Modelling of Soybean (Glycine max (L.) Merr.) Response to Blue Light Intensity in Controlled Environments
Abstract
1. Introduction
2. Results
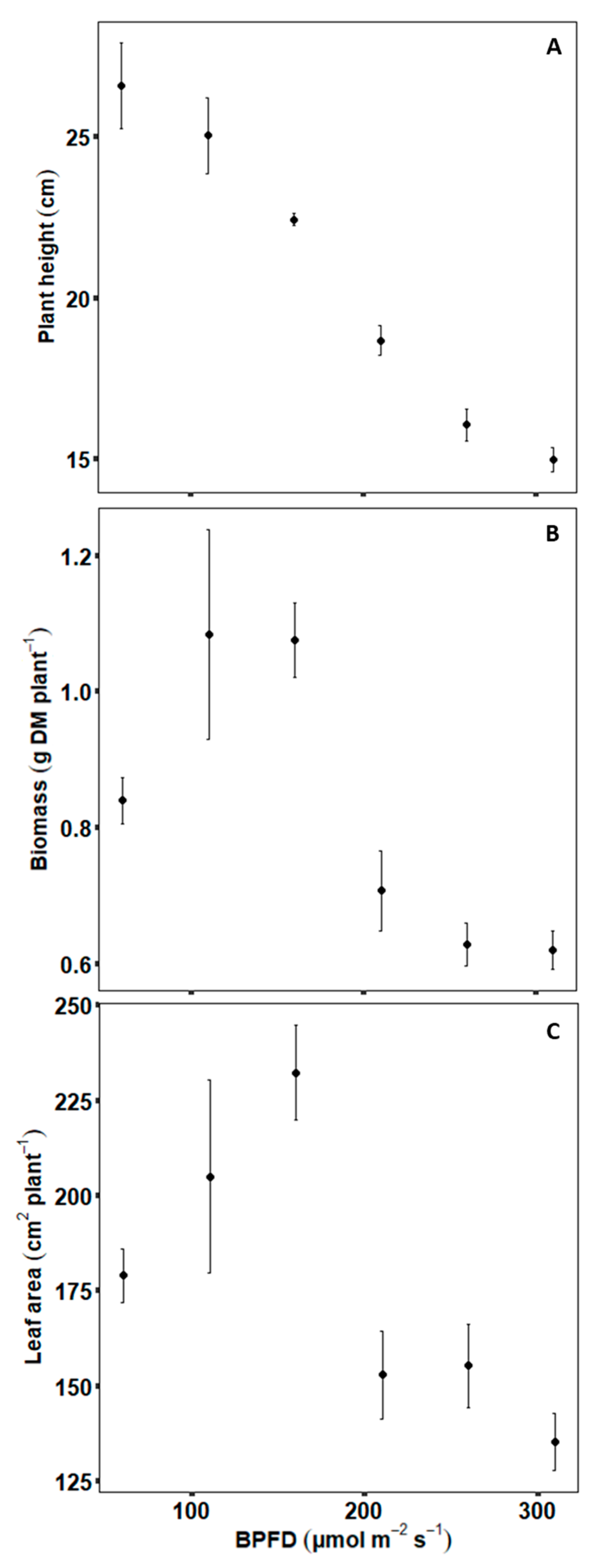
2.1. Experimental Data—Plant Scale
2.2. Experimental Data—Phytomer Scale
2.2.1. Biomass
2.2.2. Leaf Morphology and Physiology
2.2.3. Elongation
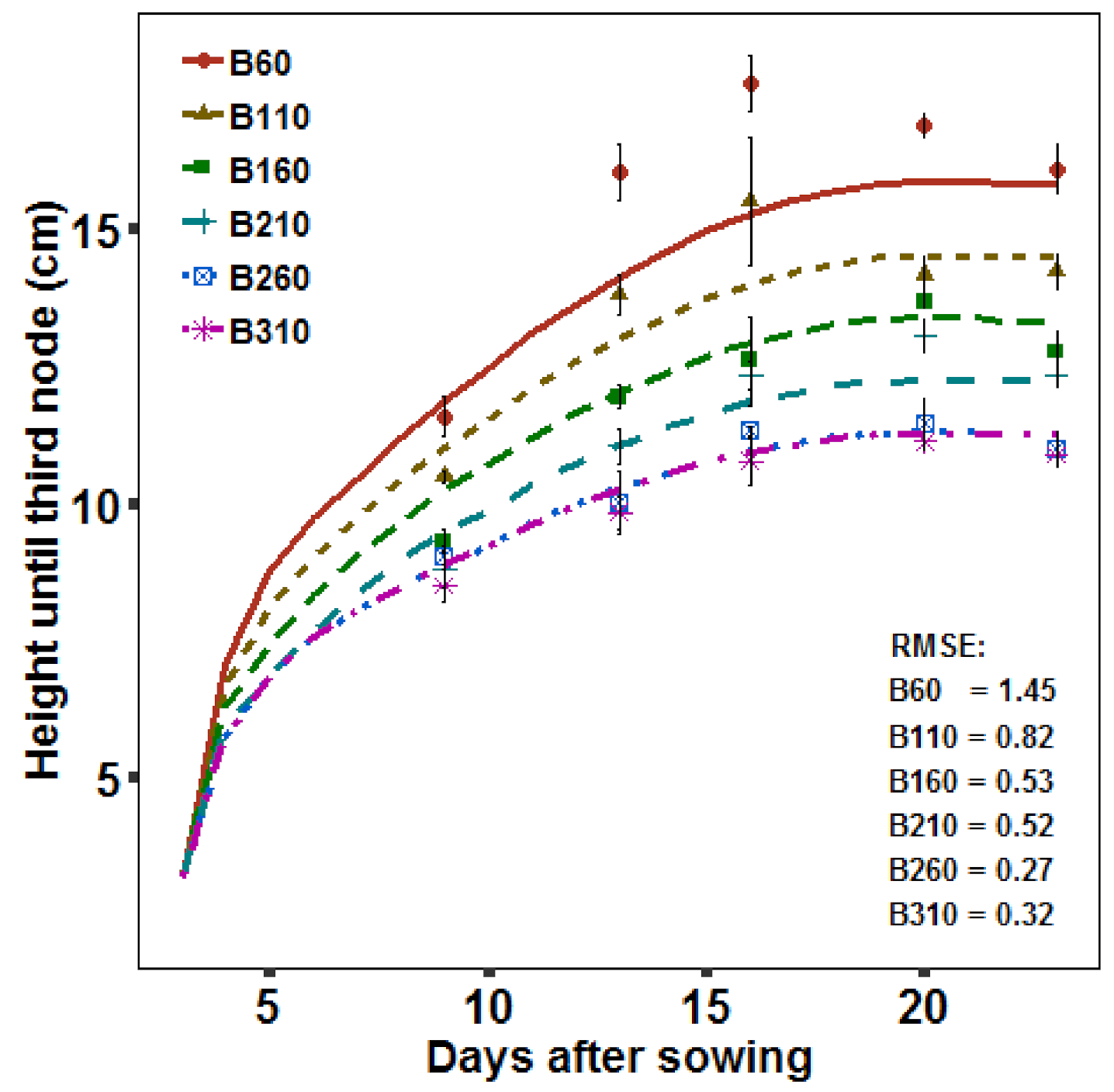
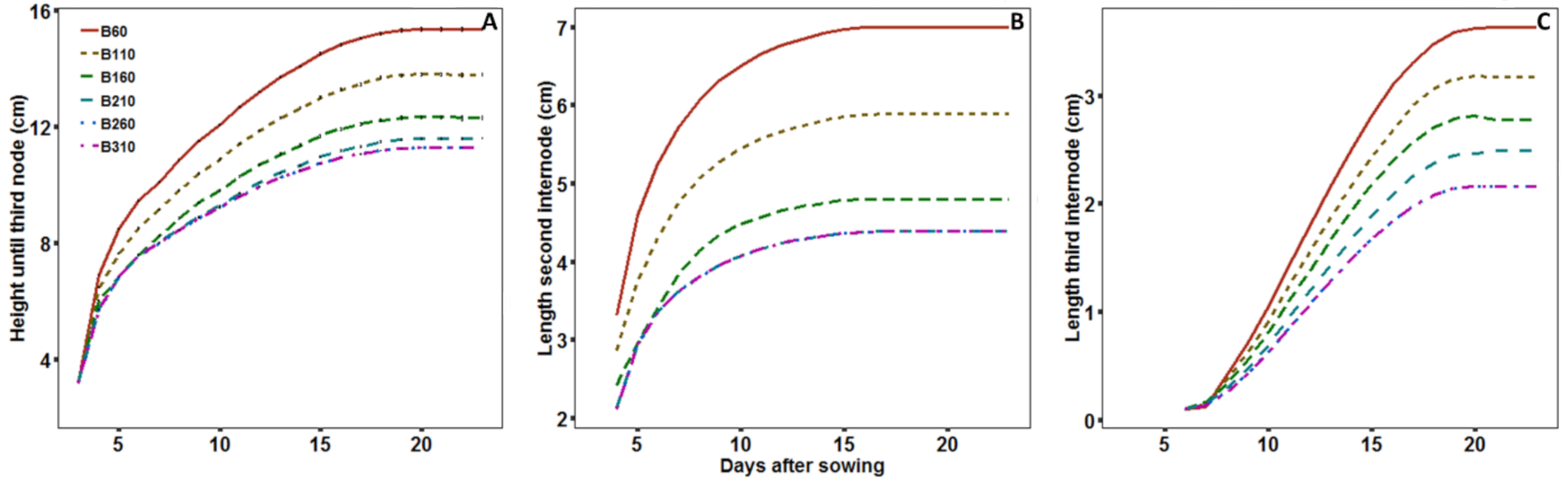
2.2.4. Growth Dynamics
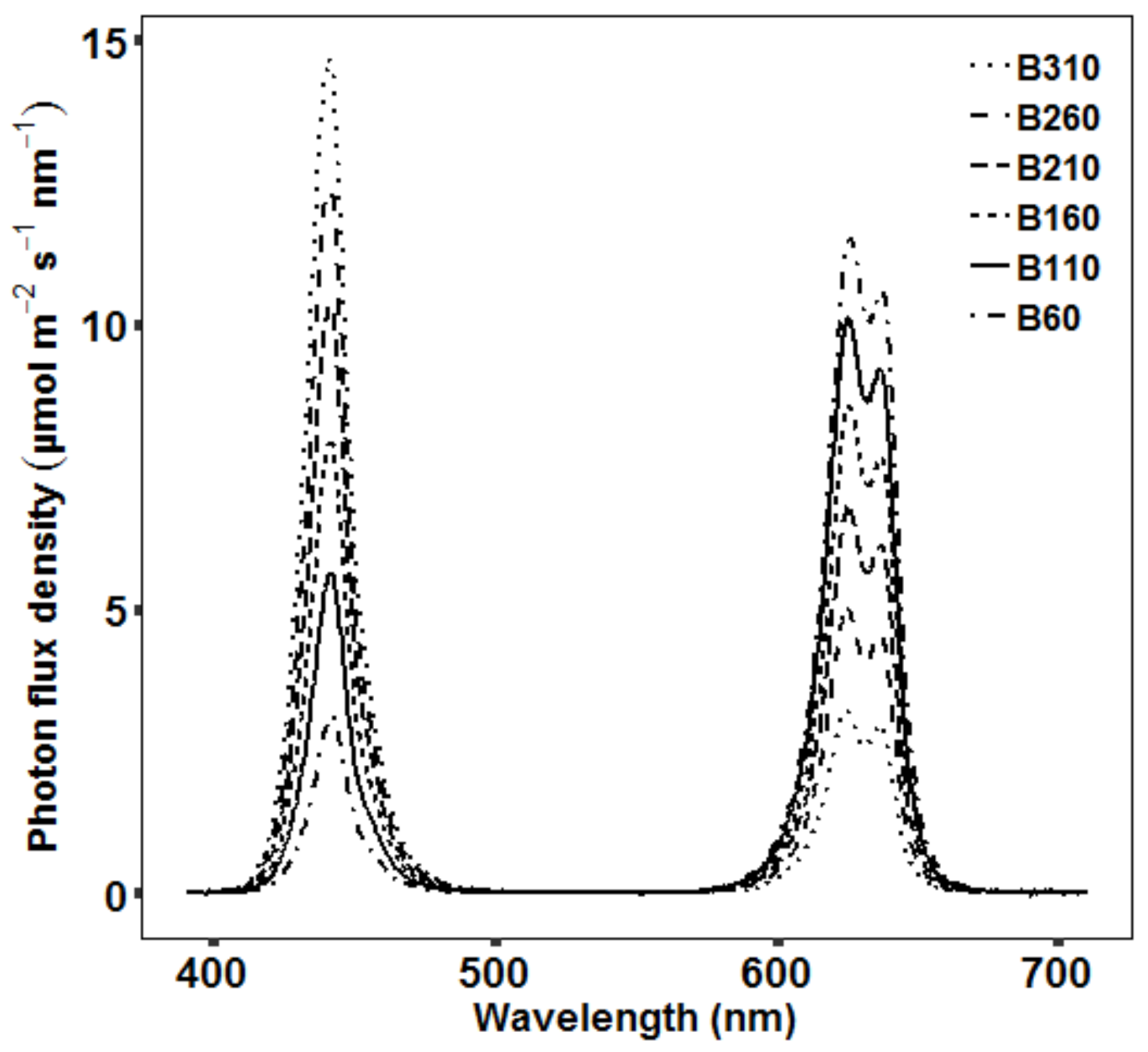
2.2.5. Energy Consumption
2.3. Modelling
2.3.1. Blue Light Response Function of Internodes
2.3.2. Evaluation and Light Optimization
3. Discussion
3.1. Biomass and Photosynthesis
3.2. Response to BPFD Under Shade
3.3. BPFD Response Function
3.4. Optimization of Light Spectrum
4. Materials and Methods
4.1. Experimental Setup
4.2. Light Treatments
4.3. Plant Measurements
4.4. Statistical Design and Analysis
4.5. FSP Model
4.6. Response Function
4.7. Model Evaluation and Alternative Scenarios
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for energy efficient greenhouse lighting. Renew. Sustain. Energy Rev. 2015, 49, 139–147. [Google Scholar] [CrossRef]
- Olle, M.; Viršile, A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013, 22, 223–234. [Google Scholar] [CrossRef]
- Ahlman, L.; Bånkestad, D.; Wik, T. Using chlorophyll a fluorescence gains to optimize LED light spectrum for short term photosynthesis. Comput. Electron. Agric. 2017, 142, 224–234. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the role of red:blue LED lights on resource use efficiency and nutritional properties of indoor grown sweet basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef]
- Schulze, P.S.C.; Barreira, L.A.; Pereira, H.G.C.; Perales, J.A.; Varela, J.C.S. Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.-D.; Asyraf Md Hatta, M.; Hinchliffe, A.; Steed, A.; Reynolds, D.; et al. Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef]
- Jähne, F.; Hahn, V.; Würschum, T.; Leiser, W.L. Speed breeding short-day crops by LED-controlled light schemes. Theor. Appl. Genet. 2020, 133, 2335–2342. [Google Scholar] [CrossRef]
- Fraser, D.P.; Hayes, S.; Franklin, K.A. Photoreceptor crosstalk in shade avoidance. Curr. Opin. Plant Biol. 2016, 33, 1–7. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Wang, H.; Deng, X.W. Phytochrome signaling mechanisms. Arab. Book 2011, 9, e0148. [Google Scholar] [CrossRef]
- Yu, X.; Liu, H.; Klejnot, J.; Lin, C. The cryptochrome blue light receptors. Arab. Book 2010, 8, e0135. [Google Scholar] [CrossRef]
- Hitz, T.; Hartung, J.; Graeff-Hönninger, S.; Munz, S. Morphological response of soybean (Glycine max (L.) Merr.) cultivars to light intensity and red to far-red ratio. Agronomy 2019, 9, 428. [Google Scholar] [CrossRef]
- Yang, F.; Huang, S.; Gao, R.; Liu, W.; Yong, T.; Wang, X.; Wu, X.; Yang, W. Growth of soybean seedlings in relay strip intercropping systems in relation to light quantity and red:far-red ratio. Field Crop. Res. 2014, 155, 245–253. [Google Scholar] [CrossRef]
- Gong, W.Z.; Jiang, C.D.; Wu, Y.S.; Chen, H.H.; Liu, W.Y.; Yang, W.Y. Tolerance vs. avoidance: Two strategies of soybean (Glycine max) seedlings in response to shade in intercropping. Photosynthetica 2015, 53, 259–268. [Google Scholar] [CrossRef]
- Wollaeger, H.M.; Runkle, E.S. Growth and acclimation of impatiens, salvia, petunia, and tomato seedlings to blue and red light. HortScience 2015, 50, 522–529. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Wheeler, R.M.; Mackowiak, C.L.; Sager, J.C. Soybean stem growth under high-pressure sodium with supplemental blue lighting. Agron. J. 1991, 83, 903–906. [Google Scholar] [CrossRef]
- Cope, K.R.; Bugbee, B. Spectral effects of three types of white light-emitting diodes on plant growth and development: Absolute versus relative amounts of blue light. HortScience 2013, 48, 504–509. [Google Scholar] [CrossRef]
- Dougher, T.A.; Bugbee, B. Differences in the response of wheat, soybean and lettuce to reduced blue radiation. Photochem. Photobiol. 2001, 73, 199–207. [Google Scholar] [CrossRef]
- Naznin, M.; Lefsrud, M.; Gravel, V.; Azad, M. Blue light added with red LEDs enhance growth characteristics, pigments content, and antioxidant capacity in lettuce, spinach, kale, basil, and sweet pepper in a controlled environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Wientjes, E.; Douwstra, P.; Trouwborst, G.; van Ieperen, W.; Croce, R.; Harbinson, J. Photosynthetic quantum yield dynamics: From photosystems to leaves. Plant Cell 2012, 24, 1921–1935. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- He, J.; Qin, L.; Chong, E.L.C.; Choong, T.-W.; Lee, S.K. Plant growth and photosynthetic characteristics of mesembryanthemum crystallinum grown aeroponically under different blue- and red-LEDs. Front. Plant Sci. 2017, 8, 361. [Google Scholar] [CrossRef]
- Kang, W.H.; Park, J.S.; Park, K.S.; Son, J.E. Leaf photosynthetic rate, growth, and morphology of lettuce under different fractions of red, blue, and green light from light-emitting diodes (LEDs). Hortic. Environ. Biotechnol. 2016, 57, 573–579. [Google Scholar] [CrossRef]
- Vos, J.; Evers, J.B.; Buck-Sorlin, G.H.; Andrieu, B.; Chelle, M.; de Visser, P.H.B. Functional-structural plant modelling: A new versatile tool in crop science. J. Exp. Bot. 2010, 61, 2101–2115. [Google Scholar] [CrossRef]
- Evers, J.B. Simulating Crop Growth and Development Using Functional-Structural Plant Modeling; Springer: Dordrecht, The Netherlands, 2016; pp. 219–236. [Google Scholar]
- Henke, M.; Kurth, W.; Buck-Sorlin, G.H. FSPM-P: Towards a general functional-structural plant model for robust and comprehensive model development. Front. Comput. Sci. 2016, 10, 1103–1117. [Google Scholar] [CrossRef]
- Burgess, A.J.; Retkute, R.; Pound, M.P.; Mayes, S.; Murchie, E.H. Image-based 3D canopy reconstruction to determine potential productivity in complex multi-species crop systems. Ann. Bot. 2017, 119, 517–532. [Google Scholar] [CrossRef]
- Evers, J.B.; Bastiaans, L. Quantifying the effect of crop spatial arrangement on weed suppression using functional-structural plant modelling. J. Plant Res. 2016, 129, 339–351. [Google Scholar] [CrossRef]
- Chelle, M.; Evers, J.B.; Combes, D.; Varlet-Grancher, C.; Vos, J.; Andrieu, B. Simulation of the three-dimensional distribution of the red:far-red ratio within crop canopies. N. Phytol. 2007, 176, 223–234. [Google Scholar] [CrossRef]
- Evers, J.B.; Vos, J.; Chelle, M.; Andrieu, B.; Fournier, C.; Struik, P.C. Simulating the effects of localized red:far-red ratio on tillering in spring wheat (Triticum aestivum) using a three-dimensional virtual plant model. N. Phytol. 2007, 176, 325–336. [Google Scholar] [CrossRef]
- Buck-Sorlin, G.; Hemmerling, R.; Kniemeyer, O.; Burema, B.; Kurth, W. A rule-based model of barley morphogenesis, with special respect to shading and gibberellic acid signal transduction. Ann. Bot. 2008, 101, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Bongers, F.J.; Pierik, R.; Anten, N.P.R.; Evers, J.B. Subtle variation in shade avoidance responses may have profound consequences for plant competitiveness. Ann. Bot. 2018, 121, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Gautier, H.; Měch, R.; Prusinkiewicz, P.; Varlet-Grancher, C. 3D Architectural Modelling of Aerial Photomorphogenesis in White Clover (Trifolium repens L.) using L-systems. Ann. Bot. 2000, 85, 359–370. [Google Scholar] [CrossRef]
- Buck-Sorlin, G.; de Visser, P.H.; Henke, M.; Sarlikioti, V.; van der Heijden, G.W.; Marcelis, L.F.; Vos, J. Towards a functional–structural plant model of cut-rose: Simulation of light environment, light absorption, photosynthesis and interference with the plant structure. Ann. Bot. 2011, 108, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, J.A.; De Visser, P.H.B.; Meinen, E.; Grit, J.G.; Dueck, T.A. integrating morphological and physiological responses of tomato plants to light quality to the crop level by 3D modeling. Front. Plant Sci. 2019, 10, 839. [Google Scholar] [CrossRef]
- Kalaitzoglou, P.; van Ieperen, W.; Harbinson, J.; van der Meer, M.; Martinakos, S.; Weerheim, K.; Nicole, C.C.S.; Marcelis, L.F.M. Effects of continuous or end-of-day far-red light on tomato plant growth, morphology, light absorption, and fruit production. Front. Plant Sci. 2019, 10, 322. [Google Scholar] [CrossRef]
- Hitz, T.; Henke, M.; Graeff-Honninger, S.; Munz, S. Simulating light spectrum within a soybean canopy in an LED growth chamber. In Proceedings of the 6th International Symposium on Plant Growth Modeling, Simulation, Visualization and Applications (PMA), Hefei, China, 4–8 November 2018; pp. 120–125. [Google Scholar]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1972, 9, 191–216. [Google Scholar] [CrossRef]
- Lanoue, J.; Leonardos, E.D.; Grodzinski, B. Effects of light quality and intensity on diurnal patterns and rates of photo-assimilate translocation and transpiration in tomato leaves. Front. Plant Sci. 2018, 9, 756. [Google Scholar] [CrossRef]
- Pierik, R.; de Wit, M. Shade avoidance: Phytochrome signaling and other aboveground neighbor detection cues. J. Exp. Bot. 2014, 65, 2815–2824. [Google Scholar] [CrossRef]
- Feng, L.; Raza, M.A.; Li, Z.; Chen, Y.; Khalid, M.H.B.; Du, J.; Liu, W.; Wu, X.; Song, C.; Yu, L.; et al. The influence of light intensity and leaf movement on photosynthesis characteristics and carbon balance of soybean. Front. Plant Sci. 2019, 9, 1952. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation and photosynthetic photon flux density independently regulate seedling growth but interactively regulate flowering. Environ. Exp. Bot. 2018, 155, 206–216. [Google Scholar] [CrossRef]
- Kim, G.-T.; Yano, S.; Kozuka, T.; Tsukaya, H. Photomorphogenesis of leaves: Shade-avoidance and differentiation of sun and shade leaves. Photochem. Photobiol. Sci. 2005, 4, 770. [Google Scholar] [CrossRef] [PubMed]
- Weston, E.; Thorogood, K.; Vinti, G.; López-Juez, E. Light quantity controls leaf-cell and chloroplast development in Arabidopsis thaliana wild type and blue-light-perception mutants. Planta 2000, 211, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Kahlen, K.; Stützel, H. Modelling photo-modulated internode elongation in growing glasshouse cucumber canopies. New Phytol. 2011, 190, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Sellaro, R.; Crepy, M.; Trupkin, S.A.; Karayekov, E.; Buchovsky, A.S.; Rossi, C.; Casal, J.J. Cryptochrome as a Sensor of the blue/green ratio of natural radiation in Arabidopsis. Plant Physiol. 2010, 154, 401–409. [Google Scholar] [CrossRef]
- Snowden, M.C.; Cope, K.R.; Bugbee, B. Sensitivity of seven diverse species to blue and green light: interactions with photon flux. PLoS ONE 2016, 11, e0163121. [Google Scholar] [CrossRef]
- Ahmad, M.; Grancher, N.; Heil, M.; Black, R.C.; Giovani, B.; Galland, P.; Lardemer, D. Action spectrum for cryptochrome-dependent hypocotyl growth inhibition in Arabidopsis. Plant Physiol. 2002, 129, 774–785. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Yin, X.; Goudriaan, J.; Lantinga, E.A.; Vos, J.; Spiertz, H.J. A flexible sigmoid function of determinate growth. Ann. Bot. 2003, 91, 361–371. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statisticalcomputing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 10 December 2020).
- Piepho, H.-P. An algorithm for a letter-based representation of all-pairwise comparisons. J. Comput. Graph. Stat. 2004, 13, 456–466. [Google Scholar] [CrossRef]
- Hitz, T.; Henke, M.; Graeff-Hönninger, S.; Munz, S. Three-dimensional simulation of light spectrum and intensity within an LED growth chamber. Comput. Electron. Agric. 2019, 156, 540–548. [Google Scholar] [CrossRef]
- Henke, M.; Buck-Sorlin, G.H. Using a full spectral raytracer for calculating light microclimate in functional-structural plant modelling. Comput. Inform. 2018, 36, 1492–1522. [Google Scholar] [CrossRef]
- Van Antwerpen, D.G. Unbiased Physically Based Rendering on the GPU. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 2011. [Google Scholar]
- Kasperbauer, M.J. Far-red light reflection from green leaves and effects on phytochrome-mediated assimilate partitioning under field conditions. Plant Physiol. 1987, 85, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Jacquemoud, S.; Baret, F.; Hanocq, J.F. Modeling spectral and bidirectional soil reflectance. Remote Sens. Environ. 1992, 41, 123–132. [Google Scholar] [CrossRef]
- Parks, B.M.; Folta, K.M.; Spalding, E.P. Photocontrol of stem growth. Curr. Opin. Plant Biol. 2001, 4, 436–440. [Google Scholar] [CrossRef]


| Phytomer Level | Organ/Ratio | Treatment | |||||
|---|---|---|---|---|---|---|---|
| B60 | B110 | B160 | B210 | B260 | B310 | ||
| Second | Internode (g) | 0.082 a | 0.082 a | 0.076 a,b | 0.058 b,c | 0.047 c | 0.049 c |
| Third | Internode (g) | 0.041 a | 0.044 a | 0.046 a | 0.030 b | 0.022 b | 0.023 b |
| Petiole (g) | 0.016 a | 0.018 a | 0.018 a | 0.015 a | 0.013 a | 0.013 a | |
| Leaf lamina (g) | 0.127 a | 0.136 a,b | 0.116 a,c | 0.100 a,c | 0.078 c | 0.081 b,c | |
| LMR | 0.69 a,b | 0.68 b,c | 0.64 c | 0.67 b,c | 0.71 a | 0.71 a | |
| IMRS | 0.72 a | 0.72 a | 0.71 a | 0.68 a,b | 0.62 b | 0.63 b | |
| Phytomer Level | Measurement | Treatment | |||||
|---|---|---|---|---|---|---|---|
| B60 | B110 | B160 | B210 | B260 | B310 | ||
| Third | SLA (cm2 g−1) | 324.35 a,b | 303.04 b | 327.68 a,b | 327.02 a,b | 341.41 a,b | 346.38 a |
| SPAD value | 26.51 a | 30.08 a | 31.65 a | 28.81 a | 30.37 a | 30.92 a | |
| Youngest fully developed | A (µmol CO2 m−2 s−1) | 27.49 a | 28.81 a | 27.78 a | 27.03 a | 26.79 a | 26.53 a |
| Phytomer Level | Organ | Treatment | |||||
|---|---|---|---|---|---|---|---|
| B60 | B110 | B160 | B210 | B260 | B310 | ||
| Second | Internode (cm) | 6.86 a | 5.86 b | 5.39 b | 4.70 c | 4.26 c | 4.33 c |
| Third | Internode (cm) | 3.81 a | 3.41 a,b | 3.28 a,b | 2.75 b,c | 2.28 c | 2.14 c |
| Petiole (cm) | 5.11 a,b | 5.08 a,b | 5.61 a | 4.80 b,c | 4.72 b,c | 4.46 c | |
| Leaf lamina (cm) | 5.25 a | 5.07 a | 5.27 a | 4.58 a | 4.49 a | 4.48 a | |
| Internode diameter (mm) | 3.09 a | 3.29 a | 3.52 a | 3.41 a | 3.46 a | 3.28 a | |
| Organ of Third Phytomer | Parameter | Treatment | |||||
|---|---|---|---|---|---|---|---|
| B60 | B110 | B160 | B210 | B260 | B310 | ||
| Internode | te | 13.96 b | 14.39 a,b | 14.21 b | 14.41 a,b | 14.67 a | 14.36 a,b |
| tm | 5.23 a | 6.86 a | 6.60 a | 6.33 a | 6.60 a | 6.13 a | |
| Petiole | te | 15.76 c | 16.03 b,c | 15.85 c | 16.47 a,b | 16.39 a,c | 16.68 a |
| tm | 9.79 b | 10.25 a,b | 10.18 a,b | 10.25 a,b | 10.74 a | 10.70 a | |
| Leaf lamina | te | 13.94 a | 14.12 a | 14.04 a | 14.11 a | 14.19 a | 13.89 a |
| tm | 5.19 a | 5.04 a | 4.57 a | 4.55 a | 5.50 a | 4.95 a | |
| Treatment | Energy Consumption (W) |
|---|---|
| B60 | 94.4 |
| B110 | 95.1 |
| B160 | 96.4 |
| B210 | 97.6 |
| B260 | 101.7 |
| B310 | 107.2 |
| Scenario | Light Spectra | Average Energy Consumption (W) |
|---|---|---|
| Experimental | B310 | 107.2 |
| First scenario | B260 | 101.7 |
| Second scenario | B210/B260 | 100.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hitz, T.; Graeff-Hönninger, S.; Munz, S. Modelling of Soybean (Glycine max (L.) Merr.) Response to Blue Light Intensity in Controlled Environments. Plants 2020, 9, 1757. https://doi.org/10.3390/plants9121757
Hitz T, Graeff-Hönninger S, Munz S. Modelling of Soybean (Glycine max (L.) Merr.) Response to Blue Light Intensity in Controlled Environments. Plants. 2020; 9(12):1757. https://doi.org/10.3390/plants9121757
Chicago/Turabian StyleHitz, Tina, Simone Graeff-Hönninger, and Sebastian Munz. 2020. "Modelling of Soybean (Glycine max (L.) Merr.) Response to Blue Light Intensity in Controlled Environments" Plants 9, no. 12: 1757. https://doi.org/10.3390/plants9121757
APA StyleHitz, T., Graeff-Hönninger, S., & Munz, S. (2020). Modelling of Soybean (Glycine max (L.) Merr.) Response to Blue Light Intensity in Controlled Environments. Plants, 9(12), 1757. https://doi.org/10.3390/plants9121757





