In Vitro Symbiotic Germination: A Revitalized Heuristic Approach for Orchid Species Conservation
Abstract
1. Introduction
2. Through the Looking Glass: How Orchids Naturally Establish
2.1. Orchid Seed Characteristics as Innate Strategies of Reproduction
2.2. Environment and Mycorrhizal Fungi as Constraints to Orchid Survival
2.3. In Vitro Orchid Symbiotic Germination as a Tool for Studying Mycorrhizal Symbiosis
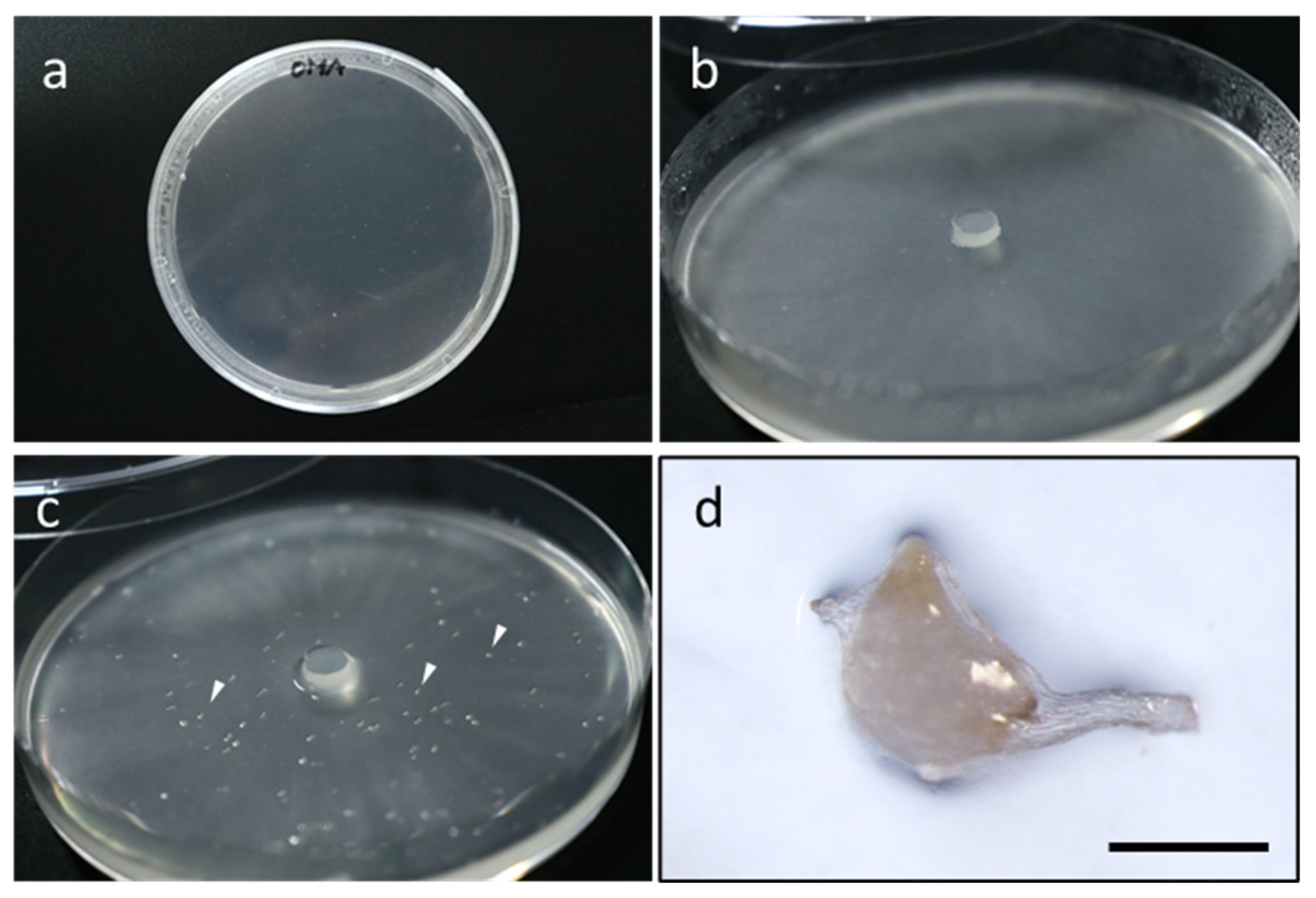
3. New Knowledge Obtained from Symbiotic Germination Using Advanced Tools
4. Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nomura, N.; Ogura-Tsujita, Y.; Gale, S.W.; Maeda, A.; Umata, H.; Hosaka, K.; Yukawa, T. The rare terrestrial orchid Nervilia nipponica consistently associates with a single group of novel mycobionts. J. Plant Res. 2013, 126, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, K. A novel seed dispersal mode of Apostasia nipponica could provide some clues to the early evolution of the seed dispersal system in Orchidaceae. Evol. Lett. 2020, 4, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Rafter, M.; Yokoya, K.; Schofield, E.J.; Zettler, L.W.; Sarasan, V. Non-specific symbiotic germination of Cynorkis purpurea (Thouars) Kraenzl., a habitat-specific terrestrial orchid from the Central Highlands of Madagascar. Mycorrhiza 2016, 26, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Oktalira, F.T.; Whitehead, M.R.; Linde, C.C. Mycorrhizal specificity in widespread and narrow-range distributed Caladenia orchid species. Fungal Ecol. 2019, 42, 100869. [Google Scholar] [CrossRef]
- Xing, X.; Jacquemyn, H.; Gai, X.; Gao, Y.; Liu, Q.; Zhao, Z.; Guo, S. The impact of life form on the architecture of orchid mycorrhizal networks in tropical forest. Oikos 2019, 128, 1254–1264. [Google Scholar] [CrossRef]
- Steinfort, U.; Verdugo, G.; Besoain, X.; Cisternas, M.A. Mycorrhizal association and symbiotic germination of the terrestrial orchid Bipinnula fimbriata (Poepp.) Johnst (Orchidaceae). Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 811–817. [Google Scholar] [CrossRef]
- Gowland, K.M.; Van Der Merwe, M.M.; Linde, C.C.; Clements, M.A.; Nicotra, A.B. The host bias of three epiphytic Aeridinae orchid species is reflected, but not explained, by mycorrhizal fungal associations. Am. J. Bot. 2013, 100, 764–777. [Google Scholar] [CrossRef]
- Zi, X.-M.; Sheng, C.-L.; Goodale, U.M.; Shao, S.-C.; Gao, J.-Y. In situ seed baiting to isolate germination-enhancing fungi for an epiphytic orchid, Dendrobium aphyllum (Orchidaceae). Mycorrhiza 2014, 24, 487–499. [Google Scholar] [CrossRef]
- Kendon, J.P.; Yokoya, K.; Zettler, L.W.; Jacob, A.S.; McDiarmid, F.; Bidartondo, M.I.; Sarasan, V. Recovery of mycorrhizal fungi from wild collected protocorms of Madagascan endemic orchid Aerangis ellisii (B.S. Williams) Schltr. and their use in seed germination in vitro. Mycorrhiza 2020, 30, 567–576. [Google Scholar] [CrossRef]
- Shao, S.-C.; Burgess, K.S.; Cruse-Sanders, J.M.; Liu, Q.; Fan, X.-L.; Huang, H.; Gao, J.-Y. Using In situ Symbiotic Seed Germination to Restore Over-collected Medicinal Orchids in Southwest China. Front. Plant Sci. 2017, 8, 888. [Google Scholar] [CrossRef]
- Khamchatra, N.; Dixon, K.; Tantiwiwat, S.; Piapukiew, J. Symbiotic seed germination of an endangered epiphytic slipper orchid, Paphiopedilum villosum (Lindl.) Stein. from Thailand. S. Afr. J. Bot. 2016, 104, 76–81. [Google Scholar] [CrossRef]
- Shimura, H.; Koda, Y. Enhanced symbiotic seed germination of Cypripedium macranthos var. rebunense following inoculation after cold treatment. Physiol. Plant. 2005, 123, 281–287. [Google Scholar] [CrossRef]
- Torrezan, M.D.A.; Da Silva, M.A.V.; Neto, V.B.P.; Padilha, D.R.C.; Santos, A.J.d.S. Precocious flowering of plants resulting from in vitro germination of Cycnoches haagii seeds on mycorrhizal fungi presence. Pesqui. Agropecu. Trop. 2018, 48, 468–475. [Google Scholar] [CrossRef]
- Guimarães, F.A.R.; Felício, C.D.S.; Torres, D.P.; Oliveira, S.F.; Veloso, T.G.R.; Pereira, M.C.; Kasuya, M.C.M. Symbiotic propagation of seedlings of Cyrtopodium glutiniferum Raddi (Orchidaceae). Acta Bot. Bras. 2013, 27, 590–596. [Google Scholar] [CrossRef][Green Version]
- Reiter, N.; Lawrie, A.C.; Linde, C.C. Matching symbiotic associations of an endangered orchid to habitat to improve conservation outcomes. Ann. Bot. 2018, 122, 947–959. [Google Scholar] [CrossRef]
- Aggarwal, S.; Nirmala, C.; Beri, S.; Rastogi, S.; Adholeya, A. In vitro symbiotic seed germination and molecular characterization of associated endophytic fungi in a commercially important and endangered Indian orchid Vanda coerulea Griff. ex Lindl. Eur. J. Environ. Sci. 2012, 2, 33–42. [Google Scholar] [CrossRef]
- Arditti, J.; Ghani, A.K.A. Tansley Review No. 110: Numerical and physical properties of orchid seeds and their biological implications. New Phytol. 2000, 145, 367–421. [Google Scholar] [CrossRef]
- Yeung, E.C. A perspective on orchid seed and protocorm development. Bot. Stud. 2017, 58, 1–14. [Google Scholar] [CrossRef]
- Barthlott, W.; Große-Veldmann, B.; Korotkova, N. Orchid seed diversity: A scanning electron microscopy survey. Englera 2014, 32, 1–239. [Google Scholar]
- Sisti, L.S.; Flores-Borges, D.N.A.; De Andrade, S.A.L.; Koehler, S.; Bonatelli, M.L.; Mayer, J.L.S. The Role of Non-Mycorrhizal Fungi in Germination of the Mycoheterotrophic Orchid Pogoniopsis schenckii. Cogn. Front. Plant Sci. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Jiao, S.; Chen, W.; Wang, J.; Du, N.; Li, Q.; Wei, G. Soil microbiomes with distinct assemblies through vertical soil profiles drive the cycling of multiple nutrients in reforested ecosystems. Microbiome 2018, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, Y.P.; Fischer, A.; Fischer, H.S. Epiphytic orchids and their ecological niche under anthropogenic influence in central Himalayas, Nepal. J. Mt. Sci. 2016, 13, 774–784. [Google Scholar] [CrossRef]
- Johansson, D. Ecology of Vascular Epiphytes in West African Rain Forest. In Acta Phytogeographica Suecica; Svenska vaxtgeografiska sallskapet: Uppsala, Sweden, 1974; Volume 59, ISBN 9172104597. [Google Scholar]
- Mondragón, D.; Maldonado, C.; Aguilar-Santelises, R. Life history and demography of a twig epiphyte: A case study of Erycina crista-galli (Orchidaceae). Selbyana 2007, 28, 137–144. [Google Scholar] [CrossRef]
- Otero, J.T.; Flanagan, N.S.; Herre, E.A.; Ackerman, J.D.; Bayman, P. Widespread mycorrhizal specificity correlates to mycorrhizal function in the neotropical, epiphytic orchid Ionopsis utricularioides (Orchidaceae). Am. J. Bot. 2007, 94, 1944–1950. [Google Scholar] [CrossRef]
- Otero, J.T.; Ackerman, J.D.; Bayman, P. Differences in mycorrhizal preferences between two tropical orchids. Mol. Ecol. 2004, 13, 2393–2404. [Google Scholar] [CrossRef]
- Shimura, H.; Matsuura, M.; Takada, N.; Koda, Y. An antifungal compound involved in symbiotic germination of Cypripedium macranthos var. rebunense (Orchidaceae). Phytochemistry 2007, 68, 1442–1447. [Google Scholar] [CrossRef]
- Akiyama, K.; Hayashi, H. Strigolactones: Chemical Signals for Fungal Symbionts and Parasitic Weeds in Plant Roots. Ann. Bot. 2006, 97, 925–931. [Google Scholar] [CrossRef]
- Selosse, M.-A.; Minasiewicz, J.; Boullard, B. An annotated translation of Noël Bernard’s 1899 article ‘On the germination of Neottia nidus-avis’. Mycorrhiza 2017, 27, 611–618. [Google Scholar] [CrossRef]
- Fracchia, S.; Aranda-Rickert, A.; Rothen, C.; Sede, S. Associated fungi, symbiotic germination and in vitro seedling development of the rare Andean terrestrial orchid Chloraea riojana. Flora 2016, 224, 106–111. [Google Scholar] [CrossRef]
- Bayman, P.; Mosquera-Espinosa, A.T.; Saladini-Aponte, C.M.; Hurtado-Guevara, N.C.; Viera-Ruiz, N.L. Age-dependent mycorrhizal specificity in an invasive orchid, Oeceoclades maculata. Am. J. Bot. 2016, 103, 1880–1889. [Google Scholar] [CrossRef]
- Umata, H. A new biological function of Shiitake mushroom, Lentinula edodes, in a myco-heterotrophic orchid, Erythrorchis ochobiensis. Mycoscience 1998, 39, 85–88. [Google Scholar] [CrossRef]
- Umata, H.; Ota, Y.; Yamada, M.; Watanabe, Y.; Gale, S.W. Germination of the fully myco-heterotrophic orchid Cyrtosia septentrionalis is characterized by low fungal specificity and does not require direct seed-mycobiont contact. Mycoscience 2013, 54, 343–352. [Google Scholar] [CrossRef]
- Maldonado, G.P.; Yarzábal, L.A.; Cevallos-Cevallos, J.M.; Chica, E.J.; Peña, D.F. Root endophytic fungi promote in vitro seed germination in Pleurothallis coriacardia (Orchidaceae). Lankesteriana 2020, 107–122. [Google Scholar] [CrossRef]
- Salazar, J.M.; Pomavilla, M.; Pollard, A.T.; Chica, E.J.; Peña, D.F. Endophytic fungi associated with roots of epiphytic orchids in two Andean forests in Southern Ecuador and their role in germination. Lankesteriana 2020, 37–47. [Google Scholar] [CrossRef]
- Johnson, T.R.; Stewart, S.L.; Dutra, D.; Kane, M.E.; Richardson, L. Asymbiotic and symbiotic seed germination of Eulophia alta (Orchidaceae)—preliminary evidence for the symbiotic culture advantage. Plant Cell Tissue Organ Cult. (PCTOC) 2007, 90, 313–323. [Google Scholar] [CrossRef]
- Frericks, J.; Munkacsi, A.; Ritchie, P.A.; Luo, Y.-B.; Lehnebach, C.A. Phylogenetic affinities and in vitro seed germination of the threatened New Zealand orchid Spiranthes novae-zelandiae. N. Z. J. Bot. 2018, 56, 91–108. [Google Scholar] [CrossRef]
- Sousa, K.C.I.; De Araújo, L.G.; Silva, C.D.S.; De Carvalho, J.C.B.; Sibov, S.T.; Gonçalves, L.D.A.; Pereira, M.C.; Gonçalves, F.J.; Da Corsi De Filippi, M.C. Seed germination and development of orchid seedlings (Cyrtopodium saintlegerianum) with fungi. Rodriguésia 2019, 70. [Google Scholar] [CrossRef]
- Decruse, S.; Neethu, R.; Pradeep, N. Seed germination and seedling growth promoted by a Ceratobasidiaceae clone in Vanda thwaitesii Hook.f., an endangered orchid species endemic to South Western Ghats, India, and Sri Lanka. S. Afr. J. Bot. 2018, 116, 222–229. [Google Scholar] [CrossRef]
- Umata, H. Formation of endomycorrhizas by an achlorophyllous orchid, Erythrorchis ochobiensis, and Auricularia polytricha. Mycoscience 1997, 38, 335–339. [Google Scholar] [CrossRef]
- Taylor, D.L.; Bruns, T.D. Population, habitat and genetic correlates of mycorrhizal specialization in the ’cheating’ orchids Corallorhiza maculata and C. mertensiana. Mol. Ecol. 1999, 8, 1719–1732. [Google Scholar] [CrossRef]
- Suetsugu, K.; Matsubayashi, J.; Tayasu, I. Some mycoheterotrophic orchids depend on carbon from dead wood: Novel evidence from a radiocarbon approach. New Phytol. 2020, 227, 1519–1529. [Google Scholar] [CrossRef]
- Sebastián, F.; Vanesa, S.; Eduardo, F.; Graciela, T.; Silvana, S. Symbiotic seed germination and protocorm development of Aa achalensis Schltr., a terrestrial orchid endemic from Argentina. Mycorrhiza 2013, 24, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Valadares, R.B.S.; Perotto, S.; Santos, E.C.; Lambais, M.R. Proteome changes in Oncidium sphacelatum (Orchidaceae) at different trophic stages of symbiotic germination. Mycorrhiza 2013, 24, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Yagame, T.; Funabiki, E.; Yukawa, T.; Nagasawa, E. Identification of mycobionts in an achlorophyllous orchid, Cremastra aphylla (Orchidaceae), based on molecular analysis and basidioma morphology. Mycoscience 2018, 59, 18–23. [Google Scholar] [CrossRef]
- Yeh, C.-M.; Chung, K.; Liang, C.-K.; Tsai, W.-C. New Insights into the Symbiotic Relationship between Orchids and Fungi. Appl. Sci. 2019, 9, 585. [Google Scholar] [CrossRef]
- Valadares, R.B.S.; Perotto, S.; Lucheta, A.R.; Santos, E.C.; Oliveira, R.M.; Lambais, M.R. Proteomic and Transcriptomic Analyses Indicate Metabolic Changes and Reduced Defense Responses in Mycorrhizal Roots of Oeceoclades maculata (Orchidaceae) Collected in Nature. J. Fungi 2020, 6, 148. [Google Scholar] [CrossRef]
- Yamamoto, T.; Miura, C.; Fuji, M.; Nagata, S.; Otani, Y.; Yagame, T.; Yamato, M.; Kaminaka, H. Quantitative evaluation of protocorm growth and fungal colonization in Bletilla striata (Orchidaceae) reveals less-productive symbiosis with a non-native symbiotic fungus. BMC Plant Biol. 2017, 17, 1–10. [Google Scholar] [CrossRef]
- López-Chávez, M.Y.; Guillén-Navarro, K.; Bertolini, V.; Encarnación, S.; Hernández-Ortiz, M.; Sánchez-Moreno, I.; Damon, A. Proteomic and morphometric study of the in vitro interaction between Oncidium sphacelatum Lindl. (Orchidaceae) and Thanatephorus sp. RG26 (Ceratobasidiaceae). Mycorrhiza 2016, 26, 353–365. [Google Scholar] [CrossRef]
- Alexander, C.; Hadley, G. Variation in symbiotic activity of Rhizoctonia isolates from Goodyera repens mycorrhizas. Trans. Br. Mycol. Soc. 1983, 80, 99–106. [Google Scholar] [CrossRef]
- Zettler, L.W.; Poulter, S.B.; McDonald, K.I.; Stewart, S.L. Conservation-driven Propagation of an Epiphytic Orchid (Epidendrum nocturnum) with a Mycorrhizal Fungus. HortScience 2007, 42, 135–139. [Google Scholar] [CrossRef]
- Hoang, N.H.; Kane, M.E.; Radcliffe, E.N.; Zettler, L.W.; Richardson, L.W. Comparative seed germination and seedling development of the ghost orchid, Dendrophylax lindenii (Orchidaceae), and molecular identification of its mycorrhizal fungus from South Florida. Ann. Bot. 2016, 119, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Zettler, L.W.; Stewart, S.L.; Bowles, M.L.; Jacobs, K.A. Mycorrhizal Fungi and Cold-assisted Symbiotic Germination of the Federally Threatened Eastern Prairie Fringed Orchid, Platanthera leucophaea (Nuttall) Lindley. Am. Midl. Nat. 2001, 145, 168–175. [Google Scholar] [CrossRef]
- Masuhara, G.; Katsuya, K. Effects of mycorrhizal fungi on seed germination and early growth of three Japanese terrestrial orchids. Sci. Hortic. 1989, 37, 331–337. [Google Scholar] [CrossRef]
- Fuji, M.; Miura, C.; Yamamoto, T.; Komiyama, S.; Suetsugu, K.; Yagame, T.; Yamato, M.; Kaminaka, H. Relative effectiveness of Tulasnella fungal strains in orchid mycorrhizal symbioses between germination and subsequent seedling growth. Symbiosis 2020, 81, 1–11. [Google Scholar] [CrossRef]
- Hajong, S.; Kumaria, S.; Tandon, P. Compatible fungi, suitable medium, and appropriate developmental stage essential for stable association of Dendrobium chrysanthum. J. Basic Microbiol. 2013, 53, 1025–1033. [Google Scholar] [CrossRef]
- Alomia, Y.; Mosquera-Espinosa, A.; Flanagan, N.; Otero, J. Seed Viability and Symbiotic Seed Germination in Vanilla spp. (Orchidaceae). Res. J. Seed Sci. 2017, 10, 43–52. [Google Scholar] [CrossRef]
- Stewart, S.L.; Zettler, L.W. Symbiotic germination of three semi-aquatic rein orchids (Habenaria repens, H. quinquiseta, H. macroceratitis) from Florida. Aquat. Bot. 2002, 72, 25–35. [Google Scholar] [CrossRef]
- Fracchia, S.; Rickert, A.M.A.; Flachsland, E.; Terada, G.; Sede, S.M. Mycorrhizal compatibility and symbiotic reproduction of Gavilea australis, an endangered terrestrial orchid from south Patagonia. Mycorrhiza 2014, 24, 627–634. [Google Scholar] [CrossRef]
- De Carvalho, O.C.; Neto, V.B.P.; Padilha, D.R.C.; Veloso, T.G.R.; Bocayuva, M.F.; Soares, D.C.O.; Kasuya, M.C.M. Cyrtopodium paludicolum germination with two Tulasnella isolates. Acta Bot. Bras. 2017, 32, 107–112. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Z.; Li, J.; Liu, N.; Jacquemyn, H.; Guo, S.; Xing, X. Do fungal associates of co-occurring orchids promote seed germination of the widespread orchid species Gymnadenia conopsea? Mycorrhiza 2020, 30, 221–228. [Google Scholar] [CrossRef]
- Izuddin, M.; Yam, T.W.; Webb, E.L. Germination niches and seed persistence of tropical epiphytic orchids in an urban landscape. J. Plant Res. 2019, 132, 383–394. [Google Scholar] [CrossRef]
- Yang, W.-K.; Li, T.-Q.; Wu, S.-M.; Finnegan, P.M.; Gao, J.-Y. Ex situ seed baiting to isolate germination-enhancing fungi for assisted colonization in Paphiopedilum spicerianum, a critically endangered orchid in China. Glob. Ecol. Conserv. 2020, 23, e01147. [Google Scholar] [CrossRef]
- Batty, A.L.; Dixon, K.W.; Brundrett, M.; Sivasithamparam, K. Constraints to symbiotic germination of terrestrial orchid seed in a Mediterranean bushland. New Phytol. 2001, 152, 511–520. [Google Scholar] [CrossRef]
- Meng, Y.-Y.; Zhang, W.-L.; Selosse, M.-A.; Gao, J.-Y. Are fungi from adult orchid roots the best symbionts at germination? A case study. Mycorrhiza 2019, 29, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Andrews, L.; Sharma, J. High specificity of a rare terrestrial orchid toward a rare fungus within the North American tallgrass prairie. Fungal Biol. 2019, 123, 895–904. [Google Scholar] [CrossRef]
- Zettler, L.W.; Corey, L.L.; Jacks, A.L.; Gruender, L.T.; Lopez, A.M. Tulasnella irregularis (Basidiomycota: Tulasnellaceae) from roots of Encyclia tampensis in south Florida, and confirmation of its mycorrhizal significance through symbiotic seed germination. Lankesteriana 2013, 13, 119–128. [Google Scholar] [CrossRef][Green Version]
- Zettler, L.W.; Hofer, C.J. Propagation of the little club-spur orchid (Platanthera clavellata) by symbiotic seed germination and its ecological implications. Environ. Exp. Bot. 1998, 39, 189–195. [Google Scholar] [CrossRef]
- Miura, C.; Saisho, M.; Yagame, T.; Yamato, M.; Kaminaka, H. Bletilla striata (Orchidaceae) Seed Coat Restricts the Invasion of Fungal Hyphae at the Initial Stage of Fungal Colonization. Plants 2019, 8, 280. [Google Scholar] [CrossRef]
- Sathiyadash, K.; Muthukumar, T.; Murugan, S.B.; Sathishkumar, R.; Pandey, R.R.; Muthukumar, T. In vitro symbiotic seed germination of South Indian endemic orchid Coelogyne nervosa. Mycoscience 2014, 55, 183–189. [Google Scholar] [CrossRef]
- Freitas, E.F.S.; Da Silva, M.; Cruz, E.D.S.; Mangaravite, É.; Bocayuva, M.F.; Veloso, T.G.R.; Selosse, M.-A.; Kasuya, M.C.M. Diversity of mycorrhizal Tulasnella associated with epiphytic and rupicolous orchids from the Brazilian Atlantic Forest, including four new species. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.-C.; Qin, L.-Y.; He, H.-Y.; Yu, X.-L.; Yang, M.-Z.; Zhang, H. Dynamics of fungal communities during Gastrodia elata growth. BMC Microbiol. 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Beyrle, H.F.; Smith, S.E.; Franco, C.M.M.; Peterson, R.L. Colonization of Orchis morio protocorms by a mycorrhizal fungus: Effects of nitrogen nutrition and glyphosate in modifying the responses. Can. J. Bot. 1995, 73, 1128–1140. [Google Scholar] [CrossRef]
- Harvais, G.; Hadley, G. The development of Orchis purpurella in asymbiotic and inoculated cultures. New Phytol. 1967, 66, 217–230. [Google Scholar] [CrossRef]
- Perotto, S.; Rodda, M.; Benetti, A.; Sillo, F.; Ercole, E.; Rodda, M.; Girlanda, M.; Murat-Furminieux, C.; Balestrini, R. Gene expression in mycorrhizal orchid protocorms suggests a friendly plant–fungus relationship. Planta 2014, 239, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, S.S.; Kohler, A.; Yan, B.; Luo, H.M.; Chen, X.M.; Guo, S.-X. iTRAQ and RNA-Seq Analyses Provide New Insights into Regulation Mechanism of Symbiotic Germination of Dendrobium officinale Seeds (Orchidaceae). J. Proteome Res. 2017, 16, 2174–2187. [Google Scholar] [CrossRef] [PubMed]
- Miura, C.; Yamaguchi, K.; Miyahara, R.; Yamamoto, T.; Fuji, M.; Yagame, T.; Imaizumi-Anraku, H.; Yamato, M.; Shigenobu, S.; Kaminaka, H. The Mycoheterotrophic Symbiosis Between Orchids and Mycorrhizal Fungi Possesses Major Components Shared with Mutualistic Plant-Mycorrhizal Symbioses. Mol. Plant-Microbe Interact. 2018, 31, 1032–1047. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, J.; Li, S.-C.; Zeng, X.; Meng, Z.-X.; Guo, S.-X. Comparative Transcriptome Analysis of Genes Involved in GA-GID1-DELLA Regulatory Module in Symbiotic and Asymbiotic Seed Germination of Anoectochilus roxburghii (Wall.) Lindl. (Orchidaceae). Int. J. Mol. Sci. 2015, 16, 30190–30203. [Google Scholar] [CrossRef]
- Chen, J.; Yan, B.; Tang, Y.; Xing, Y.; Li, Y.; Zhou, D.; Guo, S. Symbiotic and Asymbiotic Germination of Dendrobium officinale (Orchidaceae) Respond Differently to Exogenous Gibberellins. Int. J. Mol. Sci. 2020, 21, 6104. [Google Scholar] [CrossRef]
- Gutjahr, C. Phytohormone signaling in arbuscular mycorhiza development. Curr. Opin. Plant Biol. 2014, 20, 26–34. [Google Scholar] [CrossRef]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin Localization and Transport in Plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef]
- Selosse, M.-A.; Roy, M. Green plants that feed on fungi: Facts and questions about mixotrophy. Trends Plant Sci. 2009, 14, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Fochi, V.; Chitarra, W.; Kohler, A.; Voyron, S.; Singan, V.R.; Lindquist, E.A.; Barry, K.W.; Girlanda, M.; Grigoriev, I.V.; Martin, F.; et al. Fungal and plant gene expression in the Tulasnella calospora-Serapias vomeracea symbiosis provides clues about nitrogen pathways in orchid mycorrhizas. New Phytol. 2016, 213, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Ghirardo, A.; Fochi, V.; Lange, B.; Witting, M.; Schnitzler, J.-P.; Perotto, S.; Balestrini, R. Metabolomic adjustments in the orchid mycorrhizal fungus Tulasnella calospora during symbiosis with Serapias vomeracea. New Phytol. 2020, 228, 1939–1952. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, W.; Xie, Q.; Liu, N.; Liu, L.; Wang, D.; Zhang, X.; Yang, C.; Chen, X.-Y.; Tang, D.; et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef]
- Keymer, A.; Pimprikar, P.; Wewer, V.; Huber, C.; Brands, M.; Bucerius, S.L.; Delaux, P.-M.; Klingl, V.; Von Röpenack-Lahaye, E.; Wang, T.L.; et al. Lipid transfer from plants to arbuscular mycorrhiza fungi. eLife 2017, 6, 1–33. [Google Scholar] [CrossRef]
- Kuga, Y.; Sakamoto, N.; Yurimoto, H. Stable isotope cellular imaging reveals that both live and degenerating fungal pelotons transfer carbon and nitrogen to orchid protocorms. New Phytol. 2014, 202, 594–605. [Google Scholar] [CrossRef]
- Bougoure, J.; Ludwig, M.; Brundrett, M.; Cliff, J.; Clode, P.; Kilburn, M.; Grierson, P. High-resolution secondary ion mass spectrometry analysis of carbon dynamics in mycorrhizas formed by an obligately myco-heterotrophic orchid. Plant, Cell Environ. 2013, 37, 1223–1230. [Google Scholar] [CrossRef]
- Waud, M.; Brys, R.; Van Landuyt, W.; Lievens, B.; Jacquemyn, H. Mycorrhizal specificity does not limit the distribution of an endangered orchid species. Mol. Ecol. 2017, 26, 1687–1701. [Google Scholar] [CrossRef]
- Hossain, M.M.; Rahi, P.; Gulati, A.; Sharma, M. Improved ex vitro survival of asymbiotically raised seedlings of Cymbidium using mycorrhizal fungi isolated from distant orchid taxa. Sci. Hortic. 2013, 159, 109–116. [Google Scholar] [CrossRef]
- Rasmussen, H.N.; Rasmussen, F.N. The epiphytic habitat on a living host: Reflections on the orchid–tree relationship. Bot. J. Linn. Soc. 2018, 186, 456–472. [Google Scholar] [CrossRef]
| Orchid | Fungus | Reference | ||||
|---|---|---|---|---|---|---|
| Subfamily | Tribe | Species | Family | Species | Isolate Source | |
| Vanilloideae | Pogonieae | Cyrtosia septentrionalis (Rchb.f.) Garay | Physalacriaceae | Armillaria mellea subsp. nipponica J.Y.Cha et Igarashi | basidiome of A. mellea | [33] |
| Armillaria gallica Marxm. | tuber of Gastrodia elata, and roots of C. septentrionalis | |||||
| Armillaria tabescens (Scop.) Emel | roots of C. septentrionalis | |||||
| Meripilaceae | unknown species | protocorms from in situ seed baiting | ||||
| Pogoniopsis schenckii Cogn. | Bionectriaceae | Clonostachys sp. | roots of P. schenckii | [20] | ||
| Vanilleae | Erythrorchis ochobiensis (Hayata) Garay | Auriculariaceae | Auricularia polytricha (Mont.) Sacc. | a dead trunk of Morus australis in Japan, and an unknown source in Mexico (donated by the Institute for Fermentation, Osaka, Japan) | [40] | |
| Omphalotaceae | Lentinula edodes (Berk.) Pegler | fruiting body of L. edodes | [32] | |||
| Vanilla calyculata Schltr. | Ceratobasidiaceae | Ceratobasidium sp. | roots of Vanilla odorata | [57] | ||
| Vanilla rivasii Molineros | Ceratobasidiaceae | Ceratobasidium sp. | roots of V. odorata and V. calyculata | |||
| Tulasnellaceae | Tulasnella sp. | roots of V. rivasii and V. odorata | ||||
| Cypripedioideae | Cypripedieae | Cypripedium macranthos var. rebunense (Kudoh) Miyabe et Kudoh | Ceratobasidiaceae | Rhizoctonia sp., unknown anastomosis group | roots of C. macranthos var. rebunense | [12] |
| Paphiopedilum villosum (Lindl.) Stein | Tulasnellaceae | Tulasnella sp. | roots of P. villosum | [11] | ||
| Orchidioideae | Chloraeeae | Bipinnula fimbriata (Poepp.) I.M.Johnst | Ceratobasidiaceae | Ceratobasidium sp. | roots of B. fimbriata | [6] |
| Tulasnellaceae | Tulasnella calospora (Boud.) Juel | roots of B. fimbriata | ||||
| Gavilea australis (Skottsb.) M.N.Correa | Ceratobasidiaceae | Thanatephorus cucumeris (A.B.Frank) Donk | roots of Aa achalensis | [59] | ||
| Ceratobasidium sp. | roots of G. lutea, G. australis, and Sacoila lanceolata | |||||
| Tulasnellaceae | Tulasnella calospora (Boud.) Juel | roots of Codonorchis lessonii | ||||
| Cranichideae | Aa achalensis Schltr. | Magnaporthaceae | Gaeumannomyces cylindrosporus D.Hornby, Slope, Gutter. et Sivan | roots of A. achalensis | [43] | |
| Pezizaceae | uncultured Pezizaceae | roots of A. achalensis | ||||
| Ceratobasidiaceae | Thanatephorus cucumeris (A.B.Frank) Donk | roots of A. achalensis | ||||
| Spiranthes novae-zelandiae Hook.f. | Tulasnellaceae | Tulasnella sp. | roots of S. novae-zelandiae | [37] | ||
| Cynorkis purpurea (Thouars) Kraenzl. | Ceratobasidiaceae | Ceratobasidium sp. | roots of Aerangis sp. and C. purpurea | [3] | ||
| Serendipitaceae | Serendipita sp. | seedlings of Polystachya concreta | ||||
| Tulasnellaceae | Tulasnella sp. | roots of Angraecum magdalenae, C. purpurea, and Tylostigma sp. | ||||
| Gymnadenia conopsea (L.) R.Br. | Ceratobasidiaceae | Ceratobasidium sp. a | roots of G. conopsea | [61] | ||
| Habenaria macroceratitis Willd. | Ceratobasidiaceae | Ceratorhiza sp. | roots of H. quinqueseta and H. macroceratitis | [58] | ||
| Tulasnellaceae | Epulorhiza sp. | roots of Spiranthes brevilabris and Epidendrum conopseum | ||||
| Habenaria quinqueseta (Michx.) Eaton | Ceratobasidiaceae | Ceratorhiza sp. | roots of H. quinqueseta | [58] | ||
| Habenaria repens Nutt. | Ceratobasidiaceae | Ceratorhiza sp. | roots of H. quinqueseta and H. macroceratitis | [58] | ||
| Tulasnellaceae | Epulorhiza sp. | roots of Spiranthes brevilabris and Epidendrum conopseum | ||||
| Platanthera clavellata (Michx.) Luer | Tulasnellaceae | Epulorhiza inquilina Currah, Zettler et McInnis | roots of P. clavellata, P. cristata, and P. integrilabia | [68] | ||
| Epulorhiza sp. | roots of P. ciliaris | [68] | ||||
| Platanthera leucophaea (Nutt.) Lindl. | Ceratobasidiaceae | Ceratorhiza sp. | roots of P. leucophaea | [53] | ||
| Tulasnellaceae | Tulasnella calospora (Boud.) Juel | roots of in situ Anacamptis laxiflora | ||||
| Epidendroideae | Arethuseae | Arundina graminifolia (D.Don) Hochr. | Tulasnellaceae | Tulasnella sp. | roots of A. graminifolia and seedlings from ex situ bating | [65] |
| Bletilla striata (Thunb.) Rchb.f | Tulasnellaceae | Tulasnella calospora (Boud.) Juel | roots of Diuris maculata, Thelymitra aristata, Paphiopedilum, and unknown source | [55] | ||
| Tulasnellaceae | Tulasnella sp. | roots of Habenaria radiata | [48] | |||
| Serendipitaceae | Serendipita vermifera (Oberw.) P. Roberts | roots of Thelymitra sp. | [69] | |||
| Coelogyne nervosa A.Rich | Tulasnellaceae | Epulorhiza sp. | roots of Eulophia epidendreae | [70] | ||
| Epidendreae | Cycnoches haagii Barb.Rodr. | Tulasnellaceae | Tulasnella sp. | roots of Cyrtopodium paludicola and Hoffmannseggella caulescens | [13] | |
| Cyrtopodium glutiniferum Raddi | Tulasnellaceae | Epulorhiza epiphytica O.L. Pereira, Rollemb. et Kasuya | roots of Epidendrum rigidum and Polystachya concreta | [14] | ||
| Epulorhiza sp. | roots of Laelia milleri | |||||
| Cyrtopodium paludicola Hoehne | Tulasnellaceae | Tulasnella sp. | roots of C. paludicola and Epidendrum secundum | [60] | ||
| Cyrtopodium saintlegerianum Rchb.f. | Tulasnellaceae | Tulasnella sp. | roots of C. saintlegerianum | [38] | ||
| Eulophia alta (L.) Fawc. | Physalacriaceae | Armillaria sp. | roots of E. alta | [36] | ||
| Ionopsis utricularioides (Sw.) Lindl | Ceratobasidiaceae | Ceratobasidium sp. | roots of I. utricularioides | [25,26] | ||
| Oeceoclades maculata (Lindl.) Lindl. | Psathyrellaceae | Psathyrella candolleana (Fr.) Maire | roots of O. maculata | [31] | ||
| Oncidium sphacelatum Lindl. | Ceratobasidiaceae | Ceratobasidium sp. | roots of Oncidium donianum | [44] | ||
| Thanatephorus sp. | roots of Rossioglossum grande | [49] | ||||
| Tolumnia variegata (Sw.) Braem | Ceratobasidiaceae | Ceratobasidium sp. | roots of T. variegata and I. utricularioides | [26] | ||
| Dendrobieae | Dendrobium aphyllum (Roxb.) C.E.C.Fisch | Tulasnellaceae | Tulasnella sp. a | protocorms from in situ seed baiting | [8] | |
| Dendrobium chrysanthum Wall. ex. Lindl. | Ceratobasidiaceae | Rhizoctonia oryzae-sativae (Sawada) Mordue | unknown (Institute of Microbial Technology, India) | [56] | ||
| Rhizoctonia solani Kühn | infected Ipomoea batatas | |||||
| Epidendreae | Encyclia tampensis (Lindl.) Small | Tulasnellaceae | Tulasnella irregularis Warcup et P.H.B. Talbot | roots of E. tampensis seedling and mature plant | [67] | |
| Epidendrum dalstromii Dodson | Psathyrellaceae | Coprinellus radians (Desm.) Vilgalys | roots of various epiphytic orchids | [35] | ||
| Epidendrum nocturnum Jacq. | Psathyrellaceae | Coprinellus radians (Desm.) Vilgalys | roots of various epiphytic orchids | [35] | ||
| Tulasnellaceae | Tulasnella calospora (Boud.) Juel | roots of Spiranthes brevilabris | [53] | |||
| Tulasnella irregularis Warcup et P.H.B. Talbot | roots of E. tampensis seedling and mature plant | [67] | ||||
| Pleurothallis coriacardia Rchb.f. | Hypocreaceae | Ilyonectria sp. | roots of P. coriacardia | [34] | ||
| Psathyrellaceae | Coprinellus sp. | roots of P. coriacardia | ||||
| Vandeae | Aerangis ellisii (B.S.Williams) Schltr. | Ceratobasidiaceae | Ceratobasidium sp. | protocorm of A. ellisii | [9] | |
| Dendrophylax lindenii (Lindl.) Benth. ex Rolfe | Ceratobasidiaceae | Ceratobasidium sp. | roots of D. lindenii | [52] | ||
| Vanda coerulea Griff. ex Rolfe | Ceratobasidiaceae | Rhizoctonia zeae Voorhees | roots of V. coerulea | [16] | ||
| Vanda thwaitesii Hook.f. | Ceratobasidiaceae | Ceratobasidium sp. | roots of V. thwaitesii | [39] | ||
| Orchid Species | Fungal Species | Germination | Technique/Method | Reference |
|---|---|---|---|---|
| Anoectochilus roxburghii (Wall.) Lindl. | Unpublished | in vitro | Transcriptome (Illumina HiSeq 4000) | [78] |
| Bletilla striata (D.Don) Hochr. | Tulasnella sp. | in vitro | Transcriptome (Illumina HiSeq 1500) | [77] |
| Dendrobium officinale Kimura et Migo | Tulasnella sp. | in vitro | Transcriptome (Illumina HiSeq 2000) and proteome (iTRAQ) | [76] |
| Liparis loeselii (L.) Rich | (Seed baiting) | in situ | Microbiome (454 amplicon pyrosequencing) | [89] |
| Oncidium sphacelatum Lindl. | Ceratobasidium sp. | in vitro | Proteome (2D LC–MS/MS coupled to iTRAQ) | [44] |
| Rhizanthella gardneri R.S.Rogers | (Seemingly) Ceratobasidium sp. | ex situ | Stable isotope imaging (NanoSIMS) | [88] |
| Serapias vomeracea (Burm.f) Briq. | Tulasnella calospora (Boud.) Juel | in vitro | Transcriptome (454 GS-FLX pyrosequencing) | [75] |
| S. vomeracea (Burm.f) Briq. | T. calospora (Boud.) Juel | in vitro | Transcriptome (Illumina HiSeq 2000) | [83] |
| S. vomeracea (Burm.f) Briq. | T. calospora (Boud.) Juel | in vitro | Proteome (UPLC-UHR-QqToF-MS) | [84] |
| Spiranthes sinensis (Pers.) Ames | Ceratobasidium sp. | in vitro | Stable isotope imaging (NanoSIMS) | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pujasatria, G.C.; Miura, C.; Kaminaka, H. In Vitro Symbiotic Germination: A Revitalized Heuristic Approach for Orchid Species Conservation. Plants 2020, 9, 1742. https://doi.org/10.3390/plants9121742
Pujasatria GC, Miura C, Kaminaka H. In Vitro Symbiotic Germination: A Revitalized Heuristic Approach for Orchid Species Conservation. Plants. 2020; 9(12):1742. https://doi.org/10.3390/plants9121742
Chicago/Turabian StylePujasatria, Galih Chersy, Chihiro Miura, and Hironori Kaminaka. 2020. "In Vitro Symbiotic Germination: A Revitalized Heuristic Approach for Orchid Species Conservation" Plants 9, no. 12: 1742. https://doi.org/10.3390/plants9121742
APA StylePujasatria, G. C., Miura, C., & Kaminaka, H. (2020). In Vitro Symbiotic Germination: A Revitalized Heuristic Approach for Orchid Species Conservation. Plants, 9(12), 1742. https://doi.org/10.3390/plants9121742







