Phytochemical Profile, Safety Assessment and Wound Healing Activity of Artemisia absinthium L.
Abstract
1. Introduction
2. Results
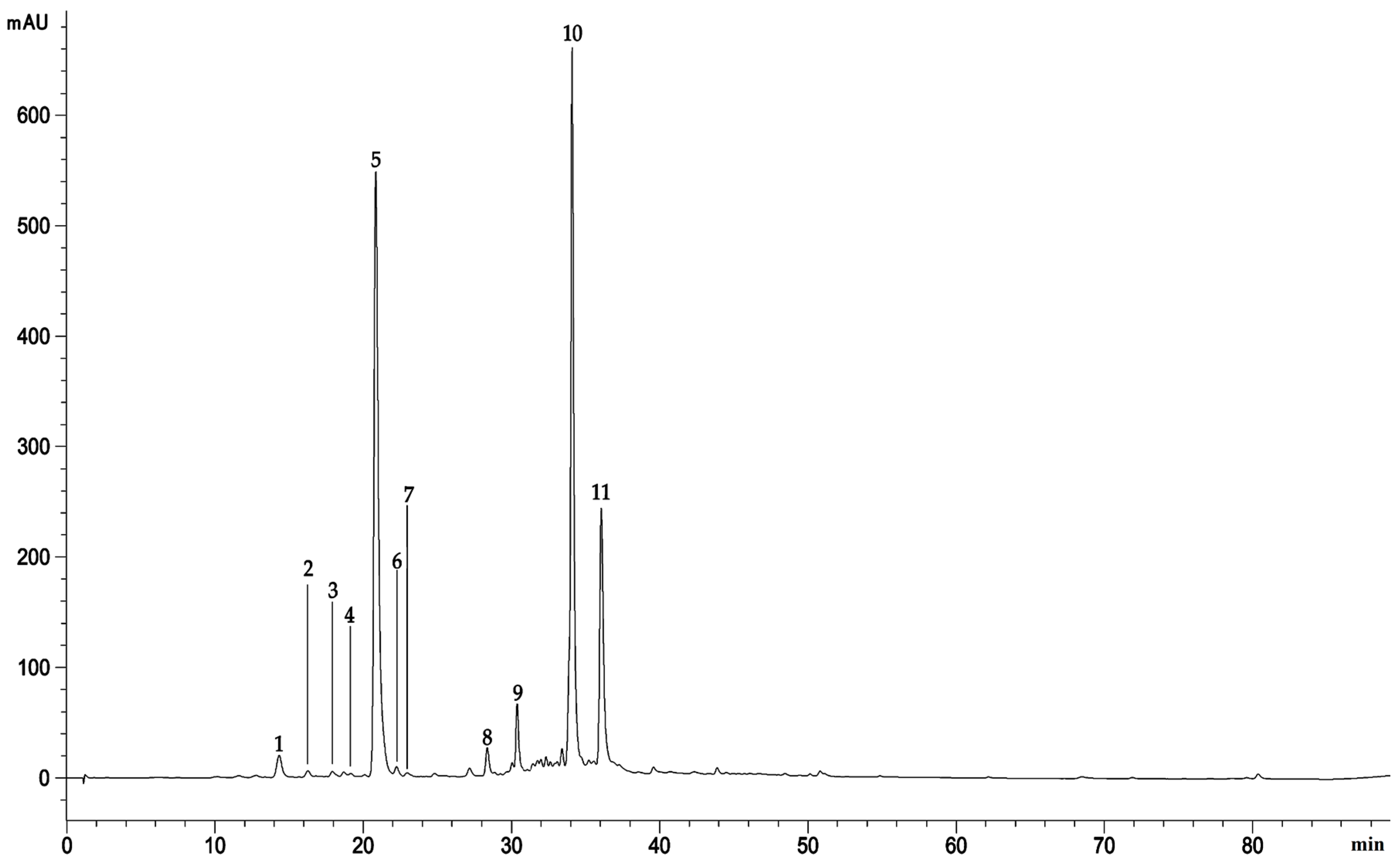
2.1. Extraction Yield and Phytochemical Characterization
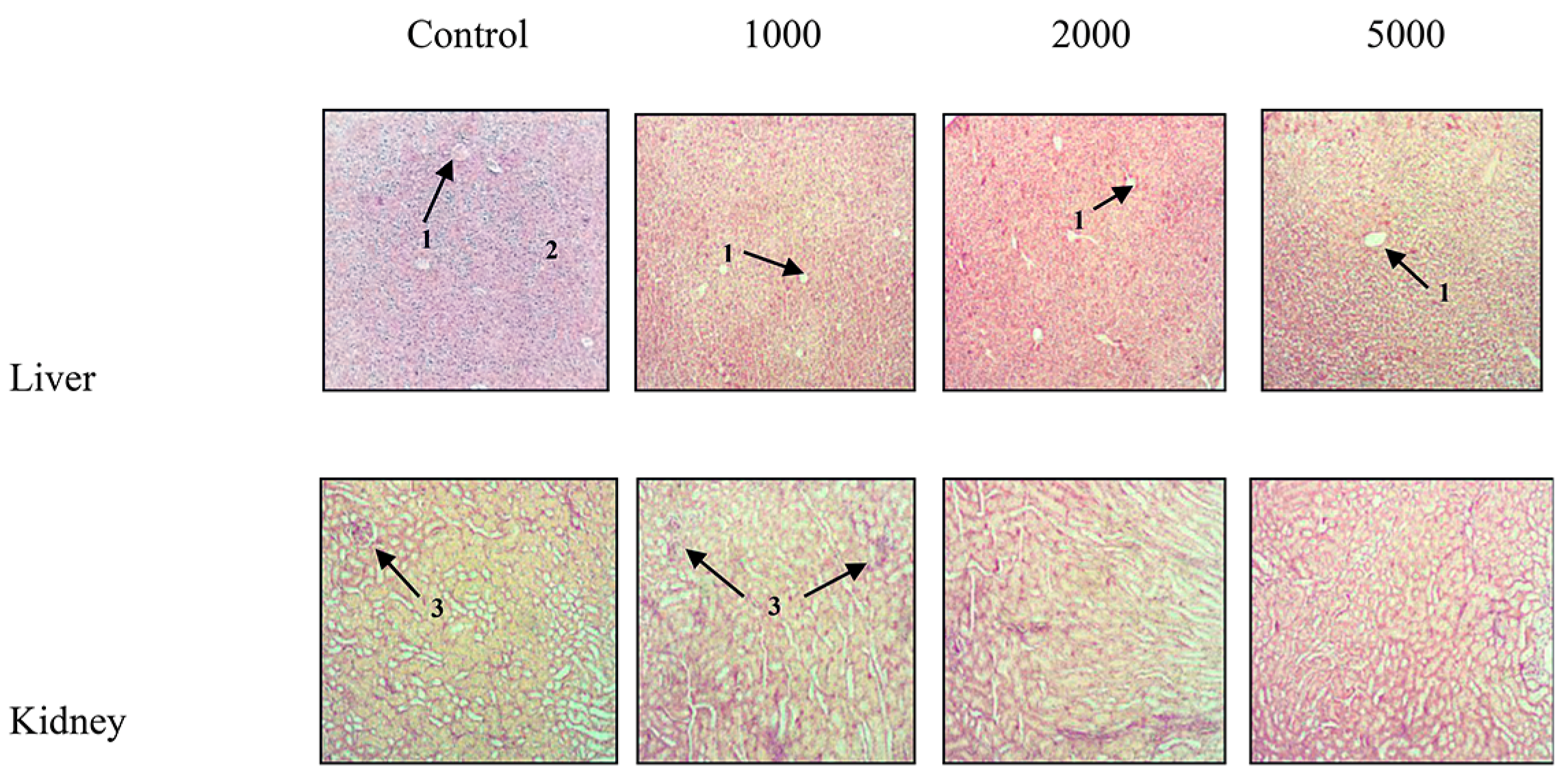
2.2. Toxicity Tests
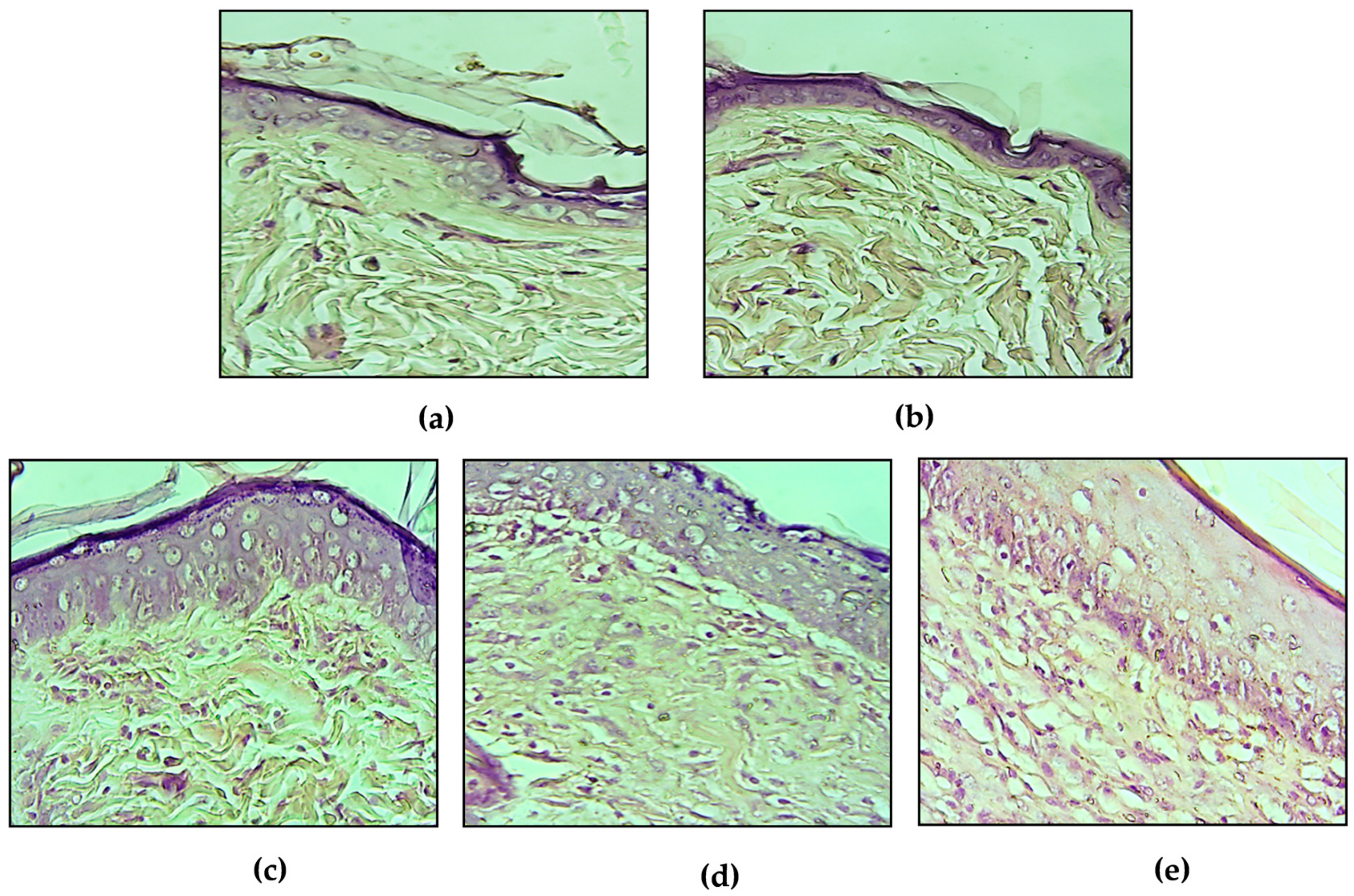
2.2.1. Acute Dermal Irritation
2.2.2. Acute Oral Toxicity
2.3. Biological Properties
2.3.1. Antibacterial Activity
2.3.2. Evaluation of the Wound Healing Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction
4.2. Ointments Preparation
4.3. Animals
4.4. Phytochemical Studies
4.4.1. Total Phenols Content
4.4.2. Total Flavonoids Content
4.4.3. Vanillin Index Determination
4.4.4. Proanthocyanidins Content
4.4.5. Polyphenol Profile Characterization
4.5. Toxicity Assays
4.5.1. Acute Dermal Irritation
4.5.2. Acute Oral Toxicity
4.6. Biological Properties
4.6.1. Antibacterial Activity
4.6.2. Wound-Healing Activity
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ahmmad, A.; Torequl, I.; Sultana, I.; Mahmood, A.; Akther Hossain, J.; Homa, Z.; Ibrahim, M.; Chowdhury, M.M. Pharmacological and Phytochemical Screening of Ethanol Extract of Litsea monopetala (Roxb.) Pers. J. Pharm. 2012, 2, 398–402. [Google Scholar] [CrossRef]
- Ammar, S.; Noui, H.; Djamel, S.; Madani, S.; Maggi, F.; Bruno, M.; Romano, D.; Canale, A.; Pavela, R.; Benelli, G. Essential oils from three Algerian medicinal plants (Artemisia campestris, Pulicaria arabica, and Saccocalyx satureioides) as new botanical insecticides? Environ. Sci. Pollut. Res. 2020, 27, 26594–26604. [Google Scholar] [CrossRef] [PubMed]
- Quezel, P.; Santa, S. Nouvelle Flore de l’Algérie et des Régions Désertiques Méridionales; Editions du Centre National de la Recherche Scientifique: Paris, France, 1963; Volume 2, pp. 798–990. [Google Scholar]
- Carvalho, A.R.J.; Diniz, R.M.; Suarez, M.A.M.; Figueiredo, C.S.S.E.S.; Zagmignan, A.; Grisotto, M.A.G.; Fernandes, E.S.; Da Silva, L.C.N. Use of Some Asteraceae Plants for the Treatment of Wounds: From Ethnopharmacological Studies to Scientific Evidences. Front. Pharmacol. 2018, 9, 784. [Google Scholar] [CrossRef] [PubMed]
- Azzi, R.; Djaziri, R.; Lahfa, F.; Sekkal, F.Z.; Benmehdi, H.; Belkacem, N. Ethnopharmacological survey of medicinal plants used in the traditional treatment ofdiabetes mellitus in the North Western and South Western Algeria. J. Med. Plants Res. 2012, 6, 2041–2050. [Google Scholar]
- Boudjelal, A.; Henchiri, C.; Sari, M.; Sarri, D.; Hendel, N.; Benkhaled, A.; Ruberto, G. Herbalists and wild medicinal plants in M’Sila (North Algeria): An ethnopharmacology survey. J. Ethnopharmacol. 2013, 148, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Sarri, M.; Boudjelal, A.; Hendel, N.; Sarri, D.J.; Benkhaled, A. Flora and ethnobotany of medicinal plants in the southeast of the capital of Hodna (Algeria). Flora 2015, 1, 24–30. [Google Scholar]
- Bouasla, A.; Bouasla, I. Ethnobotanical survey of medicinal plants in northeastern of Algeria. Phytomedicine 2017, 36, 68–81. [Google Scholar] [CrossRef]
- Msaada, K.; Salem, N.; Bachrouch, O.; Bousselmi, S.; Tammar, S.; AlFaify, A.; Al Sane, K.; Ben Ammar, W.; Azeiz, S.; Brahim, A.H.; et al. Chemical Composition and Antioxidant and Antimicrobial Activities of Wormwood (Artemisia absinthium L.) Essential Oils and Phenolics. J. Chem. 2015, 2015, 804658. [Google Scholar] [CrossRef]
- Harmanescu, M.; Moisuc, A.; Radu, F.; Drăgan, S.; Gergen, I. Total polyphenols content determination in complex matrix of medicinal plants from Romania by NIR spectroscopy. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Agric. 2008, 65, 123–128. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Nabavi, S.F.; Nabavi, S.M.; Pourmorad, F. Nitric oxide radical scavenging potential of some Elburz medicinal plants. Afr. J. Biotechnol. 2010, 9, 5212–5217. [Google Scholar]
- Tan, R.X.; Zheng, W.F.; Tang, H.Q. Biologically Active Substances from the GenusArtemisia. Planta Med. 1998, 64, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, J.; Mir, S.R.; Amin, S. A pharmacognostic review on artemisia absinthium. Int. Res. J. Pharm. 2019, 10, 25–31. [Google Scholar] [CrossRef]
- Szopa, A.; Pajor, J.; Klin, P.; Rzepiela, A.; Elansary, H.O.; Al-Mana, F.A.; Mattar, M.A.; Ekiert, H. Artemisia absinthium L.—Importance in the History of Medicine, the Latest Advances in Phytochemistry and Therapeutical, Cosmetological and Culinary Uses. Plants 2020, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Daradka, H.M.; Abas, M.M.; Mohammad, M.A.M.; Jaffar, M.M. Antidiabetic effect of Artemisia absinthium extracts on alloxan-induced diabetic rats. Comp. Haematol. Int. 2014, 23, 1733–1742. [Google Scholar] [CrossRef]
- Daradka, H.M.; Badawneh, M.; Al jamal, J.A.; Bataineh, Y. Hypolipidemic efficacy of Artemisia absinthium extracts in rabbits. World Appl. Sci. J. 2014, 31, 1415–1421. [Google Scholar]
- Hodge, A.; Sterner, B. Toxicity Classes. In Canadian Center for Occupational Health and Safety. Available online: http://www.ccohs.ca/oshanswers/chemicals/id50.htm (accessed on 4 November 2020).
- Frank, C.L.; Lhuguenot, J.C.; Rivière, J.L. Toxicologie-Données Générales Procédures D’évaluation, Organes Cibles, Evaluation du Risqué; Masson: Paris, France, 1991. [Google Scholar]
- Muto, T.; Watanabe, T.; Okamura, M.; Moto, M.; Kashida, Y.; Mitsumori, K. Thirteen-week repeated dose toxicity study of wormwood (artemisia absinthium) extract in rats. J. Toxicol. Sci. 2003, 28, 471–478. [Google Scholar] [CrossRef]
- Busineni, J.G.; Dwarakanath, V.; Chikka Swamy, B.K. A review on history, controversy, traditional use, ethnobotany, phytochemistry and pharmacology of Artemisia absinthium Linn. Int. J. Adv. Res. Eng. Appl. Sci. 2015, 4, 77–107. [Google Scholar]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Fiamegos, Y.C.; Kastritis, P.L.; Exarchou, V.; Han, H.; Bonvin, A.; Vervoort, J.; Lewis, K.; Hamblin, M.R.; Tegos, G.P. Antimicrobial and Efflux Pump Inhibitory Activity of Caffeoylquinic Acids from Artemisia absinthium against Gram-Positive Pathogenic Bacteria. PLoS ONE 2011, 6, e18127. [Google Scholar] [CrossRef]
- Djerrou, Z.; Pacha, Y.H.; Belkhiri, A.; Djaalab, H.; Riachi, F.; Serakta, M.; Boukeloua, A.; Maameri, Z. Evaluation of Pistacia lentiscus Fatty Oil Effects on Glycemic Index, Liver Functions and Kidney Functions of New Zealand Rabbits. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 214–219. [Google Scholar] [CrossRef]
- Araújo, L.U.; Grabe-Guimarães, A.; Mosqueira, V.C.F.; Carneiro, C.M.; Silva-Barcellos, N.M. Profile of wound healing process induced by allantoin. Acta Cir. Bras. 2010, 25, 460–461. [Google Scholar] [CrossRef]
- Chen, W.-C.; Liou, S.-S.; Tzeng, T.-F.; Lee, S.-L.; Liu, I.-M. Effect of Topical Application of Chlorogenic Acid on Excision Wound Healing in Rats. Planta Med. 2013, 79, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Gul, N.Y.; Topal, A.; Tas, S.; Ozyigit, M.O.; Cinkilic, N.; Gul, Z.; Etoz, B.C.; Ziyanok, S.; Inan, S.; et al. Pharmacologic overview of systemic chlorogenic acid therapy on experimental wound healing. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 1101–1116. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zhang, Y.; Huang, L.; Xu, L.; Wang, K.; Wang, D.; Guan, L.; Zhang, Y.; Yu, F.-L.; Chen, Z.; et al. Effects and Mechanisms of Total Flavonoids from Blumea balsamifera (L.) DC. on Skin Wound in Rats. Int. J. Mol. Sci. 2017, 18, 2766. [Google Scholar] [CrossRef]
- Pereira, L.X.; Silva, H.; Longatti, T.R.; Dias, P.P.; Oliveira, C.D.L.; Proietti, A.B.D.F.C.; Thomé, R.G.; Vieira, M.D.C.; Carollo, C.A.; Demarque, D.P.; et al. Achyrocline alata potentiates repair of skin full thickness excision in mice. J. Tissue Viability 2017, 26, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.H.; Zuwaiel, A.A.; Sivakumar, S.M.; Alshahrani, S.; Alqahtani, S.S.; Madkhali, O.; Elmobark, M.E. Bioactive principles and potentiality of hot methanolic extract of the leaves from Artemisia absinthium L “in vitro cytotoxicity against human MCF-7 breast cancer cells, antibacterial study and wound healing activity”. Curr. Pharm. Biotechnol. 2020, 21, 1–13. [Google Scholar] [CrossRef]
- Smeriglio, A.; Galati, E.M.; Monforte, M.T.; Lanuzza, F.; D’Angelo, V.; Circosta, C. Polyphenolic Compounds and Antioxidant Activity of Cold-Pressed Seed Oil from Finola Cultivar of Cannabis sativa L. Phytother. Res. 2016, 30, 1298–1307. [Google Scholar] [CrossRef]
- Lenucci, M.; Cadinu, D.; Taurino, M.; Piro, G.; Dalessandro, G. Antioxidant Composition in Cherry and High-Pigment Tomato Cultivars. J. Agric. Food Chem. 2006, 54, 2606–2613. [Google Scholar] [CrossRef]
- Barreca, D.; Laganà, G.; Leuzzi, U.; Smeriglio, A.; Trombetta, D.; Bellocco, E. Evaluation of the nutraceutical, antioxidant and cytoprotective properties of ripe pistachio (Pistacia vera L.; variety Bronte) hulls. Food Chem. 2016, 196, 493–502. [Google Scholar] [CrossRef]
- The Organization of Economic Co-Operation and Development (OECD). The OECD Guideline for Testing of Chemicals: 404-Acute Dermal Irritation/Corrosion; OECD: Paris, France, 2002. [Google Scholar]
- Council for International Organizations of Medical Sciences and International Council for Laboratory Animal Science. 2012. International guiding principles for biomedical research involving animals. Available online: www.cioms.ch/images/stories/CIOMS/IGP2012.pdf (accessed on 11 November 2020).
- The Organization of Economic Co-Operation and Development (OECD). The OECD Guideline for Testing of Chemicals: 423-Acute Oral Toxicity; OECD: Paris, France, 2001. [Google Scholar]
- Abrar, H.M.; Manjusha, S.; Mohd, Y.M. An acute oral toxicity study of methanolic extract from Tridex procumbent sin Sprague Dawley’s Rats as per OECD guidelines 423. Asian J. Plant Sci. Res. 2013, 3, 16–20. [Google Scholar]
- Gandhare, B.; Kavimani, S.; Rajkapoor, B. Acute and Subacute Toxicity Study of Methanolic Extract of ceiba pentandra (Linn.) Gaertn. on Rats. J. Sci. Res. 2013, 5, 315–324. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing M100-S22; Twentieth Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard M27-A3, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Hwisa, N.A.; Katakam, P.; Chandu, B.R.; Abadi, E.G.; Shefha, E.M. Comparative in vivo evaluation of three types of honey on topical wound healing activity in rabbits. J. Appl. Pharm. Sci. 2013, 3, 139–143. [Google Scholar] [CrossRef]
- Mashreghi, M.; Bazaz, M.R.; Shahri, N.M.; Asoodeh, A.; Mashreghi, M.; Rassouli, M.B.; Golmohammadzadeh, S. Topical effects of frog “Rana ridibunda” skin secretions on wound healing and reduction of wound microbial load. J. Ethnopharmacol. 2013, 145, 793–797. [Google Scholar] [CrossRef]
- Kumar, V.; Ahmed Khan, A.; Nagarajan, K. Animal models for the evaluation of wound healing activity. Int. Bull. Drug Res. 2013, 3, 93–107. [Google Scholar]
- Pipelzadeh, M.H.; Pipelzadeh, M.R.; Husseinzadeh, P. A Study on the effects of modulation of intracellular calcium on excisional wound healing in rabbit. J. Iran. Biomedical. 2003, 7, 161–166. [Google Scholar]
- Tamri, P.; Hemmati, A.; Boroujerdnia, M.G. Wound healing properties of quince seed mucilage: In vivo evaluation in rabbit full-thickness wound model. Int. J. Surg. 2014, 12, 843–847. [Google Scholar] [CrossRef]
- Marque, V. Manuel de Techniques D’anatomo-Cytopathologique, 1st ed.; Elsevier: Masson, France, 2010; pp. 1–196. [Google Scholar]
| Assays | Methanol Extract |
|---|---|
| Total phenols (mg GAE 1/g DE 2) | 180.33 ± 16.25 |
| Total flavonoids (mg RE 3/g DE) | 165.47 ± 13.32 |
| Proanthocyanidyns (mg CyE 4/g DE) | 43.11 ± 2.02 |
| Vanillin index (mg CE 5/g DE) | 11.17 ± 0.68 |
| Polimerization index (VI 6/PC 7) | 0.26 |
| Compound | RT | λmax | [M-H]- | MW | Area% |
|---|---|---|---|---|---|
| 4-Caffeoylquinic acid | 14.323 | 232; 262; 300 | 353 | 354 | 1.65 |
| Homorientin | 16.261 | 232; 262; 300 | 447 | 448 | 0.35 |
| 5-Caffeoylquinic acid | 17.909 | 232; 264; 312 | 353 | 354 | 0.30 |
| Apigenin-7-O-glucoside | 19.147 | 232; 264; 310 | 431 | 432 | 0.18 |
| Chlorogenic acid | 20.840 | 232; 264; 310 | 353 | 354 | 37.01 |
| Artemetin | 22.240 | 232; 264; 310 | 387 | 388 | 0.37 |
| 1-O-β-D-glucopyranosyl sinapate | 22.986 | 232; 264; 310 | 385 | 386 | 0.05 |
| 2′,6′-dihydroxy-4-Methoxychalcone-4′-O-neohesperidoside | 28.361 | 232; 270; 310 | 593 | 594 | 1.42 |
| Luteolin-6-C-[β-D-glucosyl-(1->2)-α-L-arabinoside | 30.715 | 232; 322 | 579 | 580 | 1.40 |
| 3,4-Dicaffeoylquinic acid | 34.086 | 232; 294; 324 | 515 | 516 | 31.60 |
| 3,5-Dicaffeoylquinic acid | 36.052 | 232; 296; 326 | 515 | 516 | 13.38 |
| Total identified compounds | 87.71 |
| Parameters | Treatment (mg/kg b.wt) | |||
|---|---|---|---|---|
| Control | 1000 | 2000 | 5000 | |
| Urea (mg/L) | 37.31 ± 1.75 | 33.85 ± 1.52 | 32.99 ± 0.62 | 38.97 ± 4.14 |
| Creatinine (mg/L) | 4.09 ± 0.62 | 4.31 ± 0.64 | 4.77 ± 0.28 | 4.69 ± 0.40 |
| Uric Acid (mg/dL) | 38.57 ± 5.72 | 37.14 ± 3.46 | 34.76 ± 3.51 | 39.05 ± 3.33 |
| AST (IU/L) | 66.08 ± 6.61 | 67.96 ± 7.80 | 69.27 ± 6.10 | 72.63 ± 8.34 |
| ALT (IU/L) | 22.02 ± 3.95 | 17.65 ± 1.53 | 19.83 ± 0.86 | 26.54 ± 1.77 |
| ALP (IU/L) | 45.67 ± 5.84 | 56.58 ± 6.36 | 57.15 ± 8.09 | 59.34 ± 4.84 |
| Total Protein (g/dL) | 60.00 ± 2.64 | 56.12 ± 7.55 | 62.52 ± 3.70 | 52.38 ± 2.31 |
| Bacteria | Methanol Extract | Reference Antibiotics | ||
|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (µg/mL) | MBC (µg/mL) | |
| Gram-negative | Tobramycin | |||
| Escherichia coli ATCC 10536 | 2.5–1.255 | >2.5 | 0.50 | 0.50 |
| Pseudomona aeruginosa ATCC 9027 | >2.5 | >2.5 | 0.25 | 0.25 |
| Gram-positive | Vancomycin | |||
| Staphylococcus aureus ATCC 6538 | 0.625–0.31 | >2.5 | 0.31–0.62 | 0.31–0.62 |
| Staphylococcus epidermidis ATCC 35984 | >2.5 | >2.5 | ||
| S. aureus 526 | >2.5 | >2.5 | ||
| S. aureus 530 | >2.5 | >2.5 | ||
| S. aureus 808 | >2.5 | >2.5 | ||
| S. aureus 581 | >2.5 | >2.5 | ||
| S. aureus 8 | >2.5 | >2.5 | ||
| S. aureus 14 | >2.5 | >2.5 | ||
| S. aureus 3 | >2.5 | >2.5 | ||
| S. aureus 32 | >2.5 | >2.5 | ||
| S. aureus 84 | >2.5 | >2.5 | ||
| Group | Wound Contraction % during Time (days) | |||||
|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 15 | 18 | |
| UT | 11.58 ± 1.67 | 17.82 ± 1.2 | 19.77 ± 2.83 | 20.54 ± 2.39 | 22.32 ± 2.3 | 27.54 ± 1.10 |
| CIC | 13.02 ± 0.96 | 29.81 ± 3.29 *** | 49.91 ± 1.55 *** | 70.53 ± 1.19 *** | 75.87 ± 1.12 *** | 84.52 ± 0.80 *** |
| MEO 5% | 14.46 ± 2.01 | 30.27 ± 0.47 *** | 43.18 ± 1.27 *** | 57.59 ± 1.43 *** | 68.41 ± 2.49 *** | 76.73 ± 2.97 *** |
| MEO 10% | 14.60 ± 0.59 | 25.75 ± 0.91 ** | 35.83 ± 1.51 *** | 61.66 ± 1.24 *** | 75.45 ± 1.70 *** | 81.49 ± 2.83 *** |
| PJ | 12.99 ± 2.27 | 24.79 ± 1.6 ** | 34.85 ± 1.61 *** | 36.51 ± 1.37 *** | 44.75 ± 1.85 *** | 50.56 ± 1.52 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudjelal, A.; Smeriglio, A.; Ginestra, G.; Denaro, M.; Trombetta, D. Phytochemical Profile, Safety Assessment and Wound Healing Activity of Artemisia absinthium L. Plants 2020, 9, 1744. https://doi.org/10.3390/plants9121744
Boudjelal A, Smeriglio A, Ginestra G, Denaro M, Trombetta D. Phytochemical Profile, Safety Assessment and Wound Healing Activity of Artemisia absinthium L. Plants. 2020; 9(12):1744. https://doi.org/10.3390/plants9121744
Chicago/Turabian StyleBoudjelal, Amel, Antonella Smeriglio, Giovanna Ginestra, Marcella Denaro, and Domenico Trombetta. 2020. "Phytochemical Profile, Safety Assessment and Wound Healing Activity of Artemisia absinthium L." Plants 9, no. 12: 1744. https://doi.org/10.3390/plants9121744
APA StyleBoudjelal, A., Smeriglio, A., Ginestra, G., Denaro, M., & Trombetta, D. (2020). Phytochemical Profile, Safety Assessment and Wound Healing Activity of Artemisia absinthium L. Plants, 9(12), 1744. https://doi.org/10.3390/plants9121744








