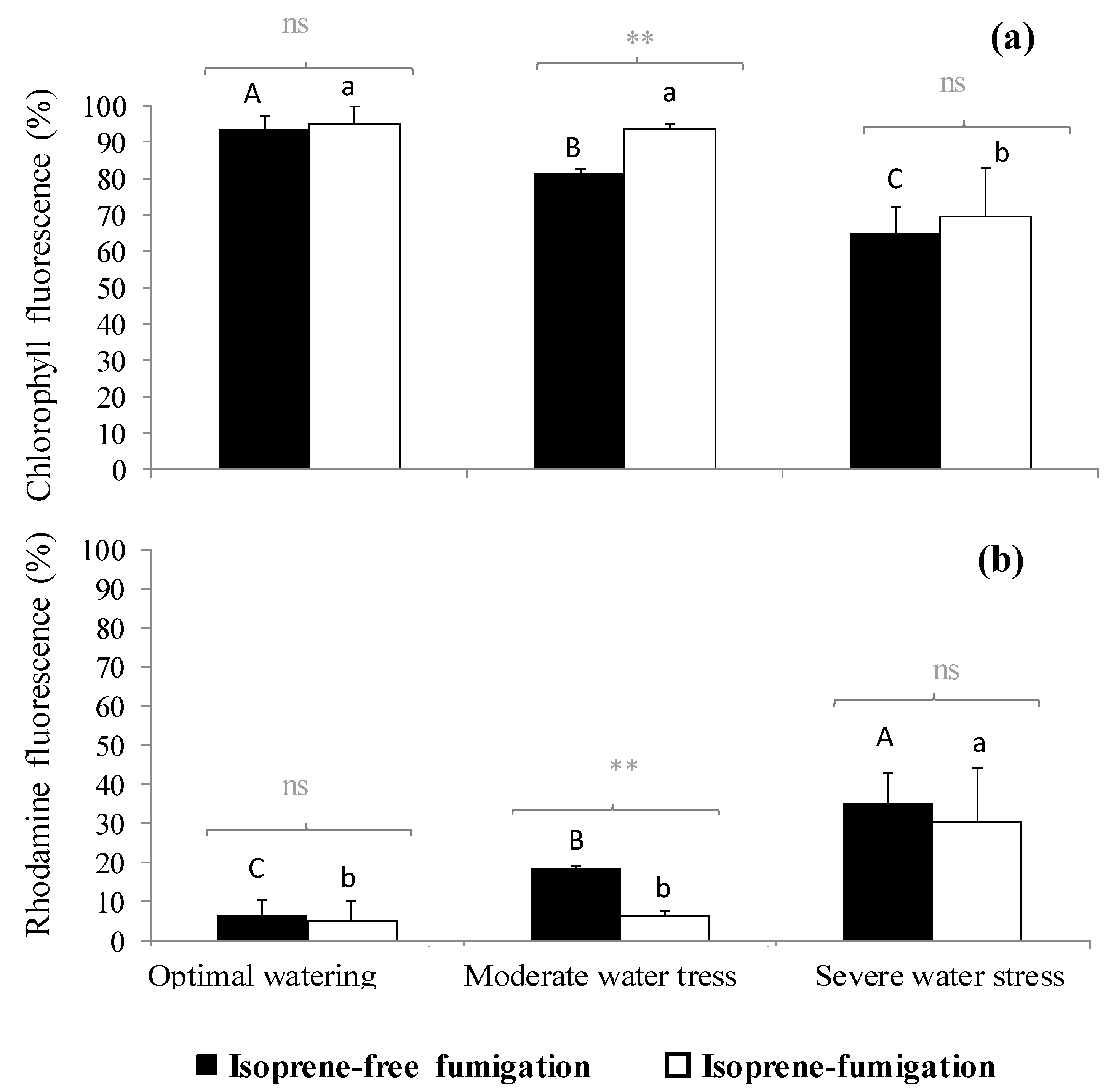
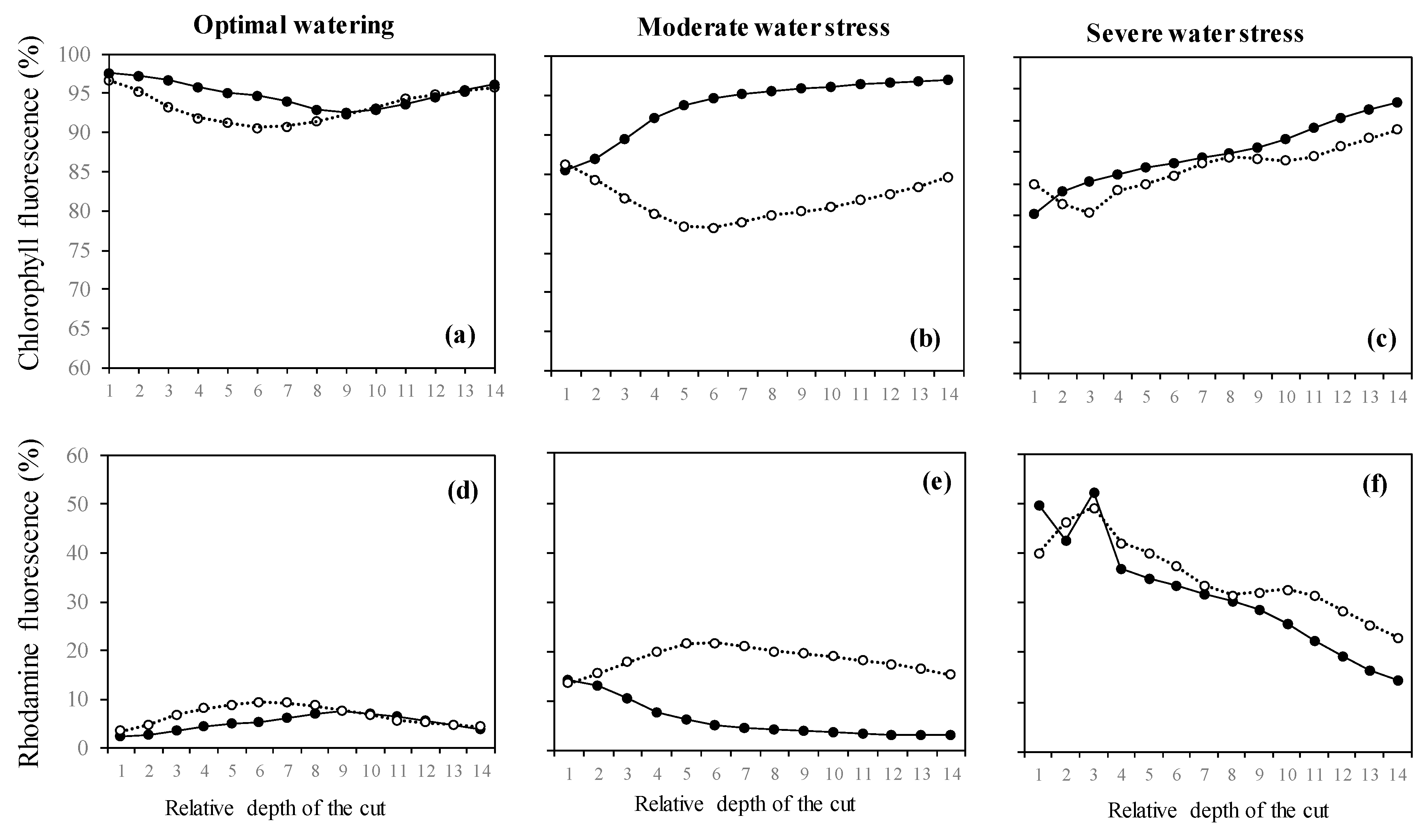
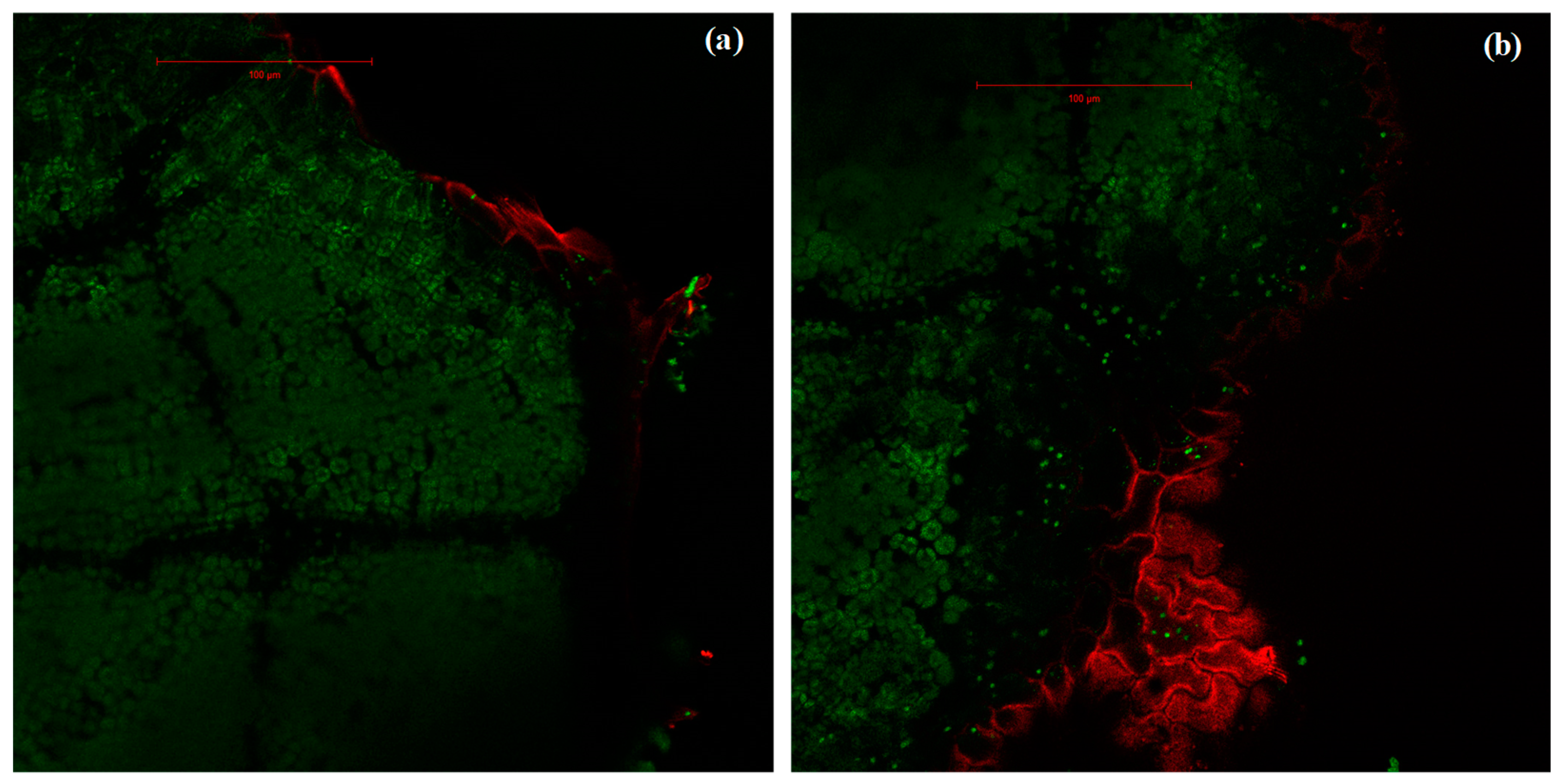
2.3. Variation of the Oxidative Pressure (H2O2) According to Watering and Fumigation Treatments
3. Discussion
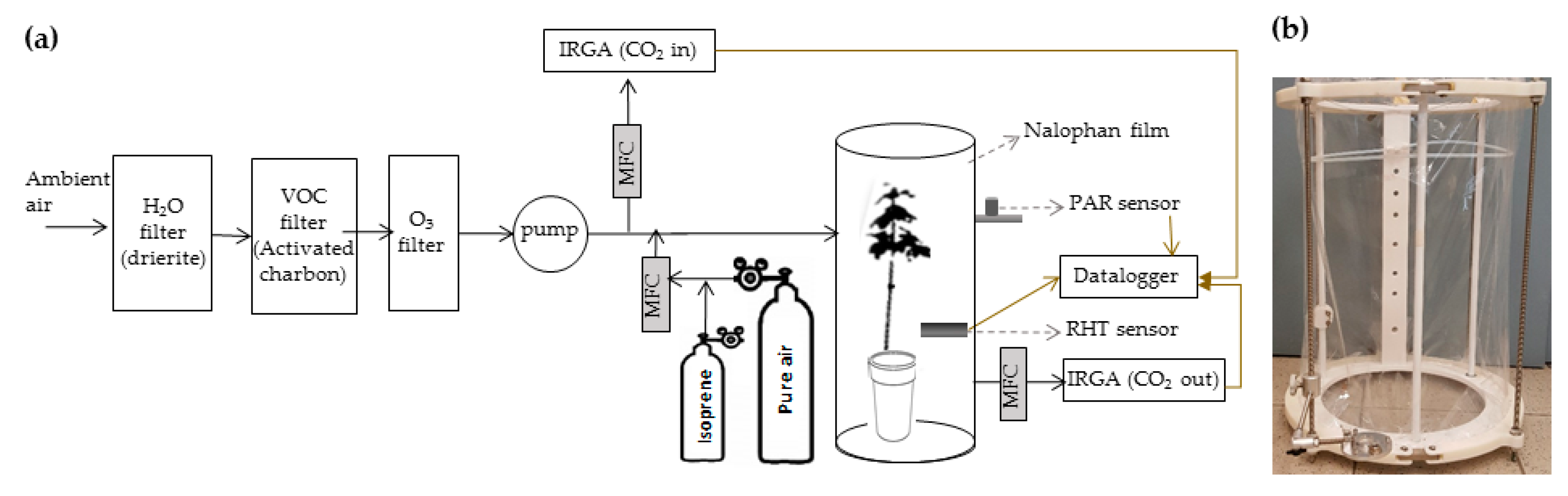
4. Materials and Methods
4.1. Acer Monspessulanum Saplings
4.2. Watering Treatments
4.3. Acer Monspessulanum Fumigation Treatments
4.4. Physiological Performances of A. Monspessulanum at the Leaf Scale
4.5. Estimation of ROS Presence Using Laser-Scanning Confocal Microscopy
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Keenan, T.; Niinemets, U.; Sabate, S.; Gracia, C.; Peñuelas, J. Process based inventory of isoprenoid emissions from European forests: model comparisons, current knowledge and uncertainties. Atmos. Chem. Phys. Discuss. 2009, 9, 6147–6206. [Google Scholar] [CrossRef] [Green Version]
- Owen, S.M.; Boissard, C.; Hewitt, C.N. Volatile organic compounds (VOCs) emitted from 40 Mediterranean plant species: VOC speciation and extrapolation to habitat scale. Atmos. Envrion. 2001, 35, 5393–5409. [Google Scholar] [CrossRef]
- Sindelarova, K.; Granier, C.; Bouarar, I.; Guenther, A.; Tilmes, S.; Stavrakou, T.; Muller, J.F.; Kuhn, U.; Stefani, P.; Knorr, W. Global data set of biogenic VOC emissions calculated by the MEGAN model over the last 30 years. Atmos. Chem. Phys. 2014, 14, 9317–9341. [Google Scholar] [CrossRef] [Green Version]
- Velikova, Violeta. Isoprene as a tool for plant protection against abiotic stresses. J. Plant Interact. 2008, 3, 1–15. [Google Scholar] [CrossRef]
- Visakorpi, K.; Gripenberg, S.; Malhi, Y.; Bolas, C.; Oliveras, I.; Harris, N.; Rifai, S.; Riutta, T. Small-scale indirect plant responses to insect herbivory could have major impacts on canopy photosynthesis and isoprene emission. New Phytol. 2018, 220, 799–810. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Monson, R.K. Isoprene research - 60 years later, the biology is still enigmatic. Plant Cell Envrion. 2017, 40, 1671–1678. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusià, J. Plant VOC emissions: making use of the unavoidable. Trends Plant Sci. 2004, 19, N° 8. [Google Scholar]
- Velikova, V.; Sharkey, T.D.; Loreto, F. Stabilization of thylakoid membranes in isoprene-emitting plants reduces formation of reactive oxygen species. Plant Signal. Behav. 2012, 7, 139–141. [Google Scholar] [CrossRef] [Green Version]
- Ryan, A.C.; Hewitt, C.N.; Possell, M.; Vickers, C.E.; Purnell, A.; Mullineaux, P.M.; Davies, W.J.; Dodd, I.C. Isoprene emission protects photosynthesis but reduces plant productivity during drought in transgenic tobacco (Nicotiana tabacum) plants. New Phytol. 2014, 201, 205–216. [Google Scholar] [CrossRef]
- Velikova, V.; Edreva, A.; Loreto, F. Endogenous isoprene protects Phragmites australis leaves against singlet oxygen. Physiol. Plant. 2004, 122, 219–225. [Google Scholar] [CrossRef]
- Affek, H.P.; Yakir, D. Protection by isoprene against singlet oxygen in leaves. Plant Physiol. 2002, 129, 269–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharkey, T.D.; Yeh, S. Isoprene emissions from plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 32. [Google Scholar]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Fares, S.; Loreto, F. Isoprene and nitric oxide reduce damages in leaves exposed to oxidative stress. Plant Cell Envrion. 2008, 31, 1882–1894. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Chen, X.Y.; Yeh, S. Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiol. 2001, 125, 2001–2006. [Google Scholar] [CrossRef] [Green Version]
- Peñuelas, J.; Llusia, J.; Asensio, D.; Munne-Bosch, S. Linking isoprene with plant thermotolerance, antioxidants and monoterpene emissions. Plant Cell Envrion. 2005, 28, 278–286. [Google Scholar] [CrossRef]
- Genard-Zielinski, A.C.; Fernandez, C.; Boissard, C.; Kalokridis, C.; Gros, V.; Lathière, J.; Orts, J.P.; Greff, S.; Bonnaire, N.; Ormenõ, E. Variability of BVOC emissions from a Mediterranean mixed forest in southern France with a focus on Quercus pubescens. Atmos. Chem. Phys. 2015, 14, 17225–17261. [Google Scholar] [CrossRef] [Green Version]
- Polade, S.D.; Pierce, D.W.; Cayan, D.R.; Gershunov, A.; Dettinger, M.D. The key role of dry days in changing regional climate and precipitation regimes. Sci. Rep. 2014. [Google Scholar] [CrossRef] [Green Version]
- Borges, R.M.; Bessière, J.-M.; Hossaert-McKey, M. The chemical ecology of seed dispersal in monoecious and dioecious Figures. Funct. Ecol. 2008. [Google Scholar] [CrossRef]
- Himanen, S.J.; Blande, J.D.; Klemola, T.; Pulkkinen, J.; Heijari, J.; Holopainen, J.K. Birch (Betula spp.) leaves adsorb and re-release volatiles specific to neighbouring plants - a mechanism for associational herbivore resistance? New Phytol. 2010, 186, 722–732. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Fares, S.; Harley, P.; Jardine, K. Bidirectional exchange of biogenic volatiles with vegetation: emission sources, reactions, breakdown and deposition (English). Plant Cell Envrion. 2014, 37, 1790–1809. [Google Scholar] [CrossRef]
- Tissier, J.; Lambs, L.; Peltier, J.P.; Marigo, G. Relationships between hydraulic traits and habitat preference for six Acer species occurring in the French Alps. Ann. Sci. 2004, 61, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Saunier, A.; Ormeño, E.; Havaux, M.; Wortham, H.; Ksas, B.; Temime-Roussel, B.; Blande, J.D.; Lecareux, C.; Mevy, J.-P.; Bousquet-Melou, A.; et al. Resistance of native oak to recurrent drought conditions simulating predicted climatic changes in the Mediterranean region. Plant Cell Envrion. 2018, 41, 2299–2312. [Google Scholar] [CrossRef] [Green Version]
- Genard-Zielinski, A.C.; Boissard, C.; Ormeño, E.; Lathière, J.; Reiter, I.M.; Wortham, H.; Orts, J.P.; Temime-Roussel, B.; Guenet, B.; Bartsch, S.; et al. Seasonal variations of Quercus pubescens isoprene emissions from an in natura forest under drought stress and sensitivity to future climate change in the Mediterranean area. Biogeosciences 2018, 15, 4711–4730. [Google Scholar] [CrossRef]
- Saunier, A.; Ormeño, E.; Boissard, C.; Wortham, H.; Temime-Roussel, B.; Lecareux, C.; Armengaud, A.; Fernandez, C. Effect of mid-term drought on Quercus pubescens BVOCs’ emission seasonality and their dependency on light and/or temperature. Atmos. Chem. Phys. 2017, 17, 7555–7566. [Google Scholar] [CrossRef] [Green Version]
- Kalogridis, C.; Gros, V.; Sarda-Esteve, R.; Langford, B.; Loubet, B.; Bonsang, B.; Bonnaire, N.; Nemitz, E.; Genard, A.-C.; Boissard, C.; et al. Concentrations and fluxes of isoprene and oxygenated VOCs at a French Mediterranean oak forest. Atmos. Chem. Phys. 2014, 14, 10085–10102. [Google Scholar] [CrossRef] [Green Version]
- Singsaas, E.L.; Lerdau, M.; Winter, K.; Sharkey, T.D. Isoprene Increases Thermotolerance of Isoprene-Emitting Species. Plant Physiol. 1997, 115, 1413. [Google Scholar] [CrossRef] [Green Version]
- Delfine, S.; Csiky, O.; Seufert, G.; Loreto, F. Fumigation with exogenous monoterpenes of a non-isoprenoid-emitting oak (Quercus suber): monoterpene acquisition, translocation, and effect on the photosynthetic properties at high temperatures. New Phytol. 2000, 146, 27–36. [Google Scholar] [CrossRef]
- Copolovici, L.O.; Filella, I.; Llusia, J.; Niinemets, U.; Penuelas, J. The capacity for thermal protection of photosynthetic electron transport varies for different monoterpenes in Quercus ilex. Plant Physiol. 2005, 139, 485–496. [Google Scholar] [CrossRef] [Green Version]
- Loreto, F.; Forster, A.; Durr, M.; Csiky, O.; Seufert, G. On the monoterpene emission under heat stress and on the increased thermotolerance of leaves of Quercus ilex fumigated with selected monoterpenes. Plant Cell Envrion. 1998, 21, 101–107. [Google Scholar] [CrossRef]
- Loreto, F.; Fineschi, S. Reconciling functions and evolution of isoprene emission in higher plants. New Phytol. 2015, 206, 578–582. [Google Scholar] [CrossRef]
- Pollastri, S.; Tsonev, T.; Loreto, F. Isoprene improves photochemical efficiency and enhances heat dissipation in plants at physiological temperatures. J. Exp. Bot. 2014, 65, 1565–1570. [Google Scholar] [CrossRef] [Green Version]
- Velikova, V.; Müller, C.; Ghirardo, A.; Rock, T.M.; Aichler, M.; Walch, A.; Schmitt-Kopplin, P.; Schnitzler, J.-P. Knocking Down of Isoprene Emission Modifies the Lipid Matrix of Thylakoid Membranes and Influences the Chloroplast Ultrastructure in Poplar1. Plant Physiol. 2015, 168, 859–870. [Google Scholar] [CrossRef] [Green Version]
- Siwko, M.E.; Marrink, S.J.; de Vries, A.H.; Kozubek, A.; Uiterkamp, A.; Mark, A.E. Does isoprene protect plant membranes from thermal shock? A molecular dynamics study. Biochim. Biophys. Acta-Biomembr. 2007, 1768, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Zeinali, N.; Altarawneh, M.; Li, D.; Al-Nu’airat, J.; Dlugogorski, B.Z. New Mechanistic Insights: Why Do Plants Produce Isoprene? Acs Omega 2016, 1, 220–225. [Google Scholar] [CrossRef]
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.T.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef]
- Genard-Zielinski, A.C.; Ormeño, E.; Boissard, C.; Fernandez, C. Isoprene emissions from Downy Oak under water limitation during an entire growing season: What cost for growth? PLos ONE 2014, 9, e112418. [Google Scholar] [CrossRef] [Green Version]
- Santonja, M.; Fernandez, C.; Gauquelin, T.; Baldy, V. Climate change effects on litter decomposition: intensive drought leads to a strong decrease of litter mixture interactions. Plant Soil 2015, 393, 69–82. [Google Scholar] [CrossRef]
- Saunier, A.; Ormeño, E.; Wortham, H.; Temime-Roussel, B.; Lecareux, C.; Boissard, C.; Fernandez, C. Chronic Drought Decreases Anabolic and Catabolic BVOC Emissions ofQuercus pubescensin a Mediterranean Forest. Front. Plant Sci. 2017, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Hideg, É.; Barta, C.; Kálai, T.; Vass, I.; Hideg, K.; Asada, K. Detection of Singlet Oxygen and Superoxide with Fluorescent Sensors in Leaves Under Stress by Photoinhibition or UV Radiation. Plant Cell Physiol. 2002, 43, 1154–1164. [Google Scholar] [CrossRef]
- Qin, Y.; Lu, M.; Gong, X. Dihydrorhodamine 123 is superior to 2,7-dichlorodihydrofluorescein diacetate and dihydrorhodamine 6G in detecting intracellular hydrogen peroxide in tumor cells. Cell Biol. Int. 2008, 32, 224–228. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque, M.B.; dos Santos, R.C.; Lima, L.M.; Melo Filho, P.; de Melo Filho, P.A.; Mansur Custodio Nogueira, R.J.; Gomes da Camara, C.A.; de Ramos, A.R. Allelopathy, an alternative tool to improve cropping systems. A review. Agron. Sustain. Dev. 2011, 31, 379–395. [Google Scholar] [CrossRef] [Green Version]
- Bussotti, F.; Gerosa, G. Are the Mediterranean forests in Southern Europe threatened from ozone? J. Mediterr. Ecol. 2002, 3, 23–34. [Google Scholar]
| Optimal Watering | Moderate Water Stress | Severe Water Stress | F-Value | |
|---|---|---|---|---|
| Ψ (MPa) | −0.74a ± 0.18 | −2.65b ± 0.21 | −5.66c ± 0.39 | 78.63 *** |
| Leaf water content (%) | 56.23a ± 0.98 | 20.56b ± 3.65 | 7.14c ± 1.33 | 120.15 *** |
| Pn | gs | Ci | |
|---|---|---|---|
| Fumigation | 3.2 * | 0.01ns | 0.3ns |
| Watering | 143.7 *** | 47.8 *** | 386.3 *** |
| Watering x Fumigation | 4.9 * | 0.16ns | 0.5ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ormeño, E.; Viros, J.; Mévy, J.-P.; Tonetto, A.; Saunier, A.; Bousquet-Mélou, A.; Fernandez, C. Exogenous Isoprene Confers Physiological Benefits in a Negligible Isoprene Emitter (Acer monspessulanum L.) under Water Deficit. Plants 2020, 9, 159. https://doi.org/10.3390/plants9020159
Ormeño E, Viros J, Mévy J-P, Tonetto A, Saunier A, Bousquet-Mélou A, Fernandez C. Exogenous Isoprene Confers Physiological Benefits in a Negligible Isoprene Emitter (Acer monspessulanum L.) under Water Deficit. Plants. 2020; 9(2):159. https://doi.org/10.3390/plants9020159
Chicago/Turabian StyleOrmeño, Elena, Justine Viros, Jean-Philippe Mévy, Alain Tonetto, Amélie Saunier, Anne Bousquet-Mélou, and Catherine Fernandez. 2020. "Exogenous Isoprene Confers Physiological Benefits in a Negligible Isoprene Emitter (Acer monspessulanum L.) under Water Deficit" Plants 9, no. 2: 159. https://doi.org/10.3390/plants9020159
APA StyleOrmeño, E., Viros, J., Mévy, J.-P., Tonetto, A., Saunier, A., Bousquet-Mélou, A., & Fernandez, C. (2020). Exogenous Isoprene Confers Physiological Benefits in a Negligible Isoprene Emitter (Acer monspessulanum L.) under Water Deficit. Plants, 9(2), 159. https://doi.org/10.3390/plants9020159








