Relationship between the Content of β-D-Glucans and Infection with Fusarium Pathogens in Oat (Avena sativa L.) Plants
Abstract
1. Introduction
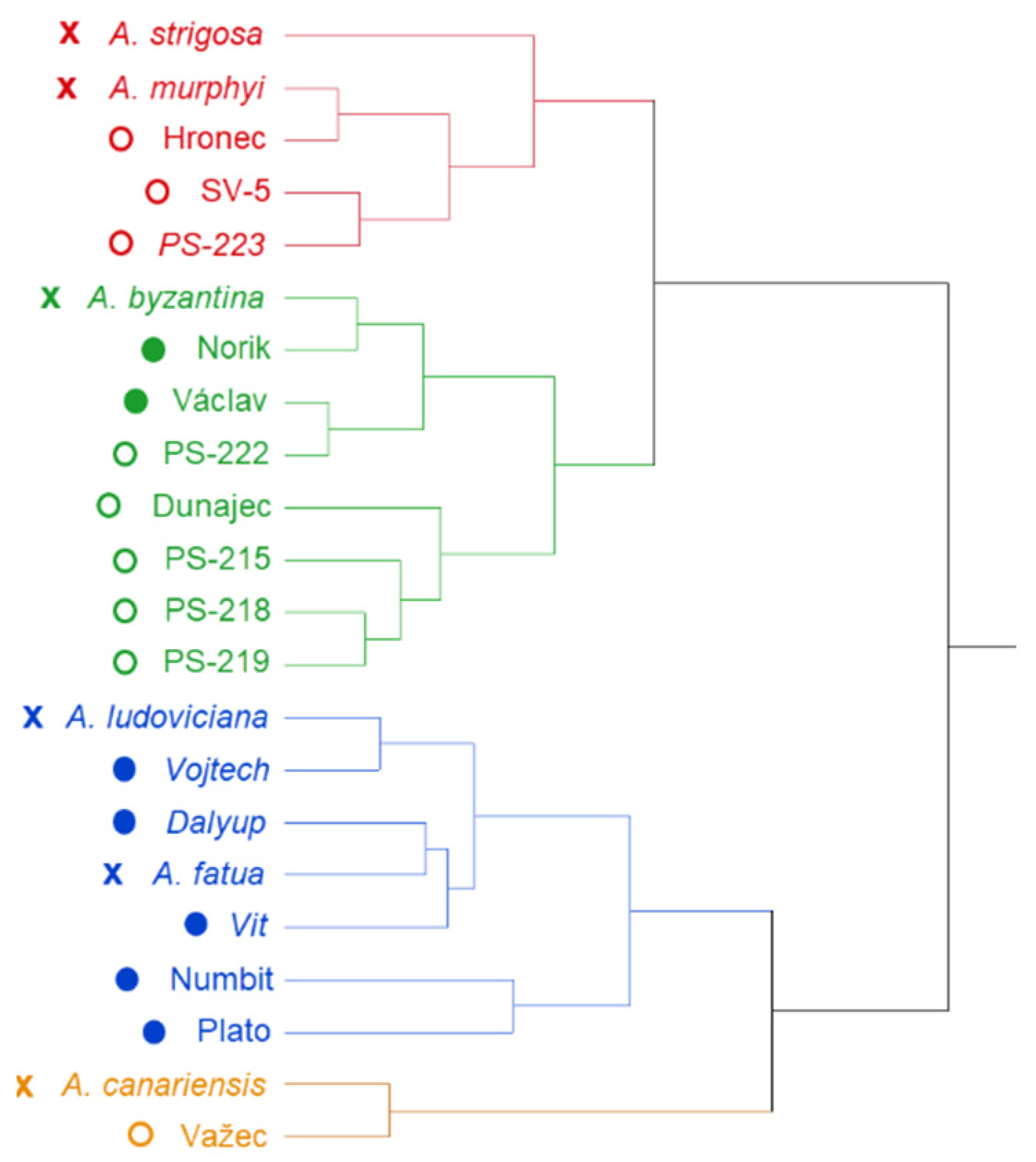
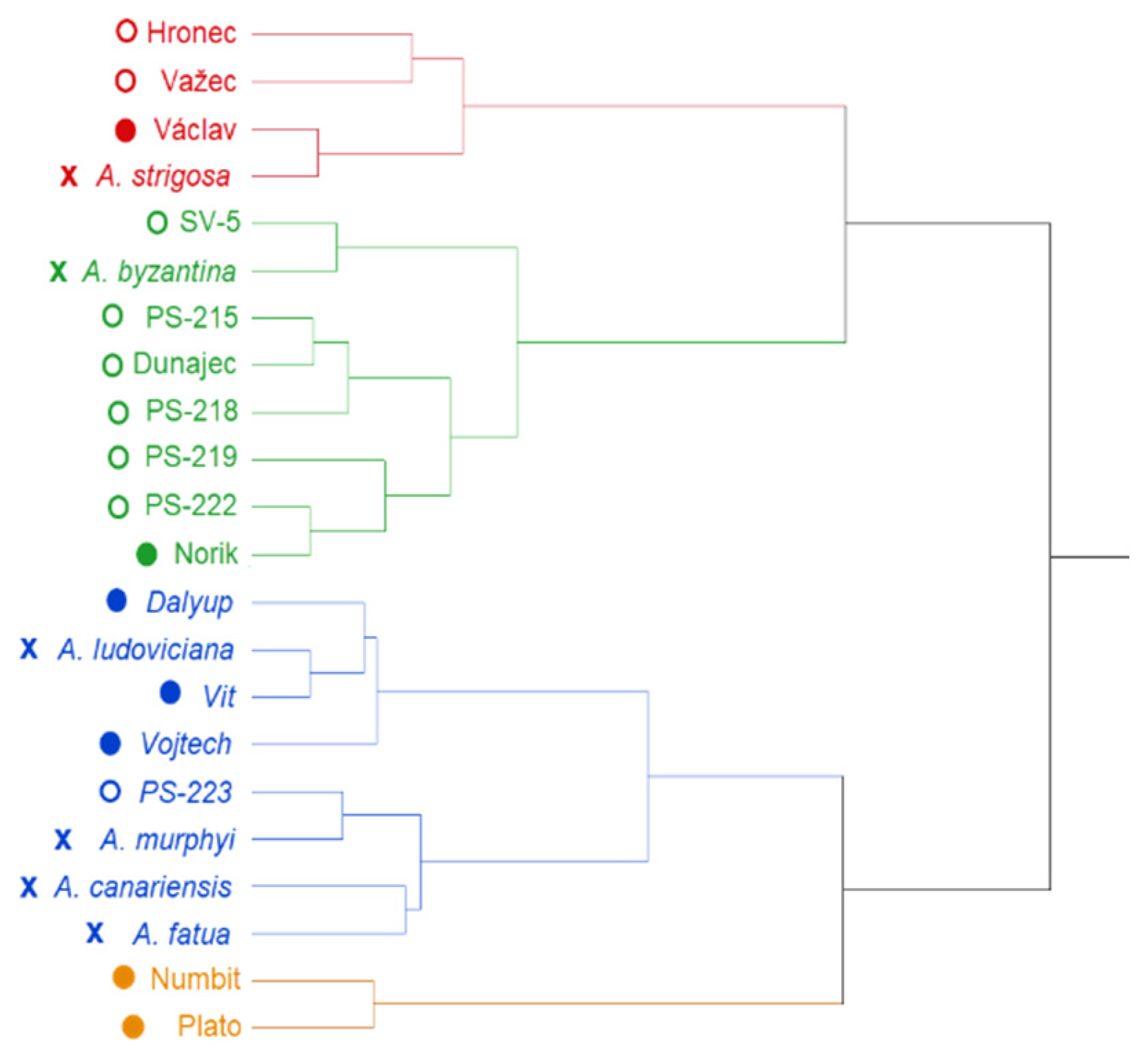
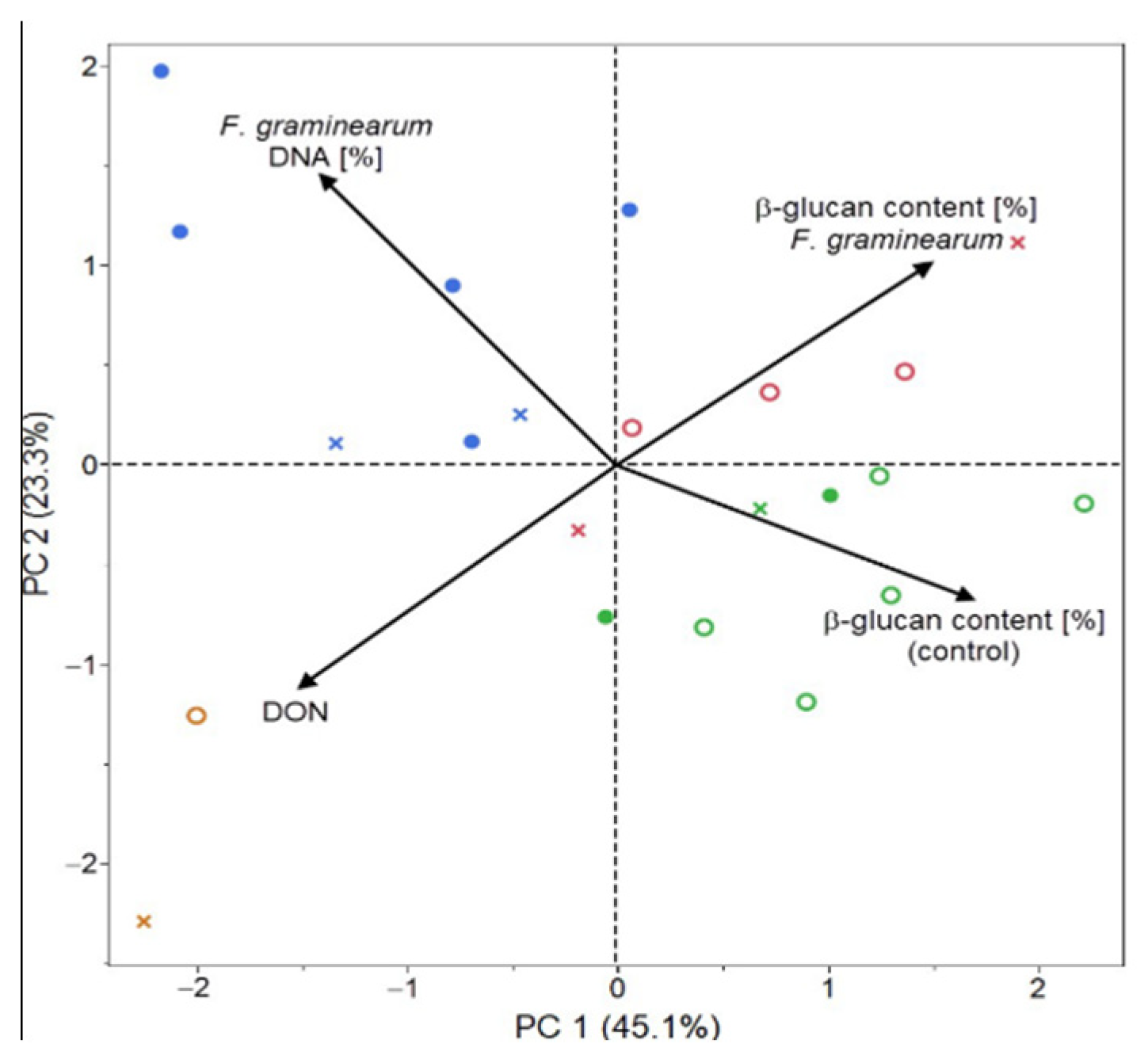
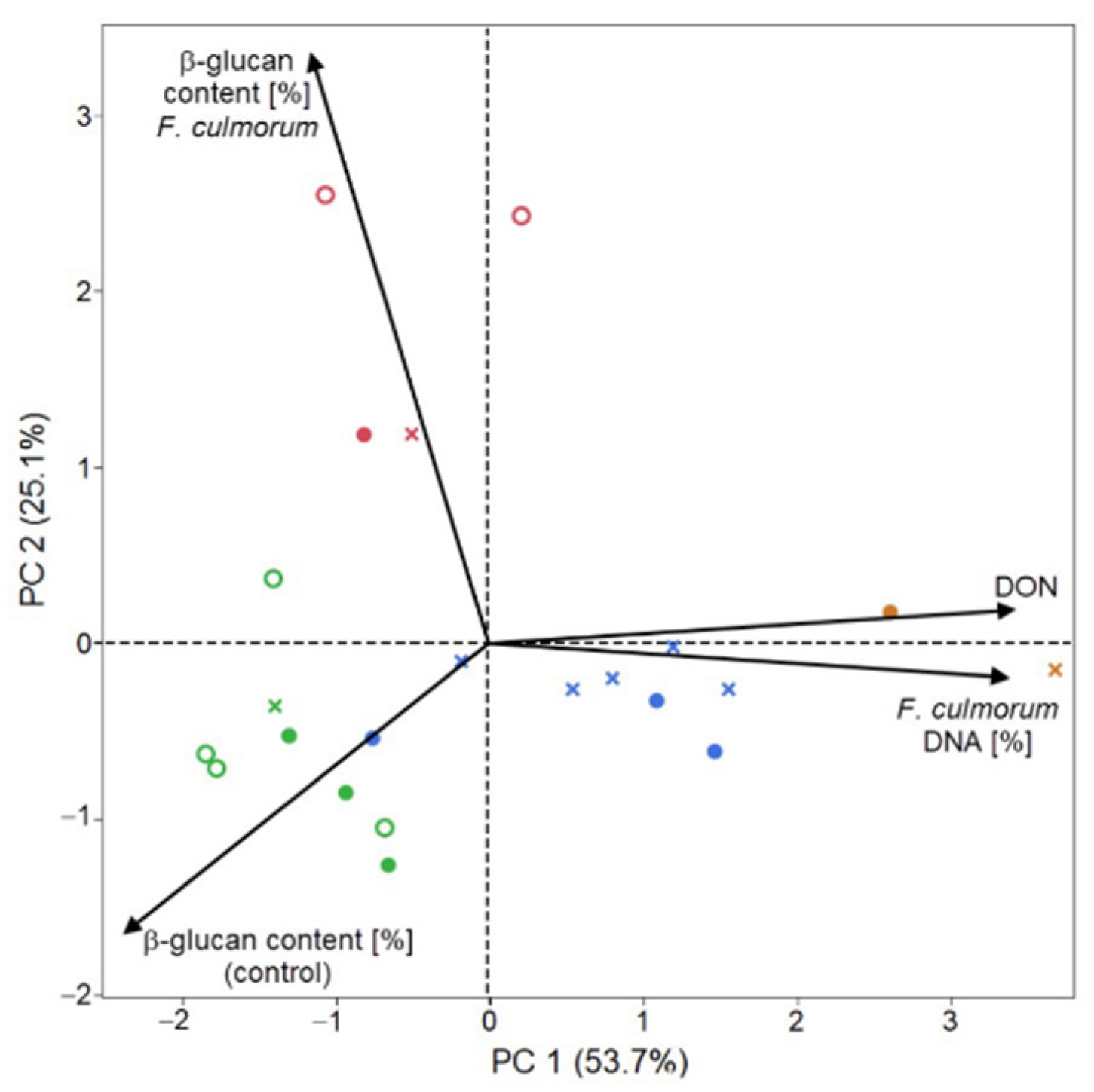
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Artificial Inoculation
4.3. Determination of β-D-Glucans
4.4. Quantification of DON
4.5. Extraction and Quantification of DNA
4.6. Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rasane, P.; Jha, A.; Sabikhi, L.; Kumar, A.; Unnikrishnan, V.S. Nutritional advantages of oats and opportunities for its processing as value added foods—A review. J. Food Sci. Technol. 2015, 52, 662–675. [Google Scholar] [CrossRef] [PubMed]
- Doehlert, D.C.; McMullen, M.S. Genotypic and environmental effects on oat milling characteristics and groat hardness. Cereal Chem. 2000, 77, 148–154. [Google Scholar] [CrossRef]
- FDA. 21 CFR Part 101. Food labeling, health claims: Soluble dietary fiber from certain foods and coronary heart disease. Fed. Regist. 1997, 62, 3584–3601. Available online: https://ecfr.federalregister.gov/current/title-21/chapter-I/subchapter-B/part-101/subpart-E/section-101.81 (accessed on 10 August 2020).
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium Ear blight (scab) in small-grain cereals—A review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Noworolnik, K. Yielding and grain quality of oat depending on soil moisture and nitrogen rate. Zywnosc Nauka Technol. Jakosc/Food Sci. Technol. Qual. 2010, 70, 190–196. [Google Scholar] [CrossRef]
- Cyrkler-Degulis, M.; Bulinska-Radomska, Z. Value of old and modern oats cultivars for organic farming. J. Res. Appl. Agric. Eng. (Pol.) 2007, 3, 27–31. Available online: https://www.pimr.eu/wp-content/uploads/2019/05/2007_3_MCZB.pdf (accessed on 12 September 2020).
- Nganje, W.E.; Bangsund, D.A.; Leistritz, F.L.; Wilson, W.W.; Tiapo, N.M. Regional economic impacts of Fusarium Head Blight in wheat and barley. Appl. Econ. Perspect. Policy 2004, 26, 332–347. [Google Scholar] [CrossRef]
- Schöneberg, T.; Kiblera, K.; Sulyokc, M.; Musaa, T.; Thomas, D.B.; Maschere, F.; Bertossa, M.; Voegele, R.T.; Vogelgsang, S. Can plant phenolic compounds reduce Fusarium growth and mycotoxin production in cereals? Food Addit. Contam. 2018, 35, 2455–2470. [Google Scholar] [CrossRef]
- Faltusová, Z.; Vaculová, K.; Pavel, J.; Svobodová, I.; Hajšlová, J.; Ovesná, J. Identification of suitable reference gene for quantitative transcription analysis (RT-qPCR) of Fusarium culmorum genes in infected barley plants. J. Cereal Sci. 2018, 79, 418–423. [Google Scholar] [CrossRef]
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vanderbroucke, V.; Li, S.; Haesebrouck, F.; Van Immerseel, F.; Croubels, S. The impact of Fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins 2014, 6, 430–452. [Google Scholar] [CrossRef]
- Pleadin, J.; Vahcic, N.; Persi, N.; Sevelj, D.; Markov, K.; Frece, J. Fusarium mycotoxins’ occurrence in cereals harvested from Croatian. Food Control 2013, 32, 49–54. [Google Scholar] [CrossRef]
- WHO. Evaluation of certain mycotoxins in food. Who Tech. Rep. Ser. 2002, 906, 35–42. [Google Scholar]
- Kosová, K.; Chrpová, J.; Šantrůček, J.; Hynek, R.; Štěrbová, L.; Vítámvás, P.; Bradová, J.; Prášil, I.T. The effect of Fusarium culmorum infection and deoxynivalenol (DON) application on proteome response in barley cultivars chevron and pedant. J. Proteom. 2017, 169, 112–124. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission recommendation on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229, 7–9. [Google Scholar]
- European Commission. Commission Recommendation on the presence of T-2 and HT-2 toxin in cereals and cereal products. Off. J. Eur. Union 2013, L91/12, 1–4. [Google Scholar]
- Farrokhi, N.; Burton, R.A.; Brownfield, L.; Hrmova, M.; Wilson, S.M.; Bacic, A.; Fincher, G.B. Plant cell wall biosynthesis: Genetic, biochemical and functional genomics approaches to the identification of key genes. Plant. Biotechnol. J. 2006, 4, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Hoson, T. Physiological functions of plant cell coverings. J. Plant Res. 2002, 115, 277–282. [Google Scholar] [CrossRef]
- Hrmova, M.; Fincher, G.B. Structure-function relationship of β-D-glucan endo- and exohydrolases from higher plants. Plant Mol. Biol. 2001, 47, 73–91. [Google Scholar] [CrossRef]
- Luna, E.; Pastor, V.; Robert, J.; Flors, V.; Mauch-Mani, B.; Ton, J. Callose deposition: A multifaceted plant defense response. Mol. Plant. Microbe Interact. J. 2011, 24, 183–193. [Google Scholar] [CrossRef]
- Piršelová, B.; Matušíková, I. Callose: The plant cell wall polysaccharide with multiple biological functions. Acta Physiol. Plant. 2013, 35, 635–644. [Google Scholar] [CrossRef]
- Buckeridge, M.S.; Rayon, C.; Urbanowicz, B.; Tine, M.A.S.; Carpita, N. Mixed linkage (1-3),(1-4)-β-D-glucans of Grasses. Cereal Chem. J. 2004, 81, 115–127. [Google Scholar] [CrossRef]
- Havrlentová, M.; Deáková, L.; Kraic, J.; Žofajová, A. Can β-D-glucan protect oat seeds against a heat stress? Nova Biotechnol. Et Chim. 2016, 15, 107–113. [Google Scholar] [CrossRef]
- Vega- Sanchez, M.E.; Verhertbruggen, Y.; Christensen, U.; Chen, X.; Sharma, V.; Varanasi, P.; Jobling, S.A.; Talbot, M.; White, R.G.; Joo, M.; et al. Loss of Cellulose synthase-like F6 function affects mixed-linkage glucan deposition, cell wall mechanical properties and defense responses in vegetative tissues of rice. Plant Physiol. 2012, 159, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Havrlentova, M.; Kraic, J. Content of β-D-glucan in cereal grains. J. Food Nutr. Res. 2006, 45, 97–103. Available online: https://scholar.google.com/scholar_lookup?title=Content+of+beta-d-glucan+in+cereal+grains&author=Havrlentova%2C+M.&publication_year=2006 (accessed on 12 September 2020).
- Readelli, R.; Sgrulletta, D.; De Stefanis, E. Genetic variability for chemical components in sixty European oat (Avena sativa L.) cultivars. Cereal Res. Commun. 2003, 31, 185–192. [Google Scholar] [CrossRef]
- Zute, S.; Berga, L.; Vicupe, Z. Variability in endosperm β-glucan content of husked and naked oat genotypes. Acta Biol. Univ. Daugavp. 2011, 11, 192–200. Available online: http://sciences.lv/wp-content/uploads/ACTA/2011/11-2/12_Zute.pdf (accessed on 13 September 2020).
- Havrlentová, M.; Bieliková, M.; Mendel, L.; Kraic, J.; Hozlár, P. The correlation of (l-3),(l-4)-beta-D-glucan with some qualitative parameters in the oat grain. Agriculture (Poľnohospodárstvo) 2008, 54, 65–71. Available online: https://www.agriculture.sk/fileadmin/agriculture/files/2006/Issue%204/2_havrl.pdf (accessed on 12 September 2020).
- Tiwari, U.; Cummins, E. Simulation of the factors affecting β-glucan levels during the cultivation of oats. J. Cereal Sci. 2009, 2, 175–183. [Google Scholar] [CrossRef]
- Benlioğlu, B.; Adak, M.S. Importance of crop wild relatives and landraces genetic resources in plant breeding programs. J. Exp. Agric. Int. 2019, 37, 1–8. [Google Scholar] [CrossRef]
- Havrlentová, M.; Petruláková, Z.; Burgárová, A.; Gago, F.; Hlinková, A.; Šturdík, E. Cereal beta-glucans and their significance for the preparation of functional foods—A review. Czech. J. Food Sci. 2011, 29, 11–14. [Google Scholar] [CrossRef]
- Martin, C.; Schöneberg, T.; Vogelgsang, S.; Morisoli, R.; Bertossa, M.; Mauch-Mani, B.; Mascher, F. Resistance against Fusarium graminearum and the relationship to β-glucan in barley grains. Eur. J. Plant Pathol. 2018, 152, 621–634. [Google Scholar] [CrossRef]
- Kofuji, K.; Aoki, A.; Tsubaki, K.; Konishi, M.; Isobe, T.; Murata, Y. Antioxidant Activity of β-Glucan. ISRN Pharm. 2012, 2012, 125864. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Hao, J.; Griffey, C.; Chung, H.; O’Keefe, S.F.; Chen, J.; Hogan, S. Antioxidant properties of Fusarium head blight-resistant and -susceptible soft red winter wheat grains grown in Virginia. J. Agric. Food Chem. 2007, 55, 3729–3736. [Google Scholar] [CrossRef] [PubMed]
- Boutigny, A.-L.; Atanasova-Pénichon, V.; Benet, M.; Barreau, C.; Richard-Forget, F. Natural phenolic acids from wheat bran inhibit Fusarium culmorum trichothecene biosynthesis in vitro by repressing Tri gene expression. Eur. J. Plant. Pathol. 2010, 127, 275–286. [Google Scholar] [CrossRef]
- Pani, G.; Scherm, B.; Azara, E.; Balmas, V.; Jahanshiri, Z.; Carta, P.; Fabbri, D.; Dettori, M.A.; Fadda, A.; Dessì, A.; et al. Natural and natural-like phenolic inhibitors of type B trichothecene in vitro production by the wheat (Triticum spp.) Pathogen Fusarium culmorum. J. Agric. Food Chem. 2014, 62, 4969–4978. [Google Scholar] [CrossRef]
- Yiannikouris, A.; André, G.; Poughon, L.; François, J.; Dussap, C.G.; Jeminet, G.; Bertin, G.; Jouany, J.P. Chemical and conformational study of the interactions involved in mycotoxin complex at ion with β-D-glucans. Biomacromolecules 2006, 7, 1147–1155. Available online: https://pubs.acs.org/doi/10.1021/bm050968t (accessed on 30 September 2020). [CrossRef]
- Yiannikouris, A.; Francois, J.; Poughon, L.; Dussap, C.G.; Bertin, G.; Jeminet, G.; Jouany, J.P. Adsorption of zearalenone by β-D-glucans in the Saccharomyces cerevisiae cell wall. J. Food Prot. 2004, 67, 1195–1200. [Google Scholar] [CrossRef]
- Schwarz, P.B.; Jones, B.L.; Steffensen, B. Enzymes associated with Fusarium infection of barley. J. Am. Soc. Brew. Chem. 2002, 60, 130–134. [Google Scholar] [CrossRef]
- Wang, J.; Wieser, H.; Pawelzik, E.; Weinert, J.; Keutgen, A.J.; Wolf, G.A. Impact of the fungal protease produced by Fusarium culmorum on the protein quality and breadmaking properties of winter wheat. Eur. Food Res. Technol. 2005, 220, 552–559. [Google Scholar] [CrossRef]
- Oliveira, P.M.; Mauch, A.; Jacob, F.; Arendt, E.K. Impact of Fusarium culmorum-infected barley malt grains on brewing and beer quality. J. Am. Soc. Brew. Chem. 2012, 70, 186–194. [Google Scholar] [CrossRef]
- Oliveira, P.M.; Mauch, A.; Jacob, F.; Waters, D.M.; Arendt, E.K. Fundamental study on the influence of Fusarium infection on quality and ultrastructure of barley malt. Int. J. Food Microbiol. 2012, 156, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Fincher, G.B. Morphology and chemical composition of barley endosperm cell walls. J. Inst. Brew. 1975, 81, 116–122. [Google Scholar] [CrossRef]
- Becker, M.; Vincent, C.; Reid, J.S.G. Biosynthesis of (1,3)(1,4)-beta-glucan and (1,3)-beta-glucan in barley (Hordeum vulgare L.). Planta 1995, 10, 331–338. [Google Scholar] [CrossRef]
- Wilson, S.M.; Burton, R.A.; Collins, H.M.; Doblin, M.S.; Pettolino, F.A.; Shirley, N.; Fincher, G.B.; Bacic, A. Pattern of deposition of cell wall polysaccharides and transcript abundance of related cell wall synthesis genes during differentiation in barley endosperm. Plant. Physiol. 2012, 159, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Linsel, K.J.; Keiper, F.J.; Forgan, A.; Oldach, K.H. New insights into the infection process of Rhynchosporium secalis in barley using GFP. Fungal Genet. Biol. 2011, 48, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Mesterházy, Á.; Bartók, T.; Kászonyi, G.; Varga, M.; Tóth, B.; Varga, J. Common resistance to different Fusarium spp. causing Fusarium Head Blight in wheat. Eur. J. Plant Pathol. 2005, 112, 267–281. [Google Scholar] [CrossRef]
- Tóth, B.; Kászonyi, G.; Bartók, T.; Varga, M.; Mesterházy, Á. Common resistance of wheat to members of the Fusarium graminearum species complex and F. culmorum. Plant. Breed. 2008, 127, 1–8. [Google Scholar] [CrossRef]
- Tekle, S.; Dill-Macky, R.; Skinnes, H.; Tronsmo, A.M.; Bjørnstad, Å. Infection process of Fusarium graminearum in oats (Avena sativa L.). Eur. J. Plant Pathol. 2012, 132, 431–442. [Google Scholar] [CrossRef]
- Martin, C.; Schöneberg, M.C.; Vogelgsang, S.; Mendes Ferreira, C.S.; Morisoli, R.; Bertossa, M.; Bucheli, T.D.; Mauch-Mani, B.; Mascher, F. Responses of Oat Grains to Fusarium poae and F. langsethiae infections and mycotoxin contaminations. Toxins 2018, 10, 47. [Google Scholar] [CrossRef]
- Miller, S.S.; Fulcher, R.G.; Sen, A.; Arnason, J.T. Oat endosperm cell walls. Isolation, composition and comparison with other tissues. Cereal Chem. 1995, 72, 421–427. Available online: https://scholar.google.com/scholar_lookup?title=Oat+endosperm+cell+walls.+Isolation,+composition,+and+comparison+with+other+tissues&author=Miller,+S.S.&author=Fulcher,+R.G.&author=Sen,+A.&author=Arnason,+J.T.&publication_year=1995&journal=Cereal+Chem.&volume=72&pages=421%E2%80%93427 (accessed on 12 September 2020).
- Tvarůžek, L.; Matušinský, P.; Vyšohlídová, M. Metodika pro zakládání a hodnocení pokusů s umělou inokulací obilnin fuzáriózami klasů. 2012. Available online: https://www.vukrom.cz/userfiles/files/vysledky_vyzkumu/Metodiky/2012_Metodika_pro_zakladani_a_hodnoceni_pokusu_s_umelou_inokulaci_obilnin_fuzariozami_klasu.pdf (accessed on 10 May 2019).
- Šliková, S.; Hozlár, P.; Gregová, E. Ovos: Infekcia Hubami Fusarium spp. v Poľných Podmienkach; Edičné Stredisko NPPC, Výskumný Ústav Pôdoznalectva a Ochrany Pôdy: Bratislava, Slovakia, 2017; p. 91. [Google Scholar]
- Nirenberg, H. Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium Sektion Liseola. Mitt. Aus Der Biol. Bundesanst. Für Land Und Forstwirtsch. 1976, 169, 1–117. [Google Scholar] [CrossRef]
- McCleary, B.V.; Codd, R. Measurement of (1 → 3),(1 → 4)-β-D-glucan in barley and oats: A streamlined enzymic procedure. J. Sci. Food Agric. 1991, 55, 303–312. [Google Scholar] [CrossRef]
- Leišová, L.; Kučera, L.; Chrpová, J.; Sýkorová, S.; Šíp, V.; Ovesná, J. Quantification of Fusarium culmorum in wheat and barley tissues using real_time PCR in comparison with DON content. J. Phytopathol. 2006, 154, 603–611. [Google Scholar] [CrossRef]
- Burlakoti, R.R.; Estrada, R.; Rivera, V.V.; Boddeda, A.; Secor, G.A.; Adhikari, T.B. Real-time PCR quantification and mycotoxin production of Fusarium graminearum in wheat inoculated with isolates collected from potato, sugar beet and wheat. Phytopathology 2007, 97, 835–841. [Google Scholar] [CrossRef]
| No. | Sample | β-D-Glucans in Control (%) | FC Inoculation | FG Inoculation | |||||
|---|---|---|---|---|---|---|---|---|---|
| β-D-Glucans after Inoculation (%) | DON (mg/kg) | Pathogen DNA (%) | β-D-Glucans after Inoculation (%) | DON (mg/kg) | Pathogen DNA (%) | ||||
| 1. | A. strigosa | 2.55 | 3.30 | 19.39 | 0.28 | 2.90 | 3.17 | 0.28 | |
| 2. | A. ludoviciana | 1.67 | 1.40 | 28.19 | 1.23 | 1.64 | 6.52 | 1.20 | |
| 3. | A. byzantina | 2.52 | 1.41 | 2.32 | 0.04 | 1.23 | 1.16 | 0.04 | |
| 4. | A. fatua | 0.71 | 0.89 | 15.29 | 1.23 | 0.93 | 4.78 | 1.23 | |
| 5. | A. canariensis | 1.30 | 0.89 | 23.33 | 0.03 | 0.72 | 14.93 | 0.03 | |
| 6. | A. murphyi | 1.29 | 1.21 | 14.14 | 0.08 | 1.33 | 4.63 | 0.08 | |
| 7. | A. sativa/hulled | Vaclav | 3.90 | 3.25 | 15.43 | 0.59 | 0.94 | 4.59 | 0.59 |
| 8. | Vojtech | 1.54 | 0.78 | 22.33 | 2.16 | 1.89 | 7.51 | 2.16 | |
| 9. | Dalyup | 2.77 | 1.48 | 25.96 | 1.98 | 1.9 | 5.32 | 1.98 | |
| 10. | Numbit | 1.16 | 1.55 | 29.20 | 3.55 | 1.68 | 10.47 | 3.55 | |
| 11. | Vit | 1.62 | 1.14 | 28.84 | 1.71 | 1.63 | 1.22 | 1.73 | |
| 12. | Plato | 0.96 | 1.11 | 37.20 | 4.29 | 0.94 | 3.22 | 4.29 | |
| 13. | Norik | 3.39 | 1.13 | 10.27 | 0.32 | 1.48 | 2.12 | 0.33 | |
| 14. | A. sativa/naked | Hronec | 1.90 | 4.60 | 7.47 | 0.23 | 1.55 | 3.24 | 0.23 |
| 15. | Važec | 3.15 | 3.94 | 21.68 | 0.92 | 1.00 | 12.77 | 0.92 | |
| 16. | SV-5 | 2.54 | 2.20 | 4.33 | 0.11 | 2.22 | 2.84 | 0.12 | |
| 17. | PS-223 | 1.61 | 0.88 | 5.93 | 0.07 | 1.89 | 2.76 | 0.08 | |
| 18. | PS-222 | 3.73 | 0.77 | 13.77 | 0.46 | 0.99 | 3.72 | 0.46 | |
| 19. | Dunajec | 4.98 | 2.00 | 14.20 | 0.44 | 1.63 | 8.34 | 0.41 | |
| 20. | PS-215 | 5.06 | 1.84 | 9.70 | 0.11 | 2.10 | 2.94 | 0.10 | |
| 21. | PS-218 | 4.43 | 1.75 | 4.39 | 0.18 | 1.54 | 3.85 | 0.17 | |
| 22. | PS-219 | 4.72 | 1.36 | 15.25 | 1.30 | 1.64 | 3.24 | 1.30 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Havrlentová, M.; Gregusová, V.; Šliková, S.; Nemeček, P.; Hudcovicová, M.; Kuzmová, D. Relationship between the Content of β-D-Glucans and Infection with Fusarium Pathogens in Oat (Avena sativa L.) Plants. Plants 2020, 9, 1776. https://doi.org/10.3390/plants9121776
Havrlentová M, Gregusová V, Šliková S, Nemeček P, Hudcovicová M, Kuzmová D. Relationship between the Content of β-D-Glucans and Infection with Fusarium Pathogens in Oat (Avena sativa L.) Plants. Plants. 2020; 9(12):1776. https://doi.org/10.3390/plants9121776
Chicago/Turabian StyleHavrlentová, Michaela, Veronika Gregusová, Svetlana Šliková, Peter Nemeček, Martina Hudcovicová, and Dominika Kuzmová. 2020. "Relationship between the Content of β-D-Glucans and Infection with Fusarium Pathogens in Oat (Avena sativa L.) Plants" Plants 9, no. 12: 1776. https://doi.org/10.3390/plants9121776
APA StyleHavrlentová, M., Gregusová, V., Šliková, S., Nemeček, P., Hudcovicová, M., & Kuzmová, D. (2020). Relationship between the Content of β-D-Glucans and Infection with Fusarium Pathogens in Oat (Avena sativa L.) Plants. Plants, 9(12), 1776. https://doi.org/10.3390/plants9121776





