Dual Effect of Nanomaterials on Germination and Seedling Growth: Stimulation vs. Phytotoxicity
Abstract
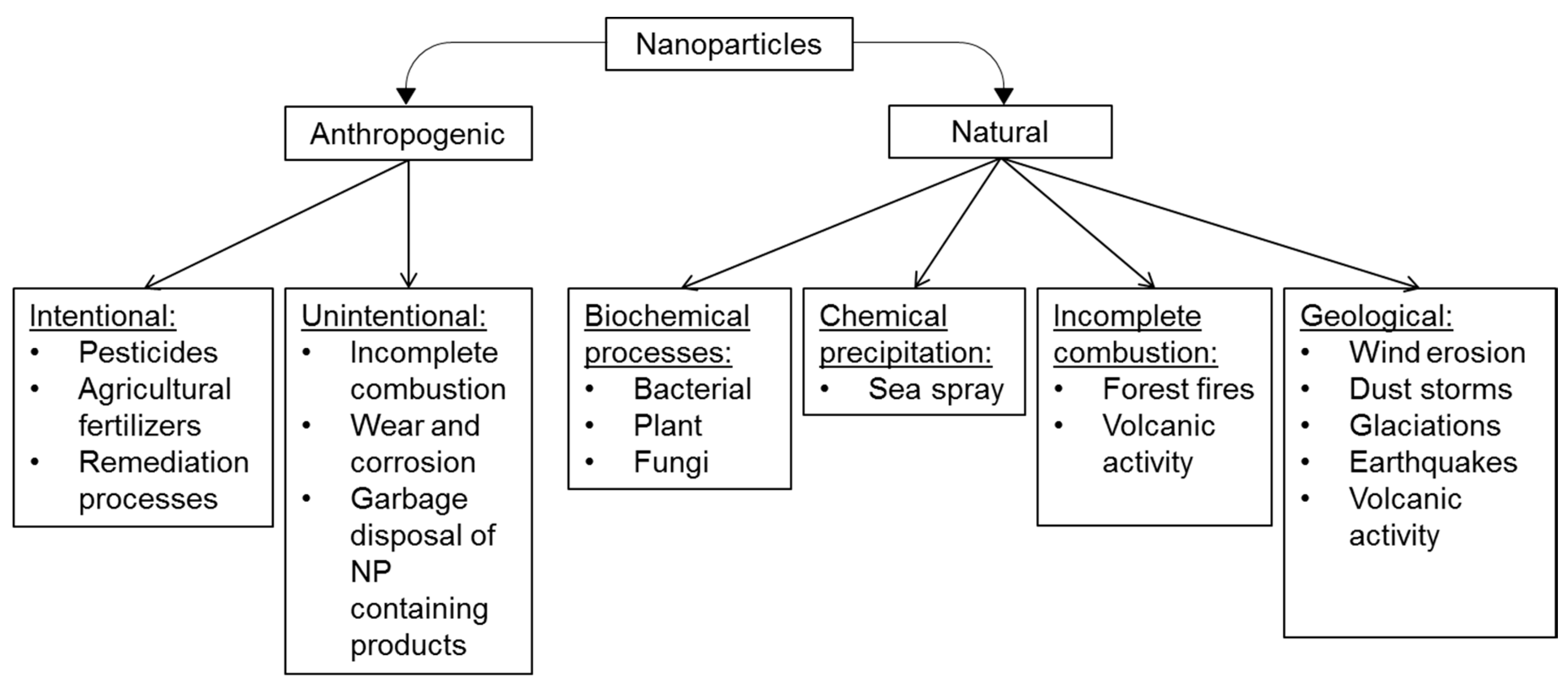
1. Introduction
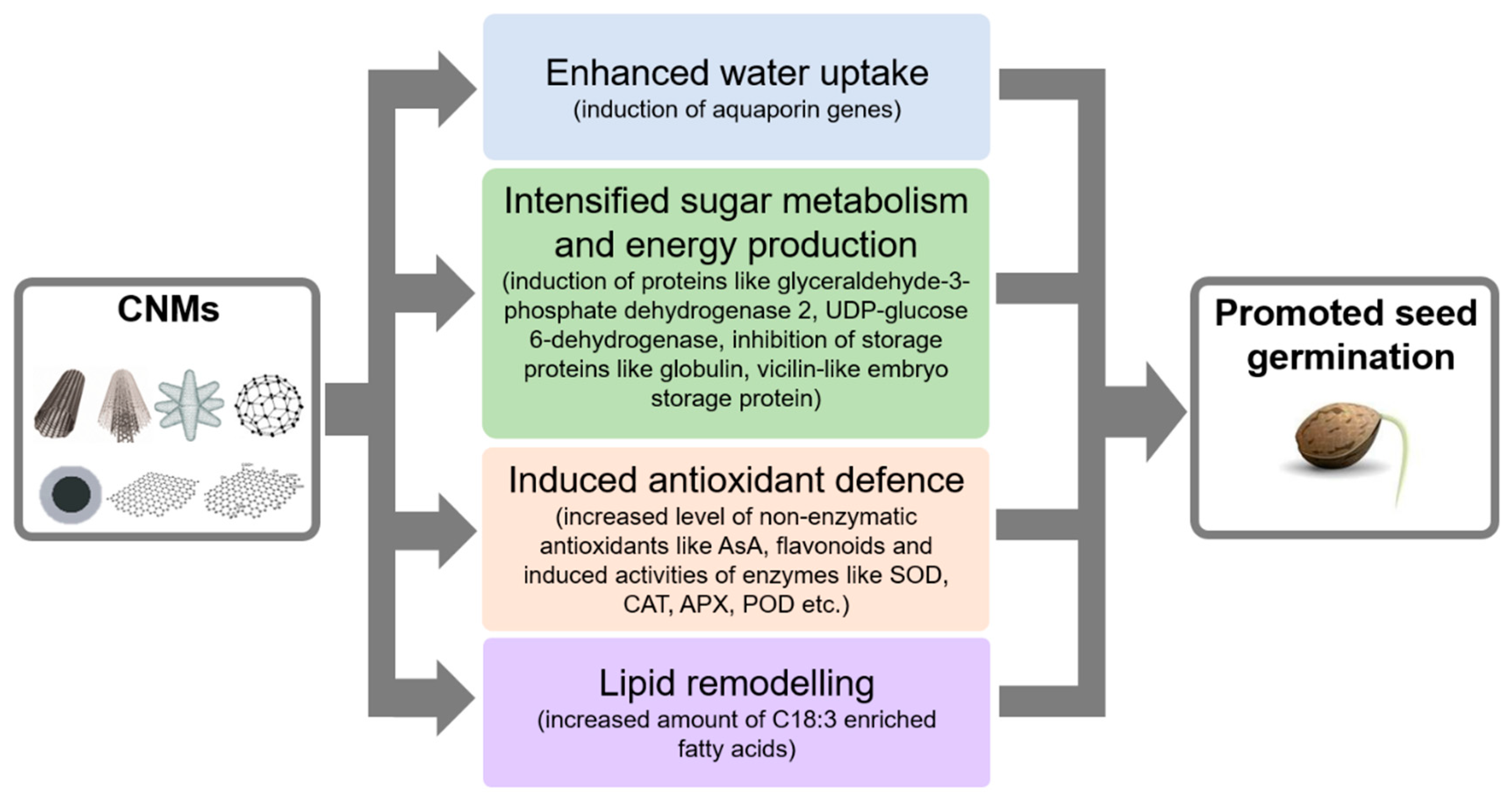
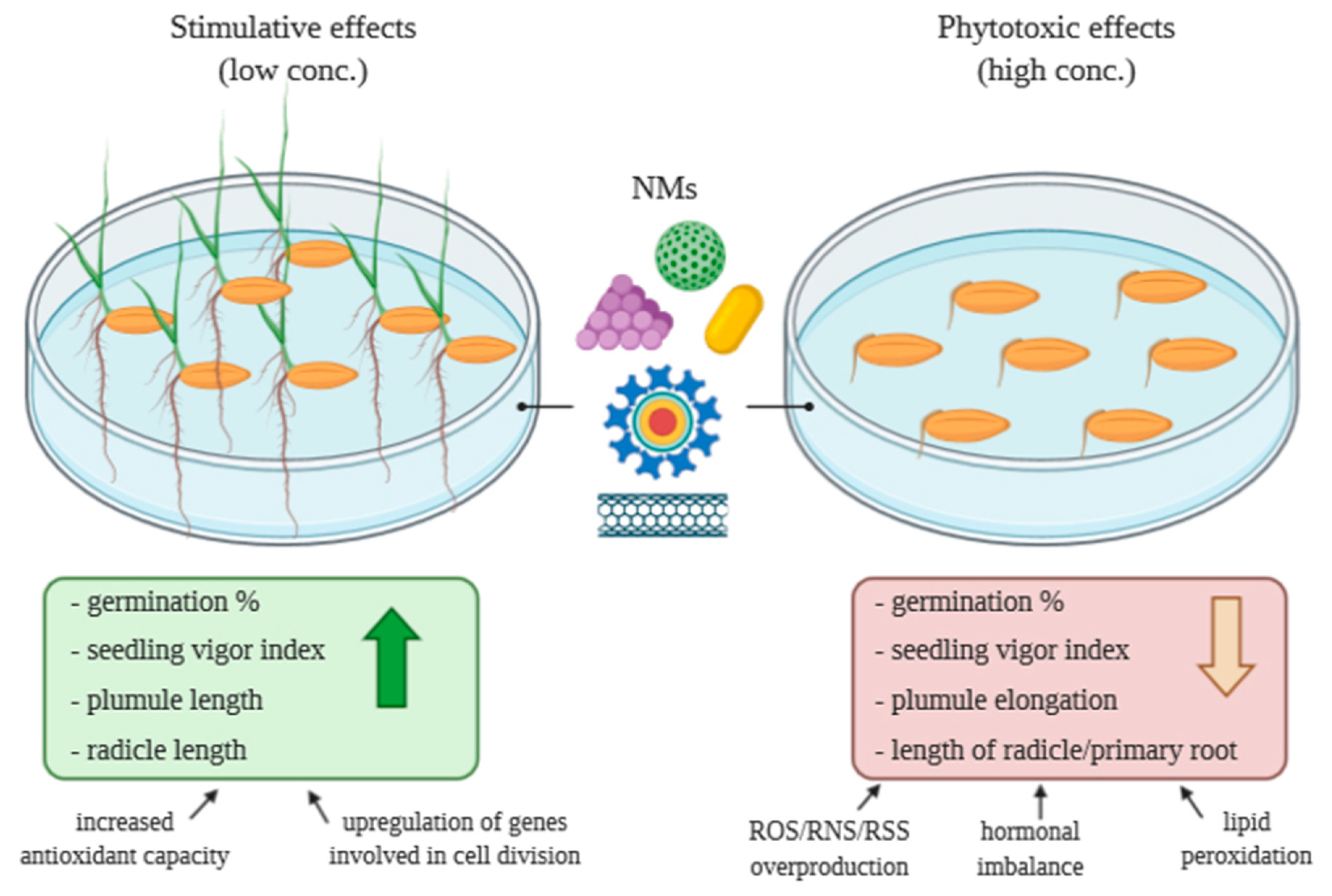
2. Effects of Nanomaterials on Seed Germination and Seedling Growth
2.1. Concentration-Dependent Effects of CNMs on Seed Germination and Seedling Growth
2.1.1. Carbon Nanotubes (CNTs)
2.1.2. Carbon Nanodots (CDs)
2.1.3. Carbon Nanohorns (CNHs)
2.1.4. Fullerenes and Fullerols
2.1.5. Graphene and Graphene Oxide (GO)
2.2. The Influences of Metal-Based NMs on Germination and Seedling Growth
2.2.1. Metallic NPs
2.2.2. Metal Oxide NPs
2.2.3. Metal Containing Quantum Dots
2.3. The Effect of Dendrimers on Seed Germination
2.4. The Effect of Composite Nanomaterials on Seed Germination
3. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Schneider, J.J. Nanomaterials: Synthesis, properties and applications. In Advanced Materials; Edelstein, A.S., Cammarata, R.C., Eds.; IOP Publishing: Bristol, UK, 1996; p. 997. [Google Scholar]
- British Standards Institution. Terminology for Nanomaterials; BSI: London, UK, 2007. [Google Scholar]
- American Society for Testing and Materials. Standard Terminology Relating to Nanotechnology; E 2456-06; ASTM International: West Conshohocken, PA, USA, 2006. [Google Scholar]
- Scientific Committee on Emerging and Newly Identified Health Risks. The Appropriateness of the Risk Assessment Methodology in Accordance with the Technical Guidance Documents for New and Existing Substances for Assessing the Risks of Nanomaterials; European Commission: Brussels, Belgium, 2007. [Google Scholar]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nano Res. 2012, 14, 1109–1112. [Google Scholar] [CrossRef]
- Brar, S.K.; Verma, M.; Tyagi, R.D.; Surampalli, R.Y. Engineered nanoparticles in wastewater and wastewater sludge—Evidence and impacts. Waste Manag. 2010, 30, 504–520. [Google Scholar] [CrossRef] [PubMed]
- Mageswari, A.; Srinivasan, R.; Subramanian, P.; Ramesh, N.; Gothandam, K.M. Nanomaterials: Classification, Biological Synthesis and Characterization. In Nanoscience in Food and Agriculture 3. Sustainable Agriculture Reviews; Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2016; Volume 23, pp. 31–71. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Simchi, A.; Milani, A.S.; Stroeve, P. Cell toxicity of superparamagnetic iron oxide nanoparticles. J. Colloid Interface Sci. 2009, 336, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Videa, J.R.; Zhao, L.; Lopez-Moreno, M.L.; de la Rosa, G.; Hong, J.; Gardea-Torresdey, J.L. Nanomaterials and the environment: A review for the biennium 2008–2010. J. Hazard. Mater. 2011, 186, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.W. Synthesis and properties of nanophase materials. Mater. Sci. Eng. A 1993, 168, 189–197. [Google Scholar] [CrossRef]
- Morton, W.J. Method of Dispersing Fluids. U.S. Patent 705,69l, 29 July 1902. [Google Scholar]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun nanofibers: New generation materials for advanced applications. Mater. Sci. Eng. B 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Hosseini, H.; Shojaee-Aliabadi, S.; Hosseini, S.M.; Mirmoghtadaie, L. Chapter 11—Nanoantimicrobials in food industry. In Nanotechnology Applications in Food; Oprea, A.E., Grumezescu, A.M., Eds.; Academic Press Elsevier: London, UK, 2017; pp. 223–243. [Google Scholar] [CrossRef]
- Acosta, E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr. Opin. Colloid Interface Sci. 2009, 14, 3–15. [Google Scholar] [CrossRef]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108, 303–318. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- Murr, L.E.; Esquivel, E.V.; Bang, J.J.; de La Rosa, G.; Gardea-Torresdey, J.L. Chemistry and nanoparticulate compositions of a 10,000 year-old ice core melt water. Water Res. 2004, 38, 4282–4296. [Google Scholar] [CrossRef]
- Verma, H.C.; Upadhyay, C.; Tripathi, A.; Tripathi, R.P.; Bhandari, N. Thermal decomposition pattern and particle size estimation of iron minerals associated with the Cretaceous-Tertiary boundary at Gubbio. Meteorit. Planet. Sci. 2002, 37, 901–909. [Google Scholar] [CrossRef]
- Handy, R.D.; Owen, R.; Valsami-Jones, E. The ecotoxicology of nanoparticles and nanomaterials: Current status, knowledge gaps, challenges, and future needs. Ecotoxicology 2008, 17, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, M. Nanomaterial and Nanoparticle: Origin and Activity. In Nanoscience and Plant–Soil Systems; Ghorbanpour, M., Manika, K., Varma, A., Eds.; Soil Biology, 48; Springer International Publishing: Cham, Switzerland, 2017; pp. 71–112. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585. [Google Scholar] [CrossRef] [PubMed]
- Ghodake, G.; Seo, Y.D.; Lee, D.S. Hazardous phytotoxic nature of cobalt and zinc oxide nanoparticles assessed using Allium cepa. J. Hazard. Mater. 2011, 186, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Hristovski, K.; Crittenden, J.C. Stability of commercial metal oxide nanoparticles in water. Water Res. 2008, 42, 2204–2212. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef]
- Du, W.; Tan, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; Ji, R.; Yin, Y.; Guo, H. Interaction of metal oxide nanoparticles with higher terrestrial plants: Physiological and biochemical aspects. Plant Phys. Biochem. 2017, 110, 210–225. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-ur-Rehman, M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Haz. Mat. 2017, 322, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Rizvi, A.; Zaidi, A.; Khan, M.S.; Musarrat, J. Understanding the phyto-interaction of heavy metal oxide bulk and nanoparticles: Evaluation of seed germination, growth, bioaccumulation, and metallothionein production. RSC Adv. 2019, 9, 4210–4225. [Google Scholar] [CrossRef]
- López-Moreno, M.L.; de la Rosa, G.; Hernandez-Viezcas, J.A.; Castillo-Michel, H.; Botez, C.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ. Sci. Technol. 2010, 44, 7315–7320. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, S.; Luo, L.; Zhang, J.; Yang, K.; Christied, P. Accumulation, speciation and uptake pathway of ZnO nanoparticles in maize. Environ. Sci. Nano 2015, 2, 68. [Google Scholar] [CrossRef]
- Molnar, A.; Papp, M.; Kovacs, D.Z.; Belteky, P.; Olah, D.; Feigl, G.; Szollosi, R.; Razga, Z.; Ordog, A.; Erdei, L.; et al. Nitro-oxidative signalling induced by chemically synthetized zinc oxide nanoparticles (ZnO NPs) in Brassica species. Chemosphere 2020, 251, 126419. [Google Scholar] [CrossRef] [PubMed]
- Serag, M.F.; Kaji, N.; Gaillard, C.; Okamoto, Y.; Terasaka, K.; Jabasini, M.; Tokeshi, M.; Mizukami, H.; Bianco, A.; Baba, Y. Trafficking and subcellular localization of multiwalled carbon nanotubes in plant cells. ACS Nano 2011, 5, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Serag, M.F.; Kaji, N.; Habuchi, S.; Bianco, A.; Baba, Y. Nanobiotechnology meets plant cell biology: Carbon nanotubes as organelle targeting nanocarriers. RSC Adv. 2013, 3, 4856–4862. [Google Scholar] [CrossRef]
- Verma, S.K.; Das, A.K.; Gantait, S.; Kumar, V.; Gurel, E. Applications of carbon nanomaterials in the plant system: A perspective view on the pros and cons. Sci. Total Environ. 2019, 667, 485–499. [Google Scholar] [CrossRef]
- Ahmad, R.; Pranaw, K.; Khare, S.K. Effect of nanomaterials and their possible implication on the plants. In Plant Biotechnology: Progress in Genomic Era; Khurana, S.M.P., Gaur, R.K., Eds.; Springer: Singapore, 2019; pp. 213–229. [Google Scholar] [CrossRef]
- Science Direct. Available online: https://www.sciencedirect.com/ (accessed on 15 October 2020).
- Miralles, P.; Church, T.L.; Harris, A.T. Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants. Environ. Sci. Technol. 2012, 46, 9224–9239. [Google Scholar] [CrossRef]
- López-Serrano, A.; Olivas, R.M.; Landaluze, J.S.; Cámara, C. Nanoparticles: A global vision. Characterization, separation, and quantification methods. Potential environmental and health impact. Anal. Methods 2014, 6, 38–56. [Google Scholar] [CrossRef]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for precision and sustainable agriculture: Current state and future perspectives. J. Agric. Food Chem. 2018, 66, 6487–6503. [Google Scholar] [CrossRef]
- Radchuk, V.; Borisjuk, L. Physical, metabolic and developmental functions of the seed coat. Front. Plant Sci. 2014, 5, 510. [Google Scholar] [CrossRef] [PubMed]
- Obroucheva, N.V. Aquaporins in seeds. Seed Sci. Res. 2013, 23, 213–216. [Google Scholar] [CrossRef]
- Molnár, Á.; Rónavári, A.; Bélteky, P.; Szőllősi, R.; Valyon, E.; Oláh, D.; Rázga, Z.; Ördög, A.; Kónya, Z.; Kolbert, Z. ZnO nanoparticles induce cell wall remodeling and modify ROS/RNS signalling in roots of Brassica seedlings. Ecotoxic. Environ. Safe 2020, 206, 111158. [Google Scholar] [CrossRef] [PubMed]
- Younes, N.A.; Hassan, H.S.; Elkady, M.F.; Hamed, A.M.; Dawood, M.F. Impact of synthesized metal oxide nanomaterials on seedlings production of three Solanaceae crops. Heliyon 2020, 6, e03188. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Dai, H. Carbon nanotubes: Synthesis, integration, and properties. Acc. Chem. Res. 2002, 35, 1035–1044. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dai, H. Carbon nanotubes: Continued innovations and challenges. MRS Bull. 2004, 29, 237–243. [Google Scholar] [CrossRef]
- Golberg, D.; Costa, P.M.F.J.; Mitome, M.; Bando, Y. Nanotubes in a gradient electric field, as revealed by STM-TEM technique. Nano Res. 2008, 1, 166–175. [Google Scholar] [CrossRef]
- Service, R.F. Superstrong nanotubes show they are smart, too. Science 1998, 281, 893–894. [Google Scholar] [CrossRef]
- Stampoulis, D.; Sinha, S.K.; White, J.C. Assay-Dependent Phytotoxicity of Nanoparticles to Plants. Environ. Sci. Technol. 2015, 43, 9473–9479. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ahn, Y.-J. Multi-walled carbon nanotubes and silver nanoparticles differentially affect seed germination, chlorophyll content, and hydrogen peroxide accumulation in carrot (Daucus carota L.). Biocatal. Agric. Biotechnol. 2016, 8, 257–262. [Google Scholar] [CrossRef]
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanabe, F.; Biris, A.S. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 2009, 3, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Khodakovskaya, M.V.; de Silva, K.; Biris, A.S.; Dervishi, E.; Villagarcia, H. Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano 2012, 6, 2128–2135. [Google Scholar] [CrossRef]
- Cañas, J.E.; Long, M.; Nations, S.; Vadan, R.; Dai, L.; Luo, M.; Ambikapathi, R.; Lee, E.H.; Olszyk, D. Effects of functionalized and nonfunctionalized single-walled carbon nanotubes on root elongation of select crop species. Environ. Toxicol. Chem. 2009, 27, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, B.; Wang, Q.; Shi, X.; Xiao, Z.; Lin, J.; Fang, X. Carbon Nanotubes as Molecular Transporters for Walled Plant Cells. Nano Lett. 2009, 9, 1007–1010. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Sharma, S.; Narayan, R.P.; Srivastava, P.; Khan, A.S.; Dubey, N.K.; Tripathi, D.K.; Chauhan, D.K. Plants and carbon nanotubes (CNTs) interface: Present status and future prospects. In Nanotechnology; Prasad, R., Kumar, V., Kumar, M., Eds.; Springer: Singapore, 2017; pp. 317–340. [Google Scholar] [CrossRef]
- Srinivasan, C.; Ramiah, S. Nano-agriculture—Carbon nanotubes enhance tomato seed germination and plant growth. Curr. Sci. 2010, 99, 274–275. [Google Scholar]
- Ghodake, G.; Seo, Y.D.; Park, D.; Lee, D.S. Phytotoxicity of Carbon Nanotubes Assessed by Brassica Juncea and Phaseolus Mungo. J. Nanoelectron. Optoelectron. 2010, 5, 157–160. [Google Scholar] [CrossRef]
- Haghighi, M.; da Silva, J.A.T. The Effect of Carbon Nanotubes on the Seed Germination and Seedling Growth of Four Vegetable Species. J. Crop Sci. Biotech. 2014, 17, 201–208. [Google Scholar] [CrossRef]
- Pourkhaloee, A.; Haghighi, M.; Saharkhiz, M.J.; Jouzi, H.; Doroodmand, M.M. Carbon Nanotubes Can Promote Seed Germination via Seed Coat Penetration. Seed Technol. 2011, 33, 155–169. [Google Scholar]
- Srivastava, A.; Rao, D.P. Enhancement of Seed Germination and Plant Growth of Wheat, Maize, Peanut and Garlic Using Multiwalled Carbon Nanotubes. Eur. Chem. Bull. 2014, 3, 502–504. [Google Scholar]
- Jiang, Y.; Hua, Z.; Zhao, Y.; Liu, Q.; Wang, F.; Zhang, Q. The Effect of Carbon Nanotubes on Rice Seed Germination and Root Growth. In Proceedings of the 2012 International Conference on Applied Biotechnology (ICAB 2012); Lecture Notes in Electrical Engineering; Zhang, T.C., Ouyang, P., Kaplan, S., Skarnes, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 250. [Google Scholar] [CrossRef]
- Lahiani, M.H.; Dervishi, E.; Chen, J.; Nima, Z.; Gaume, A.; Biris, A.S.; Khodakovskaya, M.V. Impact of Carbon Nanotube Exposure to Seeds of Valuable Crops. ACS Appl. Mater. Interfaces 2013, 5, 7965–7973. [Google Scholar] [CrossRef] [PubMed]
- Lahiani, M.H.; Chen, J.; Irin, F.; Puretzky, A.A.; Green, M.J.; Khodakovskaya, M.V. Interaction of carbon nanohorns with plants: Uptake and biological effects. Carbon 2015, 81, 607–619. [Google Scholar] [CrossRef]
- Tripathi, S.; Sonkar, S.K.; Sarkar, S. Growth stimulation of gram (Cicer arietinum) plant by water soluble carbon nanotubes. Nanoscale 2011, 3, 1176–1181. [Google Scholar] [CrossRef]
- Mukherjee, A.; Majumdar, S.; Servin, A.D.; Pagano, L.; Dhankher, O.P.; White, J.C. Carbon Nanomaterials in Agriculture: A Critical Review. Front. Plant Sci. 2016, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Vithanage, M.; Seneviratne, M.; Ahmad, M.; Sarkar, B.; Ok, Y.S. Contrasting effects of engineered carbon nanotubes on plants: A review. Environ. Geochem. Health 2017, 39, 1421–1439. [Google Scholar] [CrossRef]
- Hao, Y.; Yu, F.; Lv, R.; Ma, C.; Zhang, Z.; Rui, Y.; Liu, L.; Cao, W.; Xing, B. Carbon Nanotubes Filled with Different Ferromagnetic Alloys Affect the Growth and Development of Rice Seedlings by Changing the C:N Ratio and Plant Hormones Concentrations. PLoS ONE 2016, 11, e0157264. [Google Scholar] [CrossRef]
- Hatami, M.; Hadian, J.; Ghorbanpour, M. Mechanisms underlying toxicity and stimulatory role of single-walled carbon nanotubes in Hyoscyamus niger during drought stress simulated by polyethylene glycol. J. Hazard. Mater. 2017, 324, 306–320. [Google Scholar] [CrossRef]
- Khalifa, N.S. The Effect of Multi-walled Carbon Nanotubes on Pennycress (Thlaspi arvense L.). Plant 2018, 58, 529–537. [Google Scholar] [CrossRef]
- Pandey, K.; Lahiani, M.H.; Hicks, V.K.; Hudson, M.K.; Green, M.J.; Khodakovskaya, M. Effects of carbon-based nanomaterials on seed germination, biomass accumulation and salt stress response of bioenergy crops. PLoS ONE 2018, 13, e0202274. [Google Scholar] [CrossRef]
- González-García, Y.; López-Vargas, E.R.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; González-Morales, S.; Robledo-Olivo, A.; Alpuche-Solís, Á.G.; Juárez-Maldonado, A. Impact of Carbon Nanomaterials on the Antioxidant System of Tomato Seedlings. Int. J. Mol. Sci. 2019, 20, 5858. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.H.; Sobze, J.-M.; Pham, T.H.; Nadeem, M.; Liu, C.; Galagedara, L.; Cheema, M.; Thomas, R. Carbon Nanoparticles Functionalized with Carboxylic Acid Improved the Germination and Seedling Vigor in Upland Boreal Forest Species. Nanomaterials 2020, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- López-Vargas, E.R.; González-García, Y.; Pérez-Álvarez, M.; Cadenas-Pliego, G.; González-Morales, S.; Benavides-Mendoza, A.; Cabrera, R.I.; Juárez-Maldonado, A. Seed Priming with Carbon Nanomaterials to Modify the Germination, Growth, and Antioxidant Status of Tomato Seedlings. Agronomy 2020, 10, 639. [Google Scholar] [CrossRef]
- Chang, X.; Song, Z.; Xu, Y.; Gao, M. Effects of carbon nanotubes on growth of wheat seedlings and Cd uptake. Chemosphere 2020, 240, 124931. [Google Scholar] [CrossRef]
- Wenli, S.; Shahrajabian, M.H.; Huang, Q. Soybean Seeds Treated with Single Walled Carbon Nanotubes (SwCNTs) Showed Enhanced Drought Tolerance During Germination. Int. J. Adv. Biol. Biomed. Res. 2020, 8, 9–16. [Google Scholar]
- Baz, H.; Creech, M.; Chen, J.; Gong, H.; Bradford, K.; Huo, H. Water-Soluble Carbon Nanoparticles Improve Seed Germination and Post-Germination Growth of Lettuce under Salinity Stress. Agronomy 2020, 10, 1192. [Google Scholar] [CrossRef]
- Chen, J.; Dou, R.; Yang, Z.; Wang, X.; Mao, C.; Gao, X.; Wang, L. The effect and fate of water-soluble carbon nanodots in maize (Zea mays L.). Nanotoxicology 2016, 10, 818–828. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Song, Y.; Li, H.; Huang, H.; Shao, M.; Liu, Y.; Kang, Z. Carbon dots promote the growth and photosynthesis of mung bean sprouts. Carbon 2018, 136, 94–102. [Google Scholar] [CrossRef]
- Wang, A.; Kang, F.; Wang, Z.; Shao, Q.; Li, Z.; Zhu, G.; Lu, J.; Li, Y.Y. Facile Synthesis of Nitrogen-Rich Carbon Dots as Fertilizers for Mung Bean Sprouts. Adv. Sustain. Syst. 2019, 3, 1800132. [Google Scholar] [CrossRef]
- Qian, K.; Guo, H.; Chen, G.; Ma, C.; Xing, B. Distribution of different surface modified carbon dots in pumpkin seedlings. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Wu, Y.; Zhuang, J.; Zhang, X.; Zhang, H.; Lei, B.; Hu, C.; Liu, Y. A review on the effects of carbon dots in plant systems. Mater. Chem. Front. 2020, 4, 437–448. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, L.; Nie, X.; Man, H.; Guo, Z.; Wang, X.; Tu, J.; Jin, G.; Ci, L. Impacts of surface chemistry of functional carbon nanodots on the plant growth. Ecotox. Environ. Safe 2020, 206, 111220. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, R.; Ju, Q.; Xu, J. Physiological, Metabolic, and Transcriptomic Analyses Reveal the Responses of Arabidopsis Seedlings to Carbon Nanohorns. Environ. Sci. Technol. 2020, 54, 4409–4420. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhao, Y.; Wan, Y.; Zheng, J.; Zhang, X.; Wang, C.; Fang, X.; Lin, J. Study of the inhibitory effect of water-soluble fullerenes on plant growth at the cellular level. ACS Nano 2010, 4, 5743–5748. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, Y.; Folta, K.M.; Krishna, V.; Bai, W.; Indeglia, P.; Georgieva, A.; Nakamura, H.; Koopman, B.; Moudgil, B. Polyhydroxy Fullerenes (Fullerols or Fullerenols): Beneficial Effects on Growth and Lifespan in Diverse Biological Models. PLoS ONE 2011, 6, e19976. [Google Scholar] [CrossRef]
- Panova, G.; Ktitorova, I.; Skobeleva, O.; Sinjavina, N.; Charykov, N.; Semenov, K. Impact of polyhydroxy fullerene (fullerol or fullerol) on growth and biophysical characteristics of barley seedlings in favourable and stressful conditions. Plant Growth Regul. 2016, 79, 309–317. [Google Scholar] [CrossRef]
- Xiong, J.-L.; Li, J.; Wang, H.-C.; Zhang, C.-L.; Naeem, M.S. Fullerol improves seed germination, biomass accumulation, photosynthesis and antioxidant system in Brassica napus L. under water stress. Plant Physiol. Biochem. 2018, 129, 130–140. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, T.; Cao, J.; Zang, Z.; Wu, Q.; Wang, H.; Tai, F.; He, R. Quaternary Ammonium Salts of Iminofullerenes: Fabrication and Effect on Seed Germination. J. Agric. Food Chem. 2019, 67, 13509–13517. [Google Scholar] [CrossRef]
- Nair, R.; Mohamed, M.S.; Gao, W.; Maekawa, T.; Yoshida, Y.; Ajayan, P.M.; Kumar, D.S. Effect of carbon nanomaterials on the germination and growth of rice plants. J. Nanosci. Nanotechnol. 2012, 12, 2212–2220. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Chen, J.; Li, Y. Effects of graphene on seed germination and seedling growth. J. Nanopart. Res. 2015, 17, 78. [Google Scholar] [CrossRef]
- Liu, S.; Wei, H.; Li, Z.; Li, S.; Yan, H.; He, Y.; Tian, Z. Effects of graphene on germination and seedling morphology in rice. J. Nanosci. Nanotechnol. 2015, 15, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, R.; Fang, X.; Song, T.; Cai, X.; Liu, H.; Du, S. Toxic effects of graphene on the growth and nutritional levels of wheat (Triticum aestivum L.): Short-and longterm exposure studies. J. Hazard. Mater. 2016, 317, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Chang, H.; Teng, Y. Sulfonated graphene-induced hormesis is mediated through oxidative stress in the roots of maize seedlings. Sci. Total Environ. 2016, 572, 926–934. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Li, S.; Ding, W. Various Physiological Response to Graphene Oxide and Amine-Functionalized Graphene Oxide in Wheat (Triticum aestivum). Molecules 2018, 23, 1104. [Google Scholar] [CrossRef] [PubMed]
- Vochita, G.; Opric, L.; Gherghel, D.; Mihai, C.-T.; Boukherrou, R.; Lobiu, A. Graphene oxide effects in early ontogenetic stages of Triticum aestivum L. seedlings. Ecotoxic. Environ. Safe 2019, 181, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lu, Y.; Li, J.; Liu, R.; Zhu, X. Effect of graphene quantum dot size on plant growth. Nanoscale 2020, 12, 15045–15049. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.; Wang, J.; Zhang, Q.; Zhu, H.; Chehab, E.W.; Colvin, V.L.; Alvarez, P.J.; Braam, J. Fluorescence reports intact quantum dot uptake into roots and translocation to leaves of Arabidopsis thaliana and subsequent ingestion by insect herbivores. Environ. Sci. Technol. 2015, 49, 626–632. [Google Scholar] [CrossRef]
- Li, X.Q.; Elliott, D.W.; Zhang, W.X. Zero-valent iron nanoparticles for abatement of environmental pollutants: Materials and engineering aspects. Crit. Rev. Solid State Mater. Sci. 2006, 31, 111–122. [Google Scholar] [CrossRef]
- Zhang, W. Nanoscale iron particles for environmental remediation: An overview. J. Nanopart. Res. 2003, 5, 323–332. [Google Scholar] [CrossRef]
- Klopffer, W.; Curran, M.A.; Frankl, P.; Heijungs, R.; Kohler, A.; Olsen, S.I. Nanotechnology and life cycle assessment. In A Systems Approach to Nanotechnology and the Environment: Synthesis of Results Obtained at a Workshop; The European Commission and Woodrow Wilson International Center for Scholars: Washington, DC, USA, 2006. [Google Scholar]
- Kramer, J.R.; Benoit, G.; Bowles, K.C.; Di Toro, D.M.; Herrin, R.T.; Luther, G.W.; Manalopoulis, H.; Robilliard, K.A.; Shafer, M.M.; Shaw, J.R. Environmental chemistry of silver. In Silver in the Environment: Transport, Fate, and Effects; Andren, A.W., Bober, T.W., Eds.; SETAC: Pensacola, FL, USA, 2002; pp. 1–25. [Google Scholar]
- Doty, R.C.; Tshikhudo, T.R.; Brust, M. Extremely stablewatersoluble Ag nanoparticles. Chem. Mater. 2005, 17, 4630–4635. [Google Scholar] [CrossRef]
- Barrena, R.; Casals, E.; Colón, J.; Font, X.; Sánchez, A.; Puntes, V. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 2009, 75, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Mazhari, B.B.Z.; Rao, S. Impact of bio-nanogold on seed germination and seedling growth in Pennisetum glaucum. Enzyme Microb. Technol. 2016, 95, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, Z.M.; Alharbi, A. Effect of silver nanoparticles on seed germination of crop plants. J. Adv. Agric. 2015, 4, 283–288. [Google Scholar] [CrossRef]
- Gorka, D.E.; Liu, J. Effect of direct contact on the phytotoxicity of silver nanomaterials. Environ. Sci. Technol. 2016, 50, 10370–10376. [Google Scholar] [CrossRef] [PubMed]
- Prażak, R.; Święciło, A.; Krzepiłko, A.; Michałek, S.; Arczewska, M. Impact of Ag Nanoparticles on Seed Germination and Seedling Growth of Green Beans in Normal and Chill Temperatures. Agriculture 2020, 10, 312. [Google Scholar] [CrossRef]
- Bouguerra, S.; Gavina, A.; da Graça Rasteiro, M.; Rocha-Santos, T.; Ksibi, M.; Pereira, R. Effects of cobalt oxide nanomaterial on plants and soil invertebrates at different levels of biological organization. J. Soils Sediments 2019, 19, 3018–3034. [Google Scholar] [CrossRef]
- Hatami, M.; Ghorbanpour, M.; Salehiarjomand, H. Nano-anatase TiO2 modulates the germination behavior and seedling vigority of some commercially important medicinal and aromatic plants. J. Environ. Biol. 2014, 8, 53–59. [Google Scholar]
- Prasad, T.N.V.K.V.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sreeprasad, T.S.; Sajanlal, P.R.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant. Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Afrayeem, S.M.; Chaurasia, A.K. Effect of zinc oxide nanoparticles on seed germination and seed vigour in chilli (Capsicum annuum L.). J. Pharmacogn. Phytochem. 2017, 6, 1564–1566. [Google Scholar]
- García-López, I.J.; Lira-Saldivar, R.H.; Zavala-García, F.; Olivares-Sáenz, E.; Niño-Medina, G.; Angélica Ruiz-Torres, N.; Méndez-Argüello, B.; Díaz-Barriga, E. Effects of zinc oxide nanoparticles on growth and antioxidant enzymes of Capsicum chinense. Toxicol. Environ. Chem. 2018, 100, 560–572. [Google Scholar] [CrossRef]
- de la Rosa, G.; López-Moreno, M.L.; de Haro, D.; Botez, C.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effects of ZnO nanoparticles in alfalfa, tomato, and cucumber at the germination stage: Root development and X-ray absorption spectroscopy studies. Pure Appl. Chem. 2013, 85, 2161–2174. [Google Scholar] [CrossRef]
- Jayarambabu, N.; Kumari, B.S.; Rao, K.V.; Prabhu, Y.T. Germination and growth characteristics of mungbean seeds (Vigna radiata L.) affected by synthesized zinc oxide nanoparticles. Int. J. Curr. Eng. Technol. 2014, 4, 2347–5161. [Google Scholar]
- Srinivasan, R.; Maity, A.; Singh, K.K.; Ghosh, P.K.; Kumar, S.; Srivastava, M.K.; Radhakrishna, A.; Srivastava, R.; Kumari, B. Influence of copper oxide and zinc oxide nano-particles on growth of fodder cowpea and soil microbiological properties. Range Manag. Agrofor. 2017, 38, 208–214. [Google Scholar]
- Bayramzadeh, V.; Ghadiri, M.; Davoodi, M.H. Effects of silver nanoparticle exposure on germination and early growth of Pinus sylvestris and Alnus subcordata. Sains Malays. 2019, 48, 937–944. [Google Scholar] [CrossRef]
- Parveen, A.; Rao, S. Effect of nanosilver on seed germination and seedling growth in Pennisetum glaucum. J. Clust. Sci. 2015, 26, 693–701. [Google Scholar] [CrossRef]
- Razzaq, A.; Ammara, R.; Jhanzab, H.M.; Mahmood, T.; Hafeez, A.; Hussain, S. A novel nanomaterial to enhance growth and yield of wheat. J. Nanosci. Technol. 2016, 2, 55–58. [Google Scholar]
- López-Moreno, M.L.; de la Rosa, G.; Hernández-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species. J. Agric. Food Chem. 2010, 58, 3689–3693. [Google Scholar] [CrossRef]
- Naskar, A.; Goswami, M.; Ghosh, A.G.R. Effects of Iron Oxide Nanoparticles on Chick Pea (Cicer arietinum): Physiological Profiling, Chlorophylls Assay and Antioxidant Potential. Int. Res. J. Eng. Technol. 2020, 7, 3001–3003. [Google Scholar]
- Hao, Y.; Zhang, Z.T.; Rui, Y.K.; Ren, J.Y.; Hou, T.Q.; Wu, S.J.; Rui, M.M.; Jiang, F.P.; Liu, L.M. Effect of different nanoparticles on seed germination and seedling growth in rice. In Proceedings of the 2nd Annual International Conference on Advanced Material Engineering, AME 2016, Wuhan, China, 15–17 April 2016; Atlantis Press: Paris, France, 2016; pp. 166–173. [Google Scholar]
- Mahmoodzadeh, H.; Nabavi, M.; Kashefi, H. Effect of nanoscale titanium dioxide particles on the germination and growth of canola (Brassica napus). J. Ornam. Hortic. 2013, 3, 25–32. [Google Scholar]
- Laware, S.L.; Raskar, S. Effect of titanium dioxide nanoparticles on hydrolytic and antioxidant enzymes during seed germination in onion. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 749–760. [Google Scholar]
- Laware, S.L.; Raskar, S. Influence of Zinc Oxide nanoparticles on growth, flowering and seed productivity in onion. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 874–881. [Google Scholar]
- Maity, A.; Natarajan, N.; Vijay, D.; Srinivasan, R.; Pastor, M.; Malaviya, D.R. Influence of Metal Nanoparticles (NPs) on Germination and Yield of Oat (Avena sativa) and Berseem (Trifolium alexandrinum). Proc. Natl. Acad. Sci. USA India Sect. B Biol. Sci. 2018, 88, 595–607. [Google Scholar] [CrossRef]
- Doğaroğlu, Z.G.; Köleli, N. TiO2 and ZnO nanoparticles toxicity in barley (Hordeum vulgare L.). Clean–Soil Air Water 2017, 45, 1700096. [Google Scholar] [CrossRef]
- Boonyanitipong, P.; Kositsup, B.; Kumar, P.; Baruah, S.; Dutta, J. Toxicity of ZnO and TiO2 nanoparticles on germinating rice seed Oryza sativa L. Int. J. Biosci. Biochem. Bioinformat. 2011, 1, 282–285. [Google Scholar] [CrossRef]
- Kumar, S.; Patra, A.K.; Datta, S.C.; Rosin, K.G.; Purakayastha, T.J. Phytotoxicity of nanoparticles to seed germination of plants. Int. J. Adv. Res. 2015, 3, 854–865. [Google Scholar]
- Jain, N.; Bhargava, A.; Pareek, V.; Akhtar, M.S.; Panwar, J. Does seed size and surface anatomy play role in combating phytotoxicity of nanoparticles? Ecotoxicology 2017, 26, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Medina-Velo, I.A.; Barrios, A.C.; Zuverza-Mena, N.; Hernandez-Viezcas, J.A.; Chang, C.H.; Ji, Z.; Zink, J.I.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Comparison of the effects of commercial coated and uncoated ZnO nanomaterials and Zn compounds in kidney bean (Phaseolus vulgaris) plants. J. Hazard. Mat. 2017, 332, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Nair, R.; Chavalmane, S.; Wang, W.N.; Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 2015, 7, 1584–1594. [Google Scholar] [CrossRef]
- Geisler-Lee, J.; Wang, Q.; Yao, Y.; Zhang, W.; Geisler, M.; Li, K.; Huang, Y.; Chen, Y.; Kolmakov, A.; Ma, X. Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicology 2012, 7, 323–337. [Google Scholar] [CrossRef]
- Lee, W.M.; An, Y.J.; Yoon, H.; Kweon, H.S. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): Plant agar test for water-insoluble nanoparticles. Environ. Toxicol. Chem. 2008, 27, 1915–1921. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, J.; Dou, R.; Gao, X.; Mao, C.; Wang, L. Assessment of the phytotoxicity of metal oxide nanoparticles on two crop plants, maize (Zea mays L.) and rice (Oryza sativa L.). Int. J. Environ. Res. Public Health 2015, 12, 15100–15109. [Google Scholar] [CrossRef]
- Lee, C.W.; Mahendra, S.; Zodrow, K.; Li, D.; Tsai, Y.C.; Braam, J.; Alvarez, P.J. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ. Toxicol. Chem. 2010, 29, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, M.R.; Giorgetti, L.; Geri, C.; Cremonini, R. The effects of nano-TiO2 on seed germination, development and mitosis of root tip cells of Vicia narbonensis L. and Zea mays L. J. Nanopart. Res. 2011, 13, 2443–2449. [Google Scholar] [CrossRef]
- Mousavi Kouhi, S.M.; Lahouti, M.; Ganjeali, A.; Entezari, M.H. Comparative phytotoxicity of ZnO nanoparticles, ZnO microparticles, and Zn2+ on rapeseed (Brassica napus L.): Investigating a wide range of concentrations. Toxicol. Environ. Chem. 2014, 96, 861–868. [Google Scholar] [CrossRef]
- Hafeez, A.; Razzaq, A.; Mahmood, T.; Jhanzab, H.M. Potential of copper nanoparticles to increase growth and yield of wheat. J. Nanosci. Adv. Technol. 2015, 1, 6–11. [Google Scholar]
- Zuverza-Mena, N.; Medina-Velo, I.A.; Barrios, A.C.; Tan, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Copper nanoparticles/compounds impact agronomic and physiological parameters in cilantro (Coriandrum sativum). Environ. Sci. Process. Impacts 2015, 17, 783–1793. [Google Scholar] [CrossRef]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Balážová, Ľ.; Babula, P.; Baláž, M.; Bačkorová, M.; Bujňáková, Z.; Briančin, J.; Kurmanbayeva, A.; Sagi, M. Zinc oxide nanoparticles phytotoxicity on halophyte from genus Salicornia. Plant Phys. Biochem. 2018, 130, 30–42. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Regulation of morphological, molecular and nutrient status in Arabidopsis thaliana seedlings in response to ZnO nanoparticles and Zn ion exposure. Sci. Total Environ. 2017, 575, 187–198. [Google Scholar] [CrossRef]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Palme, K.J.; Jansen, M.A. Stress-induced morphogenic responses: Growing out of trouble? Trends Plant. Sci. 2007, 12, 98–105. [Google Scholar] [CrossRef]
- Adhikari, T.; Kundu, S.; Biswas, A.K.; Tarafdar, J.C.; Rao, A.S. Effect of copper oxide nano particle on seed germination of selected crops. J. Agric. Sci. Technol. 2012, 2, 815–823. [Google Scholar]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Agric. Rev. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Alivisatos, A.P.; Gu, W.; Larabell, C. Quantum dots as cellular probes. Annu. Rev. Biomed. Eng. 2005, 7, 55–76. [Google Scholar] [CrossRef]
- Nair, R.; Poulose, A.C.; Nagaoka, Y.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Uptake of FITC labeled silica nanoparticles and quantum dots by rice seedlings: Effects on seed germination and their potential as biolabels for plants. J. Fluoresc. 2011, 21, 2057. [Google Scholar] [CrossRef]
- Das, S.; Wolfson, B.P.; Tetard, L.; Tharkur, J.; Bazata, J.; Santra, S. Effect of N-acetyl cysteine coated CdS: Mn/ZnS quantum dots on seed germination and seedling growth of snow pea (Pisum sativum L.): Imaging and spectroscopic studies. Environ. Sci. Nano 2015, 2, 203–212. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef]
- Lee, C.C.; MacKay, J.A.; Fre’chet, J.M.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Roveda Júnior, A.C.; Franco, D.W. Nitric oxide releasing-dendrimers: An overview. Braz. J. Pharm. Sci. 2013, 49, 1–14. [Google Scholar] [CrossRef]
- Seabra, A.B.; Justo, G.Z.; Haddad, P.S. State of the art, challenges and perspectives in the design of nitric oxide-releasing polymeric nanomaterials for biomedical applications. Biotechnol. Adv. 2015, 33, 1370–1379. [Google Scholar] [CrossRef]
- Klaine, S.J.; Alvarez, P.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef]
- NAAS. Nanotechnology in Agri-Food: Scope and Current Relevance; Policy Paper No. 63; National Academy of Agricultural Sciences: New Delhi, India, 2013; p. 20. [Google Scholar]
- Ditta, A. How helpful is nanotechnology in agriculture? Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 3–5. [Google Scholar] [CrossRef]
- Dhewa, T. Nanotechnology applications in agri-food: An update. Octa J. Environ. Res. 2015, 3, 204–211. [Google Scholar]
- Etxeberria, E.; Gonzalez, P.; Bhattacharya, P.; Sharma, P.; Ke, P.C. Determining the size exclusion for nanoparticles in Citrus leaves. HortScience 2016, 51, 732–737. [Google Scholar] [CrossRef]
- Santiago-Morales, J.; Rosal, R.; Hernando, M.D.; Ulaszewska, M.M.; Calvo, E.G.; Alba, A.R.F. Fate and transformation products of amine-terminated PAMAM dendrimers under ozonation and irradiation. J. Haz. Mat. 2014, 266, 102–113. [Google Scholar] [CrossRef]
- Kaphle, A.; Navya, P.N.; Umapathi, A.; Daima, H.K. Nanomaterials for agriculture, food and environment: Applications, toxicity and regulation. Environ. Chem. Lett. 2018, 16, 43–58. [Google Scholar] [CrossRef]
- Omanović-Mikličanin, E.; Badnjević, A.; Kazlagić, A.; Hajlovac, M. Nanocomposites: A brief review. Health Technol. 2020, 10, 51–59. [Google Scholar] [CrossRef]
- Kawahara, K.; Tsuruda, K.; Morishita, M.; Uchida, M. Antibacterial effect of silver-zeolite on oral bacteria under anaerobic conditions. Dent. Mater. 2000, 16, 452–455. [Google Scholar] [CrossRef]
- Matsumura, Y.; Yoshikata, K.; Kunisaki, S.; Tsuchido, T. Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl. Environ. Microbiol. 2003, 69, 4278–4281. [Google Scholar] [CrossRef]
- Arora, A.; Padua, G.W. Review: Nanocomposites in food packaging. J. Food Sci. 2010, 75, R43–R49. [Google Scholar] [CrossRef]
- Chaudhry, Q.; Scotter, M.; Blackburn, J.; Ross, B.; Boxall, A.; Castle, L.; Aitken, R.; Watkins, R. Applications and implications of nanotechnologies for the food sector. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 241–258. [Google Scholar] [CrossRef]
- Kole, C.; Kole, P.; Randunu, K.M.; Choudhary, P.; Podila, R.; Ke, P.C.; Rao, A.M.; Marcus, R.K. Nanobiotechnology can boost crop production and quality: First evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia). BMC Biotechnol. 2013, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Lagaron, J.M.; Cabedo, L.; Cava, D.; Feijoo, J.L.; Gavara, R.; Gimenez, E. Improving packaged food quality and safety. Part 2: Nanocomposites. Food Addit. Contam. 2005, 22, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Küçükçobanoğlu, Y.; Aktaş, L.Y. Plants as a Nanocomposite Source and Field of Application. Marmara J. Pure Appl. Sci. 2018, 30, 429–436. (In Turkish) [Google Scholar] [CrossRef]
- Draz, M.S.; Fang, B.A.; Zhang, P.; Hu, Z.; Gu, S.; Weng, K.C.; Gray, J.W.; Chen, F.F. Nanoparticle-mediated systemic delivery of siRNA for treatment of cancers and viral infections. Theranostics 2014, 4, 872. [Google Scholar] [CrossRef]
- Mu, L.; Seow, P.H. Application of TPGS in polymeric nanoparticulate drug delivery system. Colloids Surfaces B Biointerfaces 2006, 47, 90–97. [Google Scholar] [CrossRef]
- Singh, P.; Singh, R.; Borthakur, A.; Srivastava, P.; Srivastava, N.; Dhanesh, T.D.; Mishra, P.K. Effect of nanoscale TiO2-activated carbon composite on Solanum lycopersicum (L.) and Vigna radiata (L.) seeds germination. Energ. Ecol. Environ. 2016, 1, 131–140. [Google Scholar] [CrossRef]
- Liu, A.X.; Lu, Q.M.; Cao, Y.J.; Liao, Z.W.; Xu, Q.H. Effects of composite nanomaterials on rice growth. Plant Nutr. Fertil. Sci. 2007, 2, 344–347. (In Chinese) [Google Scholar]
- Abdel-Aziz, H.M.M.; Hasaneen, M.N.A.; Omer, A.M. Nano chitosan-NPK fertilizer enhances the growth and productivity of wheat plants grown in sandy soil. Span. J. Agric. Res. 2016, 14, e0902. [Google Scholar] [CrossRef]
- Yılmaz, U.; Evci, C. Future of Composite Materials in Aerospace and Defence Industry. Def. Sci. J. 2015, 14, 77–109. (In Turkish) [Google Scholar]

| Plant Name | Size of NM | Type and Chemical Composition of NM * | Duration of Pre-Cultivation | Conc. of the NM Exposure | Time of Exposure | Growth Conditions | Main Effects on Germination and Early Growth ** | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Positive effects | Cucumis sativus L. | 10.1 ± 4.2 nm | Au NP | - | 62 µg mL−1 | 7 days | Petri dishes (germination test) | Germination index ↑ns | [105] |
| Lactuca sativa L. | Germination index ↑ | ||||||||
| Pennisetum glaucum L. | 14–35 nm | Au NP | Seed soaking for 2 h in test solution | 20 and 50 µg mL−1 | 5 days | Petri dishes (germination test) | Germination % ↑ns and ↑; total seedling length ↑ns | [106] | |
| Cucurbita pepo L. | 20 nm | Ag NP | 2 h seed priming | 0.05–2.5 mg L−1 | 12–16 days after priming | Petri dishes (germination test) | Germination % ↑ at 0.5–2.0 mg L−1 conc.; root length ↑ at 0.05–1.5 mg L−1 | [107] | |
| Citrullus lanatus L. | Germination % ↑ at 0.5–2.0 mg L−1 conc.; root length ↑ at 1–2.5 mg L−1 | ||||||||
| Lolium multiflorum L. | Width: 122 ± 35 nm, length: 11,908 ± 6703 nm | Ag NW | - | 10 ppm | 6 days | Petri dishes (germination test) | Root length ↑ns and physical separation from NWs caused further increment | [108] | |
| Phaseolus vulgaris L. ‘Bali’ and ‘Delfina’ | ~10 nm | Ag NP | Seed priming for 1.5 h | 0.25, 1.25 and 2.5 mg L−1 | 5 days | Petri dishes (germination test) | Germination % ↑ at all conc. | [109] | |
| Zea mays L. | <50 nm | Co3O4 | - | 269.3–1000 mg kg−1 soil (DW) | 14 days | Germination test in pot experiment | Germination % ↑ns at all conc. | [110] | |
| Alyssum homolocarpum | 10–25 nm | TiO2 NP | - | 10–80 mg L−1 | 10 days | Petri dishes (germination test) | Germination % ↑ at 10–40 mg L−1 conc. | [111] | |
| Nigella sativa L. | Germination % ↑ at 10–40 mg L−1 conc. | ||||||||
| Salvia mirzayanii Rech. f. & Esfand | Germination % ↑ at all conc. | ||||||||
| Arachis hypogea L. var. ‘K-134’ | 25 nm | ZnO NP | 100, 1000 and 2000 ppm for seed priming | 2 or 30g/15 L for foliar spraying | 3 h seed priming then 2x foliar spraying | Pot and field experiment | Germination % ↑, seedling vigour ↑ | [112] | |
| Capsicum annuum L. | no data | ZnO NP | 6 h seed priming | 0.25, 0.5 and 0.75 g | 14 days | Moistened blotter paper (in Petri dishes) | Concentration-dependent ↑ of seed germination %, root length ↑, seedling length ↑ | [113] | |
| Capsicum chinense L. var. Chichen Itza | 18 ± 8 nm | ZnO NP | - | 100–500 mg/L | 72 h seed priming; 14 day-long germination | Petri dishes (germination test) | Germination % ↑ with conc.; radicule length ↑ at 300 mg/L | [114] | |
| Cucumis sativus L. ‘Poinsett 76’ | 8 nm | ZnO NP | - | 50–1600 mg L−1 | Until 65 % of the seeds were germinated | Petri dishes (germination test) | Germination % ↑ at 400–1600 mg L−1 conc. | [115] | |
| Vigna radiata L. | ~18 nm | ZnO NP | Seed priming for 3 h | 20, 40, 60, 80 and 100 mg L−1 | Germinating for 7 days after priming | Petri dishes (germination test) | Germination % ↑ | [116] | |
| Vigna unguiculata L. | 30 nm | ZnO NP | - | 250, 500 and 750 ppm | 6 h seed treatment | Soil (pot experiment) | Seedling length ↑, germination % ↑, seedling fresh weight ↑ and vigour index ↑ | [117] |
| Plant Name | Size of NM | Type and Chemical Composition of NM * | Duration of Pre-Cultivation | Concentration of the NM Exposure | Time of Exposure | Growth Conditions | Main Effects on Germination and Early Growth ** | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Mixed or no affected | Alnus subcordata L. | no data | Ag NP | - | 10–100 mg kg−1 (soil) | 15 days | Soil (in Petri dishes) | No change of germination % and seedling length | [118] |
| - | 10 and 20 mg L−1 | Petri dishes (germination test) | Germination % ↓; seedling length ↓ns and ↓ | ||||||
| Lactuca sativa L. | 29.2 ± 1.1 nm | Ag NP | - | 100 µg mL−1 | 7 days | Petri dishes (germination test) | Germination index: no change | [105] | |
| Pennisetum glaucum L. | 13 nm | Ag NP | Seed soaking for 2 h in test solution | 20 and 50 mg L−1 | 5 days | Petri dishes (germination test) | Germination % ↑ at higher conc.; total seedling length ↓ at higher conc. | [119] | |
| Pinus sylvestris L. | no data | Ag NP | - | 10–100 mg kg−1 (soil) | 15 days | Soil (in Petri dishes) | No change of germination % and seedling length | [118] | |
| - | 10 and 20 mg L−1 | Petri dishes (germination test) | Germination % ↓; seedling length ↓ns and ↓ | ||||||
| Triticum aestivum L. cv. NARC-2009 | 10–20 nm | Ag NP | - | 25–150 ppm | 7 days | Petri dishes (germination test) | Germination % ↑ns and number of seminal roots ↑ at 25–75 ppm but ↓ at higher conc. | [120] | |
| Zea mays L. | 20 nm | Ag NP | 2 h seed priming | 0.05–2.5 mg L−1 | 12–16 days | Petri dishes (germination test) | No effect on germination %; root length ↓ at all conc. | [107] | |
| Cucumis sativus L. ‘Poincett 76’ | 7 nm | CeO2 NP | - | 500–4000 mg L−1 | 9 days | Petri dishes (germination test) | Germination % ↓ at 2000 mg L−1 conc.; root and shoot length ↑ at all conc. | [121] | |
| Glycine max L. | 7 nm | CeO2 NP | - | 500–4000 mg L−1 | Until 65% of control roots were 5 mm long | Petri dishes (germination test) | Germination % ↓ at 2000 mg L−1 conc.; root elongation ↑ at all conc. | [30] | |
| Medicago sativa L. Mesa variety | 7 nm | CeO2 NP | - | 500–4000 mg L−1 | 9 days | Petri dishes (germination test) | Germination % was not affected; root length ↓ at 2000–4000 mg L−1, while shoot length ↑ at 500–1000 mg L−1 conc. | [121] | |
| Zea mays L. | 7 nm | CeO2 NP | - | 500–4000 mg L−1 | 8 days | Petri dishes (germination test) | Germination % ↓ at 500–2000 mg L−1; root length ↑ at 4000 mg L−1, while shoot length ↑ at 2000 mg L−1 but ↓ at 4000 mg L−1 conc. | ||
| Brassica oleracea L. | <50 nm | Co3O4 | - | 269.3–1000 mg kg−1 soil (DW) | 14 days | Germination test in pot experiment | Germination % ↑ at 269–350 mg kg−1 conc. but ↓ns at 769–1000 mg kg−1conc. | [110] | |
| Cicer arietinum L. | <50 nm | Fe2O3 NP | Seed priming for 2 h | 10–200 mg L−1 | 3 days | Petri dishes (germination test) | Germination time ↓ at all conc.; root length ↓ns but shoot length ↑ns concentration-dependently | [122] | |
| Oryza sativa L. (Y Liangyou 1928) | 40–100 nm | Fe2O3 NC | Seed priming for 2 h | 5–150 mg L−1 | 10 d after priming | Petri dishes (germination test) | Germination % ↓ns while root length ↑ and shoot length ↑ns with conc. | [123] | |
| Length: 200–400 nm, diameter: 10–20 nm | Fe2O3 SR | Germination % ↓ns; root length ↑ at 5–50 mg/L and ↑ns at 100–150 mg L−1; shoot length ↑ and ↑ns at 5–50 and 100 mg L−1 | |||||||
| Length: 500 nm, diameter: 50 nm | Fe2O3 LR | Germination % ↓ at 5–100 mg L−1; root length ↑ with conc.; shoot length ↑ at 5–50 mg L−1 | |||||||
| Brassica napus L. var. RGS003 | 20 nm | TiO2 NP | - | 10–2000 mg L−1 | 7 days | Petri dishes (germination test) | No change of germination % at 100–1700 mg L−1 but ↑ns at 2000 mg L−1 conc.; no significant changes of radicle length while plumule length ↓ at 10–1000 mg L−1 and ↑ns at higher doses | [124] | |
| Allium cepa L. | 21 nm | TiO2 NP | - | 10–50 mg L−1 | 10 days | Wet filter paper (germination test) | Germination % ↑ns at 10–40 mg L−1 conc.; radicle length ↑ns at 10–30 mg L−1 but ↓ at higher doses, while shoot length ↑ | [125] | |
| Carum copticum L. | 10–25 nm | TiO2 NP | - | 10–80 mg L−1 | 10 days | Petri dishes (germination test) | Germination % ↑ at 10–20 mg L−1 but ↓ at higher conc. | [111] | |
| Oryza sativa L. (Y Liangyou 1928) | 20 nm | TiO2 NP | - | 5–150 mg L−1 | Seed priming for 2 h then cultivation for 10 d | Petri dishes (germination test) | Germination % ↓ns; root length ↑ at 5–10, 50 and 100 mg L−1; shoot length ↑ at 150 mg L−1 | [123] | |
| Sinapis alba L. | 10–25 nm | TiO2 NP | - | 10–80 mg L−1 | 10 d | Petri dishes (germination test) | Germination % ↑ at 10–20 mg L−1 but ↓ at higher conc. | [111] | |
| Allium cepa L. | 20 nm | ZnO NP | - | 10–40 mg L−1 | 10 days | Wet filter paper (germination test) | Germination % and seedling growth ↑ns at lower conc. | [126] | |
| Avena sativa L. | no data | ZnO NP | - | 750, 1000 and 1250 mg kg−1 seed | 10 min priming | Wet paper and field experiment | Germination %, seedling vigour and yield ↑ at low conc., root and shoot length ↓ at higher doses | [127] | |
| Brassica juncea L. Czern. cv. Negro Caballo | 45 nm | ZnO NP | - | 25 and 100 mg L−1 | 5 days | Petri dishes (germination test) | Primary root length ↑ at 25 mg L−1 but ↓ at 100 mg L−1 conc. | [32] | |
| Brassica napus L. cv. GK Gabriella | - | ||||||||
| Glycine max L. | 8 nm | ZnO NP | - | 500–4000 mg L−1 | Until 65% of control roots were 5 mm long | Petri dishes with wet filter paper (germination test) | Germination was not affected; root elongation ↑ at 500 mg L−1 but ↓ at 2000 mg L−1 ZnO NP | [30] | |
| Hordeum vulgare L. | 30 nm | ZnO NP | - | 5, 10, 20, 40 and 80 mg kg−1 | 7 days germination then 21 days cultivation | Petri dishes (germination) then pot experiment | No effect on seed germination and root elongation | [128] | |
| Oryza sativa L. | no data | ZnO NP | 1–3 days | 10–1000 mg L−1 | 7 days | Moistened filter paper | No change in germination % | [129] | |
| Oryza sativa L. | ≤ 50 nm | ZnO NP | 2 h | 10–500 mg L−1 | 5–12 days | Petri dishes (filter paper or soil) | No change of germination % | [130] | |
| Pennisetum glaucum L. | <50 nm | ZnO NP | - | 100–1000 mg L−1 | 7 days | Petri dishes (germination test) | Germination % ↓; root length ↑ but ↓ at 500–1000 mg L−1 conc.; shoot length ↑ ns and ↓ns | [131] | |
| Phaseolus vulgaris L. var. red hawk kidney | 93.8 or 84.1 nm | ZnO NP | - | 62.5–500 mg kg−1 (soil) | 45 days | Soil (pot experiment) | No effect on germination % | [132] | |
| Solanum lycopersicum L. hybr. ‘tomato cherry super sweet 100’ | 28 ± 0.7 nm | ZnO NP | Seed priming for 1 h | 10–1000 mg L−1 | 5 days | Petri dishes (germination test) | No change of germination % at 10–750 mg L−1 but ↓ at 1000 mg L−1 conc. | [133] | |
| Trifolium alexandrium L. | no data | ZnO NP | 10 min priming | 750, 1000 and 1250 mg kg−1 seed | no data | Wet paper and field experiment | Germination %, seedling vigour and yield ↑ at low conc., root and shoot length ↓ at higher doses | [127] |
| Plant Name | Size of NM | Type and Chemical Composition of NM * | Duration of Pre-Cultivation | Concentration of the NM Exposure | Time of Exposure | Growth Conditions | Main Effects on Germination and Early Growth ** | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Negative effects | Cucumis sativus L. | 29.2 ± 1.1 nm | Ag NP | - | 100 µg mL−1 | 7 days | Petri dishes (germination test) | Germination index ↓ | [105] |
| Lolium multiflorum L. | 35 ± 7 nm | Ag NP | - | 10 ppm | 6 days | Petri dishes (germination test) | Root length ↓ns but physical separation from NPs caused ↑ | [108] | |
| Lolium multiflorum L. | 44 ± 7 nm | Ag NC | Root length ↓ns but physical separation from NCs caused ↑ | ||||||
| Phaseolus radiatus L. | no data | Cu NP | 24 h | 200–1000 mg L−1 | 48 h | Petri dishes (germination test, agar) | Seedling growth and root growth ↓ concentration-dependently | [135] | |
| Triticum aestivum ssp. aestivum | no data | Cu NP | Seedling growth and root growth ↓ concentration-dependently | ||||||
| Solanum lycopersicum L. ‘Pomodoro’ | 7 nm | CeO2 NP | - | 500–4000 mg L−1 | 6 days | Petri dishes (germination test) | Germination % ↓ns and ↓; root elongation ↓ at 1000–4000 mg/L conc. | [121] | |
| Avena sativa L. | <50 nm | Co3O4 NP | - | 269.3–1000 mg kg−1 soil (DW) | 14 days | Germination test in pot experiment | Germination % ↓ns at higher conc. | [110] | |
| Solanum lycopersicum L. | Germination % ↓ns but ↓ at 1000 mg/kg conc. | ||||||||
| Oryza sativa L. Jijing No.6. | <50 nm | CuO NP | 2 h priming | 25–2000 mg L−1 | Germination for 5 days after priming | Petri dishes (germination test) | Root length ↓ at all conc. | [136] | |
| Zea mays L. Zhengdan No. 958. | <50 nm | CuO NP | 2 h priming | 25–2000 mg L−1 | Germination for 7 days after priming | Petri dishes (germination test) | Root length ↓ at all conc. | [136] | |
| Arabidopsis thaliana ‘Col-0’ | <50 nm | Fe3O4 NP | 5 days at 4 °C (in dark) | 400, 2000 and 4000 mg L−1 | 18 days | 1/2 MS medium | Seed germination % ↓ns at 400 and 2000 mg L−1; root elongation ↓ | [137] | |
| Cucumis sativus L. | 7.57 ± 5.6 nm | Fe3O4 NP | - | 116 µg mL−1 | 7 days | Petri dishes (germination test) | Germination index ↓ | [105] | |
| Lactuca sativa L. | Germination index ↓ | ||||||||
| Vicia narbonensis L. | <100 nm | TiO2 NP | Soaking seed for 24 h in the test solutions | 0.2–4.0‰ | 72 h | Petri dishes (germination test) | Germination % ↓ ns; root elongation ↓ ns and ↓, chromosomal aberration index in root tip meristem ↑ with conc. | [138] | |
| Zea mays L. | |||||||||
| Arabidopsis thaliana ‘Col-0’ | ~44 nm | ZnO NP | 5 days at 4 °C (in dark) | 400, 2000 and 4000 mg L−1 | 18 days | 1/2 MS medium | Seed germination % ↓ and root elongation ↓ | [137] | |
| Brassica napus L. cv. Hayola 401 | <50 nm | ZnO NP | - | 5–500 mg L−1 | 6 days | Petri dishes (germination test) | Germination % ↓ns | [139] | |
| Cucumis sativus L. | ≤50 nm | ZnO NP | 2 h | 10–500 mg/L | 5–12 days | Petri dishes (filter paper or soil) | Germination % ↓ns | [130] | |
| Oryza sativa L. Jijing No. 6. | <50 nm | ZnO NP | 2 h priming | 25–2000 mg/L | Germination for 5 days after priming | Petri dishes (germination test) | Germination % was not affected at 2000 mg L−1conc.; root length ↓ at 100–2000 mg L−1 | [136] | |
| Solanum lycopersicum L. ‘Roma FV’ | 8 nm | ZnO NP | - | 50–1600 mg L−1 | Until 65 % of the seeds were germinated | Petri dishes (germination test) | Germination % ↓ at 800–1600 mg L−1conc. | [115] | |
| Solanum lycopersicum L. | <50 nm | ZnO NP | - | 100–1000 mg L−1 | 7 days | Petri dishes (germination test) | Germination % ↓ at 750–1000 mg L−1 | [131] | |
| Triticum aestivum L. | Germination % ↓ from 250 mg L−1 ZnO NP | ||||||||
| Triticum aestivum L. | ≤50 nm | ZnO NP | 2 h | 10–500 mg L−1 | 5–12 days | Petri dishes (filter paper or soil) | Germination % ↓ ns | [130] | |
| Vigna radiata L. | |||||||||
| Zea mays L. Zhengdan No. 958. | <50 nm | ZnO NP | 2 h priming | 25–2000 mg L−1 | Germination for 7 days after priming | Petri dishes (germination test) | Germination % was not affected at 2000 mg L−1 conc.; root length ↓ at 500–2000 mg L−1 | [136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szőllősi, R.; Molnár, Á.; Kondak, S.; Kolbert, Z. Dual Effect of Nanomaterials on Germination and Seedling Growth: Stimulation vs. Phytotoxicity. Plants 2020, 9, 1745. https://doi.org/10.3390/plants9121745
Szőllősi R, Molnár Á, Kondak S, Kolbert Z. Dual Effect of Nanomaterials on Germination and Seedling Growth: Stimulation vs. Phytotoxicity. Plants. 2020; 9(12):1745. https://doi.org/10.3390/plants9121745
Chicago/Turabian StyleSzőllősi, Réka, Árpád Molnár, Selahattin Kondak, and Zsuzsanna Kolbert. 2020. "Dual Effect of Nanomaterials on Germination and Seedling Growth: Stimulation vs. Phytotoxicity" Plants 9, no. 12: 1745. https://doi.org/10.3390/plants9121745
APA StyleSzőllősi, R., Molnár, Á., Kondak, S., & Kolbert, Z. (2020). Dual Effect of Nanomaterials on Germination and Seedling Growth: Stimulation vs. Phytotoxicity. Plants, 9(12), 1745. https://doi.org/10.3390/plants9121745








