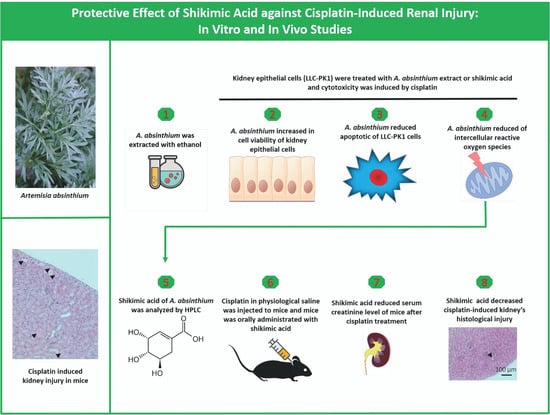
Protective Effect of Shikimic Acid against Cisplatin-Induced Renal Injury: In Vitro and In Vivo Studies
Abstract
1. Introduction
2. Results
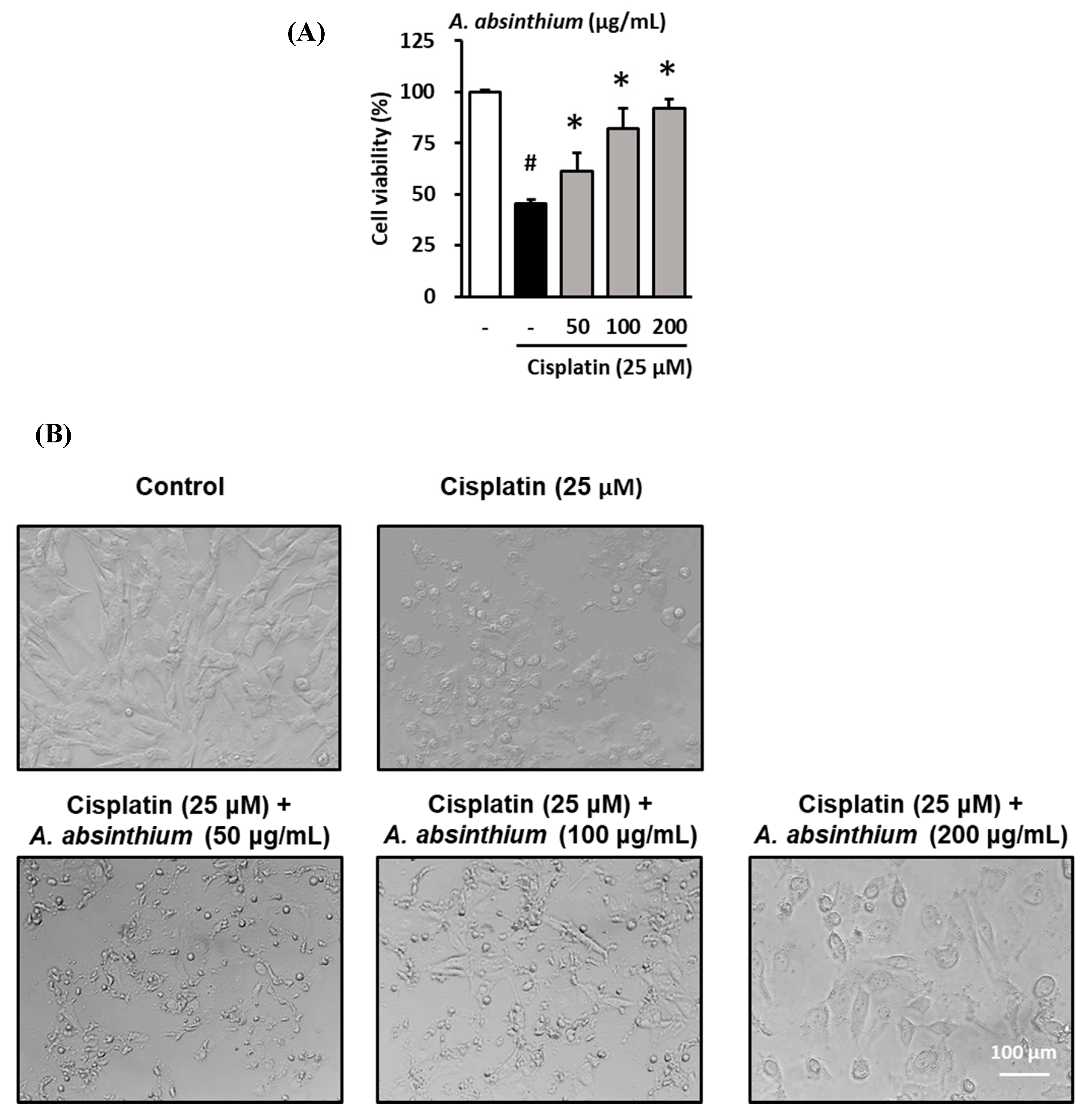
2.1. Protective Effect of A. absinthium Extract against Cisplatin-Induced Kidney Cell Death
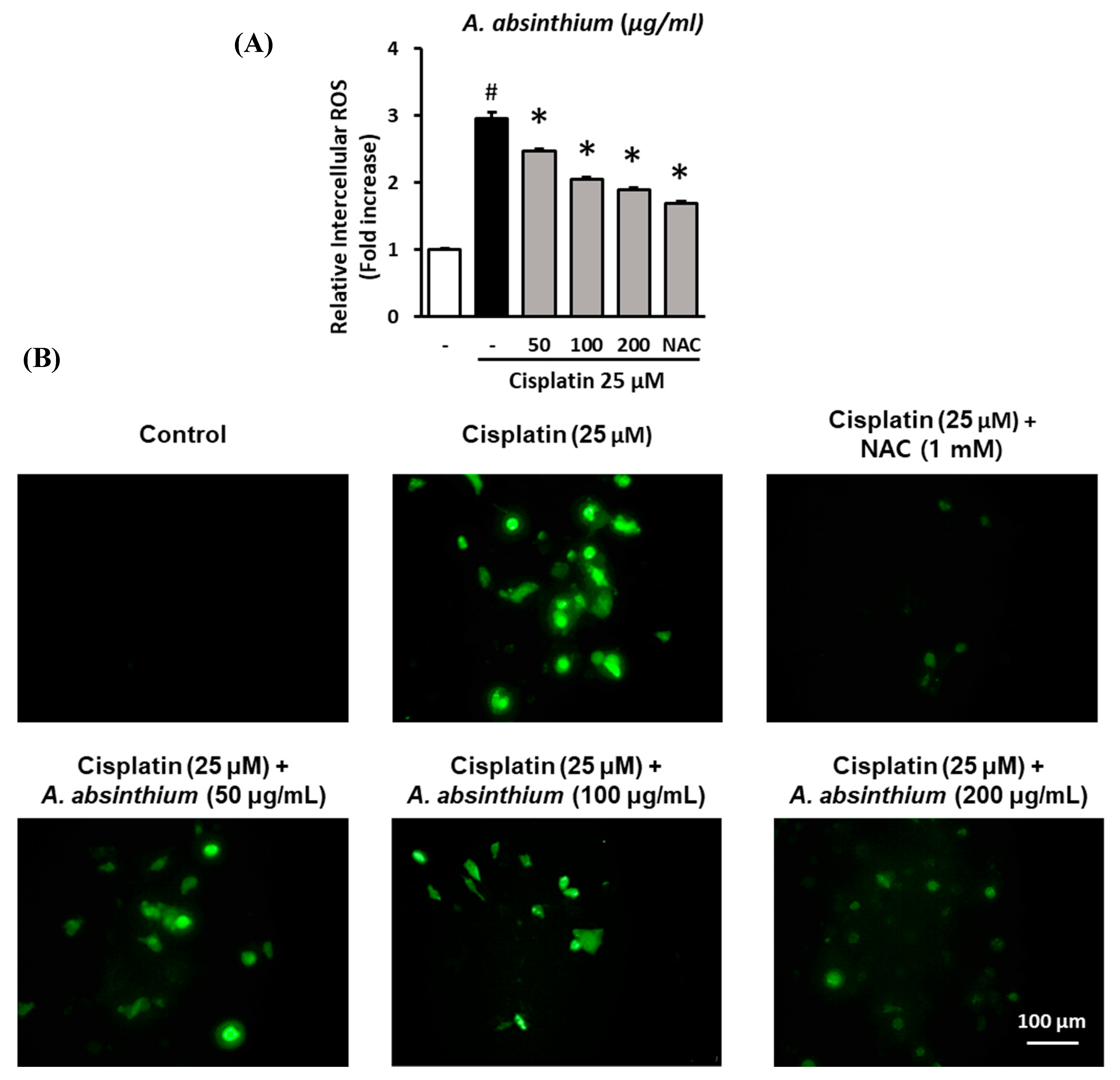
2.2. Inhibitory Effect of A. absinthium Extract on Cisplatin-Induced ROS Accumulation in LLC-PK1 Cells
2.3. Inhibitory Effect of A. absinthium Extract on Cisplatin-Induced Apoptosis in LLC-PK1 Cells
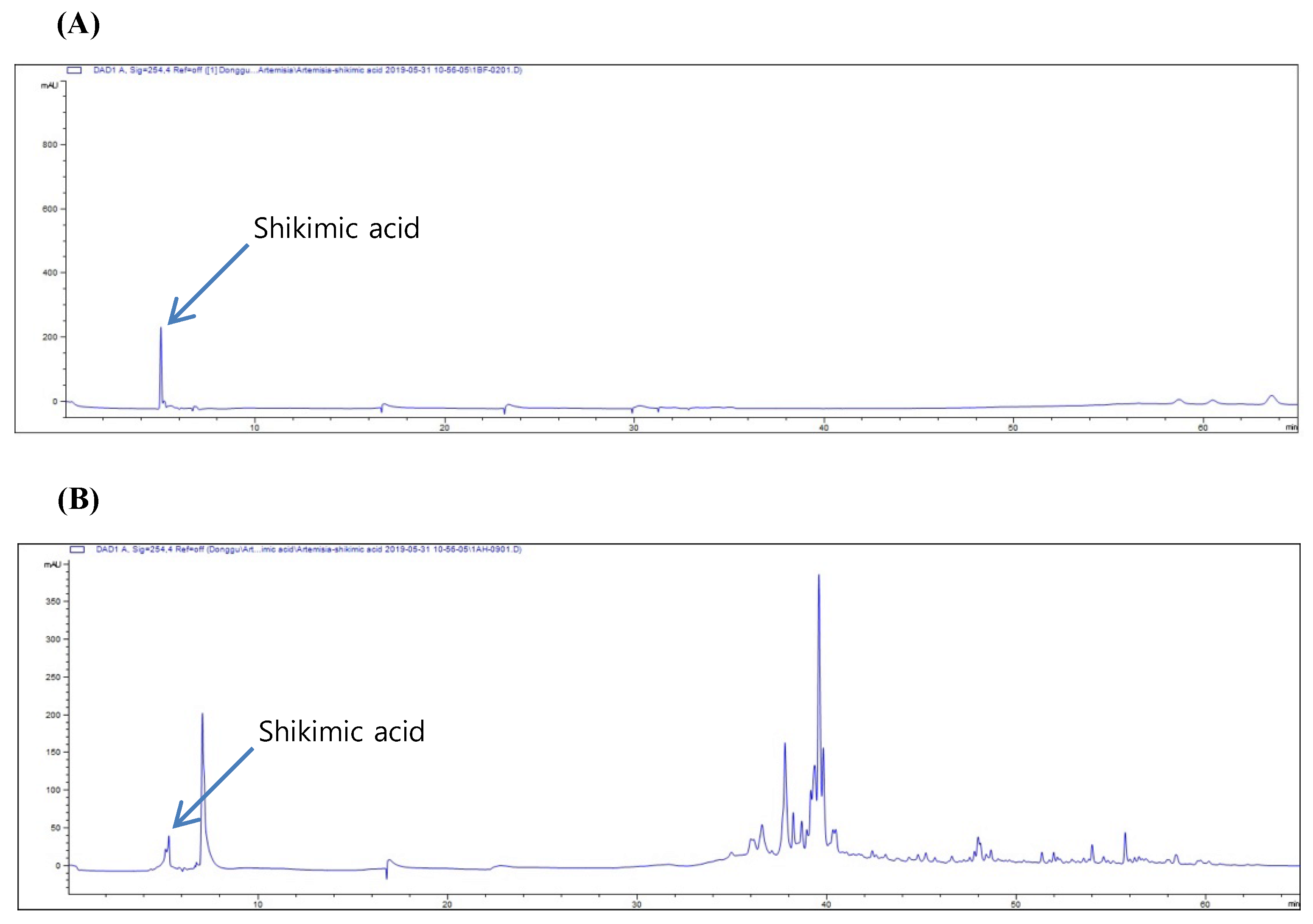
2.4. High-Performance Liquid Chromatography (HPLC) of A. absinthium Extract
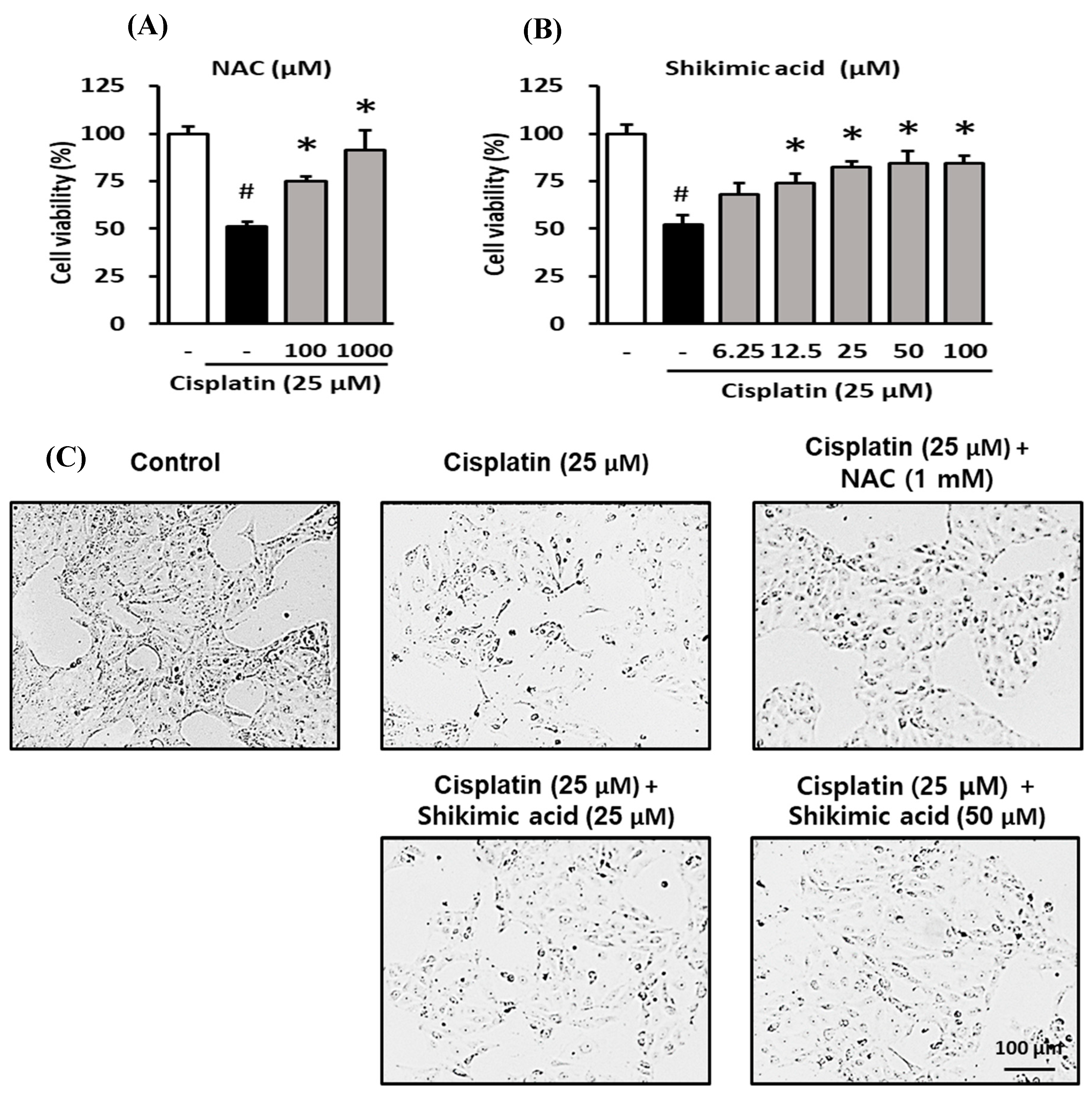
2.5. Protective Effect of Shikimic Acid against Cisplatin-Induced Kidney Cell Damage
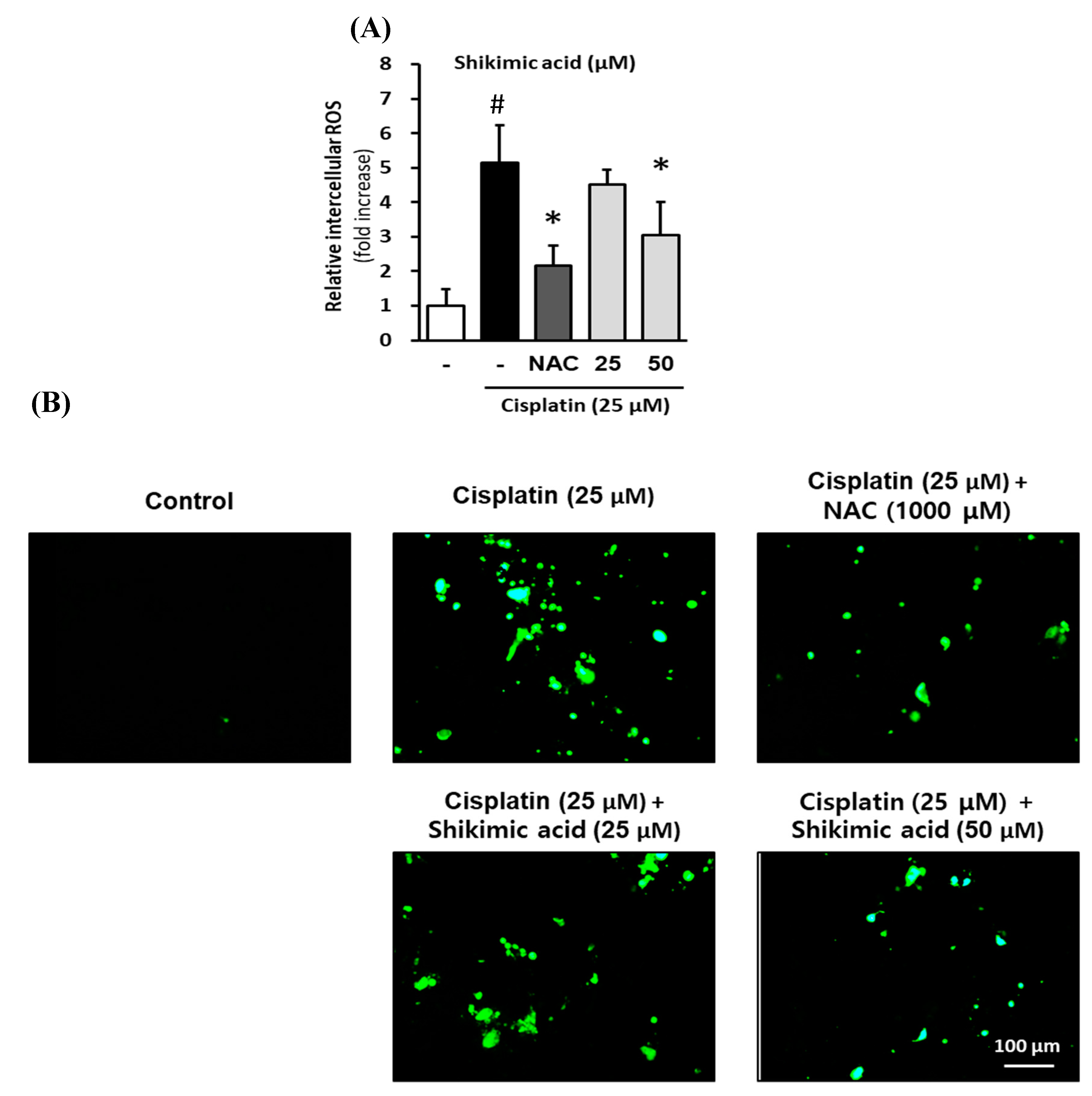
2.6. Inhibitory Effect of Shikimic Acid on Cisplatin-Induced ROS Accumulation in LLC-PK1 Cells
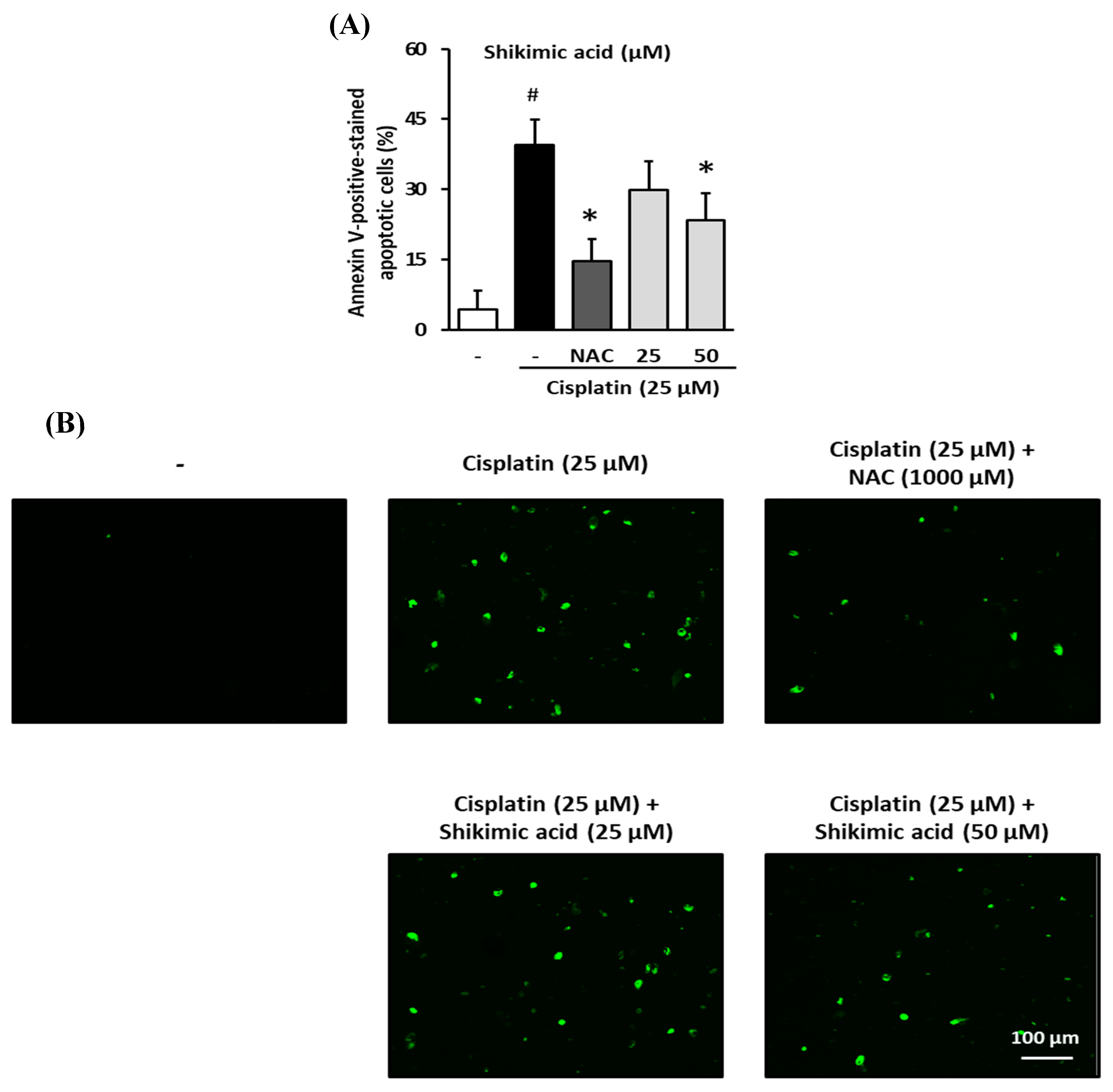
2.7. Inhibitory Effect of Shikimic Acid on Cisplatin-Induced Apoptosis in LLC-PK1 Cells
2.8. Effect of Shikimic Acid on the Cisplatin-Induced Increase in Serum Creatinine and Kidney Injury in BALB/c Mice
3. Discussion
4. Materials and Methods
4.1. Plant Extracts, Reagents and HPLC
4.2. Assessment of the Effect of A. absinthium Extract and Shikimic Acid on Cisplatin-Induced Nephrotoxicity
4.2.1. Cell Culture and Treatment
4.2.2. Evaluation of the Protective Effects of A. absinthium Extract and Shikimic Acid against Kidney Cell Damage
4.2.3. Assessment of Intracellular ROS
4.2.4. Evaluation of Apoptotic Cells
4.3. Assessment of the Renoprotective Effect of Shikimic Acid against Cisplatin-Mediated Renal Injury in Male Mice
4.4. Statistical Analysis
5. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Rosenberg, B.H.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum Compounds: A New Class of Potent Antitumour Agents. Nat. Cell Biol. 1969, 222, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.; Vancamp, L. The successful regression of large solid sarcoma 180 tumors by platinum compounds. Cancer Res. 1970, 30, 1799–1802. [Google Scholar] [PubMed]
- Higby, D.J.; Wallace, H.J., Jr.; Albert, D.; Holland, J. Diaminodichloroplatinum: A phase I study showing responses in testicular and other tumors. Cancer 1974, 33, 1219–1225. [Google Scholar] [CrossRef]
- Erdlenbruch, B.; Pekrum, A.; Roth, C.; Grunewald, R.W.; Kern, W.; Lakomek, M. Cisplatin nephrotoxicity in children after continuous 72-h and 3x1-h infusions. Pediatr. Nephrol. 2001, 16, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Leu, L.; Baribeault, D. A comparison of the rates of cisplatin (cDDP)-induced nephrotoxicity associated with sodium loading or sodium loading with forced diuresis as a preventative measure. J. Oncol. Pharm. Pract. 2009, 16, 167–171. [Google Scholar] [CrossRef]
- Duan, Z.; Cai, G.; Li, J.; Chen, X. Cisplatin-induced renal toxicity in elderly people. Ther. Adv. Med. Oncol. 2020, 12. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Berger, C.; Hartmann, J.; Kollmannsberger, C.; Schmoll, H.; Kuczyk, M.; Kanz, L. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br. J. Cancer 1998, 77, 1355. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Latcha, S.; Jaimes, E.A.; Patil, S.; Glezerman, I.G.; Mehta, S.; Flombaum, C.D. Long-Term Renal Outcomes after Cisplatin Treatment. Clin. J. Am. Soc. Nephrol. 2016, 11, 1173–1179. [Google Scholar] [CrossRef]
- Hoek, J.; Bloemendal, K.M.; Van Der Velden, L.-A.A.; Van Diessen, J.; Van Werkhoven, E.; Klop, W.M.C.; Tesselaar, M.E.T. Nephrotoxicity as a Dose-Limiting Factor in a High-Dose Cisplatin-Based Chemoradiotherapy Regimen for Head and Neck Carcinomas. Cancers 2016, 8, 21. [Google Scholar] [CrossRef]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin Nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef]
- Uozumi, J.; Koikawa, Y.; Yasumasu, T.; Tokuda, N.; Kumazawa, J. The Protective Effect of Methylprednisolone against Cisplatin-Induced Nephrotoxicity in Patients with Urothelial Tumors. Int. J. Urol. 1996, 3, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Kim, S.H.; Kim, J.W.; Kim, Y.J.; Lee, K.-W.; Na, K.Y. Effects of a DPP4 inhibitor on cisplatin-induced acute kidney injury: Study protocol for a randomized controlled trial. Trials 2015, 16, 239. [Google Scholar] [CrossRef] [PubMed]
- El-Ghiaty, M.A.; Ibrahim, O.M.H.; Abdou, S.M.; Hussein, F.Z. Evaluation of the protective effect of Cystone® against cisplatin-induced nephrotoxicity in cancer patients, and its influence on cisplatin antitumor activity. Int. Urol. Nephrol. 2014, 46, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, F.; Sadighi, S.; Dashti-Khavidaki, S.; Shahi, F.; Mirzania, M.; Abdollahi, A.; Ghahremani, M.-H. Effect of Silymarin Administration on Cisplatin Nephrotoxicity: Report from A Pilot, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Phytother. Res. 2015, 29, 1046–1053. [Google Scholar] [CrossRef]
- Karademir, L.D.; Dogruel, F.; Kocyigit, I.; Yazici, C.; Unal, A.; Sipahioglu, M.H.; Oymak, O.; Tokgoz, B. The efficacy of theophylline in preventing cisplatin-related nephrotoxicity in patients with cancer. Ren. Fail. 2016, 38, 806–814. [Google Scholar] [CrossRef]
- Momeni, A.; Hajigholami, A.; Geshnizjani, S.; Kheiri, S. Effect of Silymarin in the Prevention of Cisplatin Nephrotoxicity, a Clinical Trial Study. J. Clin. Diagn. Res. 2015, 9, 11–13. [Google Scholar] [CrossRef]
- Matsui, M.; Saito, Y.; Yamaoka, S.; Yokokawa, Y.; Morikawa, Y.; Makimoto, A.; Yuza, Y. Kidney-protective effect of magnesium supplementation in cisplatin-containing chemotherapy for pediatric cancer: A retrospective study. J. Pediatr. Hematol. Oncol. 2018, 40, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Busari, A.A.; Adejare, A.A.; Shodipe, A.F.; Oduniyi, O.A.; Ismail-Badmus, K.B.; Oreagba, I.A. Protective but Non-Synergistic Effects of Nigella Sativa and Vitamin E against Cisplatin-Induced Renal Toxicity and Oxidative Stress in Wistar Rats. Drug Res. 2018, 68, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, M.; Mou, J.; Zhao, Z.; Yang, J.; Zhu, F.; Pei, G.; Zhu, H.; Wang, Y.; Xu, G.; et al. Pretreatment of Huaiqihuang extractum protects against cisplatin-induced nephrotoxicity. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Yu, J.S.; Lee, S.R.; Hwang, G.S.; Kang, K.S.; Park, J.G.; Kim, H.Y.; Kim, K.H.; Yamabe, N. Beneficial Effects of Bioactive Compounds in Mulberry Fruits against Cisplatin-Induced Nephrotoxicity. Int. J. Mol. Sci. 2018, 19, 1117. [Google Scholar] [CrossRef]
- Kandemir, F.M.; Yildirim, S.; Caglayan, C.; Kucukler, S.; Eser, G. Protective effects of zingerone on cisplatin-induced nephrotoxicity in female rats. Environ. Sci. Pollut. Res. 2019, 26, 22562–22574. [Google Scholar] [CrossRef]
- Kpemissi, M.; Eklu-Gadegbeku, K.; Veerapur, V.P.; Negru, M.; Taulescu, M.; Chandramohan, V.; Hiriyan, J.; Banakar, S.M.; Nv, T.; Suhas, D.S.; et al. Nephroprotective activity of Combretum micranthum G. Don in cisplatin induced nephrotoxicity in rats: In-vitro, in-vivo and in-silico experiments. Biomed. Pharmacother. 2019, 116, 108961. [Google Scholar] [CrossRef] [PubMed]
- Ridzuan, N.R.A.; Rashid, N.A.; Othman, F.; Budin, S.B.; Hussan, F.; Teoh, S.L. Protective Role of Natural Products in Cisplatin-Induced Nephrotoxicity. Mini Rev. Med. Chem. 2019, 19, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.-S.; Olatunde, A.; El-Mleeh, A.; Hetta, H.F.; Al-Rejaie, S.; Alghamdi, S.; Zahoor, M.; Magdy, B.A.; Murata, T.; Zaragoza-Bastida, A. Bioactive Compounds, Pharmacological Actions, and Pharmacokinetics of Wormwood (Artemisia absinthium). Antibiotics 2020, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Bora, K.S.; Sharma, A. Neuroprotective effect of Artemisia absinthium L. on focal ischemia and reperfusion-induced cerebral injury. J. Ethnopharmacol. 2010, 129, 403–409. [Google Scholar] [CrossRef]
- Craciunescu, O.; Constantin, D.; Gaspar-Pintiliescu, A.; Toma, L.; Utoiu, E.; Moldovan, L. Evaluation of antioxidant and cytoprotective activities of Arnica montana L. and Artemisia absinthium L. ethanolic extracts. Chem. Central J. 2012, 6, 97. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Thiruvengadam, M.; Chung, I.-M.; Nagella, P. Polyphenol composition and antioxidant activity from the vegetable plant Artemisia absinthium L. Aust. J. Crop. Sci. 2013, 7, 1921. [Google Scholar]
- Krebs, S.; Omer, B.; Omer, T.N.; Fliser, D. Wormwood (Artemisia absinthium) for Poorly Responsive Early-Stage IgA Nephropathy: A Pilot Uncontrolled Trial. Am. J. Kidney Dis. 2010, 56, 1095–1099. [Google Scholar] [CrossRef]
- Omer, B.; Krebs, S.; Omer, H.; Noor, T. Steroid-sparing effect of wormwood (Artemisia absinthium) in Crohn’s disease: A double-blind placebo-controlled study. Phytomedicine 2007, 14, 87–95. [Google Scholar] [CrossRef]
- Krebs, S.; Omer, T.N.; Omer, B. Wormwood (Artemisia absinthium) suppresses tumour necrosis factor alpha and accelerates healing in patients with Crohn’s disease—A controlled clinical trial. Phytomedicine 2010, 17, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Radwan, R.R.; Abdel Fattah, S.M. Mechanisms involved in the possible nephroprotective effect of rutin and low dose gamma irradiation against cisplatin-induced nephropathy in rats. J. Photochem. Photobiol. B 2017, 169, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Bhattacharjee, A.; Hajra, S.; Samanta, A.; Bhattacharya, S. Ameliorative effect of an oxovanadium (IV) complex against oxidative stress and nephrotoxicity induced by cisplatin. Redox Rep. 2016, 22, 377–387. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Nie, J.; Zheng, Z.-L.; Zhao, J.; Wu, L.-M.; Zhu, Y.; Su, Z.-Q.; Zheng, G.-J.; Feng, B. Renoprotective effect of scutellarin on cisplatin-induced renal injury in mice: Impact on inflammation, apoptosis, and autophagy. Biomed. Pharmacother. 2019, 112, 108647. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, F.; Ebrahim, H.S.; Akbar, V. Investigating on Effect of Wormwood Extract on Reduction of Renal Toxicity in Treated Rats by Azathioprine. Biomed. Pharmacol. J. 2015, 8, 291–299. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Horváth, B.; Zsengellér, Z.; Zielonka, J.; Tanchian, G.; Holovac, E.; Kechrid, M.; Patel, V.; Stillman, I.E.; Parikh, S.M.; et al. Mitochondrial-targeted antioxidants represent a promising approach for prevention of cisplatin-induced nephropathy. Free Radic. Biol. Med. 2012, 52, 497–506. [Google Scholar] [CrossRef]
- Karasawa, T.; Steyger, P.S. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol. Lett. 2015, 237, 219–227. [Google Scholar] [CrossRef]
- Hwang, B.S.; Lee, D.; Choi, P.; Kim, K.S.; Choi, S.-J.; Song, B.G.; Kim, T.; Song, J.H.; Kang, K.S.; Ham, J. Renoprotective Effects of Hypoxylonol C and F Isolated from Hypoxylon truncatum against Cisplatin-Induced Cytotoxicity in LLC-PK1 Cells. Int. J. Mol. Sci. 2018, 19, 948. [Google Scholar] [CrossRef]
- Jung, K.; Lee, D.; Yu, J.S.; Namgung, H.; Kang, K.S.; Kim, K.H. Protective effect and mechanism of action of saponins isolated from the seeds of gac (Momordica cochinchinensis Spreng.) against cisplatin-induced damage in LLC-PK1 kidney cells. Bioorgan. Med. Chem. Lett. 2016, 26, 1466–1470. [Google Scholar] [CrossRef]
- Lee, D.; Lee, S.R.; Kang, K.S.; Kim, K.H. Benzyl salicylate from the stems and stem barks of Cornus walteri as a nephroprotective agent against cisplatin-induced apoptotic cell death in LLC-PK1 cells. RSC Adv. 2020, 10, 5777–5784. [Google Scholar] [CrossRef]
- Volarevic, V.; Djokovic, B.; Jankovic, M.G.; Harrell, C.R.; Fellabaum, C.; Djonov, V.; Arsenijevic, N. Molecular mechanisms of cisplatin-induced nephrotoxicity: A balance on the knife edge between renoprotection and tumor toxicity. J. Biomed. Sci. 2019, 26, 1–14. [Google Scholar] [CrossRef]
- Dickey, D.T.; Muldoon, L.L.; Doolittle, N.D.; Peterson, D.R.; Kraemer, D.F.; Neuwelt, E.A. Effect of N-acetylcysteine route of administration on chemoprotection against cisplatin-induced toxicity in rat models. Cancer Chemother. Pharmacol. 2008, 62, 235–241. [Google Scholar] [CrossRef]
- Sooriyaarachchi, M.; Narendran, A.; Gailer, J. N-Acetyl-l-cysteine modulates the metabolism of cis-platin in human plasma in vitro. Metallomics 2013, 5, 197. [Google Scholar] [CrossRef] [PubMed]
- Emir, S.; Özyörük, D.; Arman, Ö.; Özbek, N.; Tunç, B. Accidental cisplatin overdose in a child: Successful management with repetitive plasmapheresis and use of chemoprotective agents. Turk. J. Pediatr. 2016, 58, 315. [Google Scholar] [CrossRef]
- Daradka, H.M.; Abas, M.M.; Mohammad, M.A.M.; Jaffar, M.M. Antidiabetic effect of Artemisia absinthium extracts on alloxan-induced diabetic rats. Comp. Haematol. Int. 2014, 23, 1733–1742. [Google Scholar] [CrossRef]
- Kharoubi, O.; Slimani, M.; Aoues, A.; Seddik, L. Prophylactic effects of wormwood on lipid peroxidation in an animal model of lead intoxication. Indian J. Nephrol. 2008, 18, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Rahman, K.; Jahan, N.; Jamil, A.; Rashid, A.; Shah, S.M.A. Protection of DNA during oxidative stress and cytotoxic potential of Artemisia absinthium. Pak. J. Pharm. Sci. 2016, 29, 295–299. [Google Scholar]
- Ummat, A.; Rechkoblit, O.; Jain, R.; Choudhury, J.R.; Johnson, R.E.; Silverstein, T.D.; Buku, A.; Lone, S.; Prakash, L.; Prakash, S.; et al. Structural basis for cisplatin DNA damage tolerance by human polymerase η during cancer chemotherapy. Nat. Struct. Mol. Biol. 2012, 19, 628–632. [Google Scholar] [CrossRef]
- Hu, J.; Lieb, J.D.; Sancar, A.; Adar, S. Cisplatin DNA damage and repair maps of the human genome at single-nucleotide resolution. Proc. Natl. Acad. Sci. USA 2016, 113, 11507–11512. [Google Scholar] [CrossRef]
- Kamali, H.; Mohammadi, A.; Sani, T.A.; Ameri, A.A.; Imani, M.; Golmakani, E. Seasonal variation in the chemical composition, antioxidant activity, and total phenolic content of Artemisia absinthium essential oils. Pharmacogn. Res. 2015, 7, 329–334. [Google Scholar] [CrossRef]
- Bora, K.S.; Sharma, A. Evaluation of antioxidant and free-radical scavenging potential of Artemisia absinthium. Pharm. Biol. 2011, 49, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Shafi, G.; Hasan, T.N.; Syed, N.A.; Al-Hazzani, A.A.; Alshatwi, A.A.; Jyothi, A.; Munshi, A. Artemisia absinthium (AA): A novel potential complementary and alternative medicine for breast cancer. Mol. Biol. Rep. 2012, 39, 7373–7379. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, I. Evaluation of anticancer, antioxidant activity and phenolic compounds of Artemisia absinthium L. Extract. Cell. Mol. Biol. 2018, 64, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, M.; Mirzaie-Asl, A.; Saidijam, M.; Moradi, M. Methanolic extract of Artemisia absinthium prompts apoptosis, enhancing expression of Bax/Bcl-2 ratio, cell cycle arrest, caspase-3 activation and mitochondrial membrane potential destruction in human colorectal cancer HCT-116 cells. Mol. Biol. Rep. 2020, 47, 8831–8840. [Google Scholar] [CrossRef]
- Sultan, M.H.; Zuwaiel, A.A.; Sivakumar, S.; Alshahrani, S.; Alqahtani, S.S.; Madkhali, O.; Elmobark, M.E. Bioactive principles and potentiality of hot methanolic extract of the leaves from Artemisia absinthium L “in vitro cytotoxicity against human MCF-7 breast cancer cells, antibacterial study and wound healing activity. Curr. Pharm. Biotechnol. 2020, 21, 1–13. [Google Scholar] [CrossRef]
- Ali, M.; Abbasi, B.H. Thidiazuron-Induced Changes in Biomass Parameters, Total Phenolic Content, and Antioxidant Activity in Callus Cultures of Artemisia absinthium L. Appl. Biochem. Biotechnol. 2013, 172, 2363–2376. [Google Scholar] [CrossRef]
- Seigler, D.S. Plant Secondary Metabolism; Springer: Berlin/Heidelberg, Germany, 2012; pp. 94–105. [Google Scholar]
- Saltveit, M.E. Synthesis and Metabolism of Phenolic Compounds. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; Volume 115. [Google Scholar]
- Bochkov, D.V.; Sysolyatin, S.V.; Kalashnikov, A.I.; Surmacheva, I.A. Shikimic acid: Review of its analytical, isolation, and purification techniques from plant and microbial sources. J. Chem. Biol. 2012, 5, 5–17. [Google Scholar] [CrossRef]
- Al-Malki, A.L. Shikimic acid from Artemisia absinthium inhibits protein glycation in diabetic rats. Int. J. Biol. Macromol. 2019, 122, 1212–1216. [Google Scholar] [CrossRef]
- Nieskens, T.T.G.; Peters, J.G.P.; Dabaghie, D.; Korte, D.; Jansen, K.; Van Asbeck, A.H.; Tavraz, N.N.; Friedrich, T.; Russel, F.G.M.; Masereeuw, R.; et al. Expression of Organic Anion Transporter 1 or 3 in Human Kidney Proximal Tubule Cells Reduces Cisplatin Sensitivity. Drug Metab. Dispos. 2018, 46, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Manna, K.; Khan, A.; Das, D.K.; Kesh, S.B.; Das, U.; Ghosh, S.; Dey, R.S.; Saha, K.D.; Chakraborty, A.; Chattopadhyay, S. Protective effect of coconut water concentrate and its active component shikimic acid against hydroperoxide mediated oxidative stress through suppression of NF-κB and activation of Nrf2 pathway. J. Ethnopharmacol. 2014, 155, 132–146. [Google Scholar] [CrossRef]
- Güntürk, I.; Yazici, C.; Köse, S.K.; Dağli, F.; Yücel, B.; Yay, A.H. The effect of N-acetylcysteine on inflammation and oxidative stress in cisplatin induced nephrotoxicity: A rat model. Turk. J. Med Sci. 2019, 49, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Jing, T.; Liao, J.; Shen, K.; Chen, X.; Xu, Z.; Tian, W.; Wang, Y.; Jin, B.; Pan, H. Protective effect of urolithin a on cisplatin-induced nephrotoxicity in mice via modulation of inflammation and oxidative stress. Food Chem. Toxicol. 2019, 129, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Wei, W.; Fan, X.; Ci, X. Farrerol Attenuates Cisplatin-Induced Nephrotoxicity by Inhibiting the Reactive Oxygen Species-Mediated Oxidation, Inflammation, and Apoptotic Signaling Pathways. Front. Physiol. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Deniz, G.Y.; Laloglu, E.; Altun, S.; Yiğit, N.; Gezer, A. Antioxidant and anti-apoptotic effects of vitexilactone on cisplatin-induced nephrotoxicity in rats. Biotech. Histochem. 2020, 95, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.; Mansourian, M.; Panahi kokhdan, E.; Salehpour, Z.; Sadati, I.; Abbaszadeh-Goudarzi, K.; Asfaram, A.; Doustimotlagh, A.H. Antioxidant and protective effect of Stachys pilifera Benth against nephrotoxicity induced by cisplatin in rats. J. Food Biochem. 2020, 44, e13190. [Google Scholar] [CrossRef]
- Rabelo, T.K.; Zeidán-Chuliá, F.; Caregnato, F.F.; Schnorr, C.E.; Gasparotto, J.; Serafini, M.R.; Araújo, A.A.D.S.; Quintans, J.D.S.S.; Moreira, J.C.F.; Gelain, D.P. In Vitro Neuroprotective Effect of Shikimic Acid Against Hydrogen Peroxide-Induced Oxidative Stress. J. Mol. Neurosci. 2015, 56, 956–965. [Google Scholar] [CrossRef]
- Lieberthal, W.; Triaca, V.; Levine, J. Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: Apoptosis vs. necrosis. Am. J. Physiol. Physiol. 1996, 270, F700–F708. [Google Scholar] [CrossRef]
- Topcu-Tarladacalisir, Y.; Sapmaz-Metin, M.; Karaca, T. Curcumin counteracts cisplatin-induced nephrotoxicity by preventing renal tubular cell apoptosis. Ren. Fail. 2016, 38, 1741–1748. [Google Scholar] [CrossRef]
- Lee, R.H.; Song, J.M.; Park, M.Y.; Kang, S.K.; Kim, Y.K.; Jung, J.S. Cisplatin-induced apoptosis by translocation of endogenous Bax in mouse collecting duct. Biochem. Pharmacol. 2001, 62, 1013–1023. [Google Scholar] [CrossRef]
- Tang, L.-L.; Ye, J.-Y.; Jiang, S.-N.; Zheng, J.-S. 3,4-oxo-isopropylidene-shikimic acid inhibits cerebral ischemia-induced oxidative stress and neuronal apoptosis in rats. Am. J. Transl. Res. 2017, 9, 1764–1773. [Google Scholar]
- Chen, X.; Li, X.; Zhai, X.; Zhi, X.; Cao, L.; Qin, L.; Su, J. Shikimic Acid Inhibits Osteoclastogenesis In Vivo and In Vitro by Blocking RANK/TRAF6 Association and Suppressing NF-kappaB and MAPK Signaling Pathways. Cell Physiol. Biochem. 2018, 51, 2858–2871. [Google Scholar] [CrossRef] [PubMed]
- McMahon, K.R.; Rassekh, S.R.; Schultz, K.R.; Blydt-Hansen, T.; Cuvelier, G.D.E.; Mammen, C.; Pinsk, M.; Carleton, B.C.; Tsuyuki, R.T.; Ross, C.J.D.; et al. Epidemiologic Characteristics of Acute Kidney Injury During Cisplatin Infusions in Children Treated for Cancer. JAMA Netw. Open 2020, 3, e203639. [Google Scholar] [CrossRef]
- McMahon, K.R.; Harel-Sterling, M.; Pizzi, M.; Huynh, L.; Hessey, E.; Zappitelli, M. Long-term renal follow-up of children treated with cisplatin, carboplatin, or ifosfamide: A pilot study. Pediatr. Nephrol. 2018, 33, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Holditch, S.J.; Brown, C.N.; Lombardi, A.M.; Nguyen, K.N.; Edelstein, C.L. Recent Advances in Models, Mechanisms, Biomarkers, and Interventions in Cisplatin-Induced Acute Kidney Injury. Int. J. Mol. Sci. 2019, 20, 3011. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, P.A.; Viswanatha, G.L.; Radheshyam, N.; Mukund, H.; Belliyappa, M.S. Science behind cisplatin-induced nephrotoxicity in humans: A clinical study. Asian Pac. J. Trop. Biomed. 2012, 2, 640–644. [Google Scholar] [CrossRef]
- Geyikoglu, F.; Emir, M.; Colak, S.; Koc, K.; Turkez, H.; Bakır, M.; Hosseinigouzdagani, M.; Cerig, S.; Keles, O.N.; Ozek, N.S.; et al. Effect of oleuropein against chemotherapy drug-induced histological changes, oxidative stress, and DNA damages in rat kidney injury. J. Food Drug Anal. 2017, 25, 447–459. [Google Scholar] [CrossRef]
- Saito, Y.; Okamoto, K.; Kobayashi, M.; Narumi, K.; Furugen, A.; Yamada, T.; Iseki, K. Magnesium co-administration decreases cisplatin-induced nephrotoxicity in the multiple cisplatin administration. Life Sci. 2017, 189, 18–22. [Google Scholar] [CrossRef]
- Won, A.J.; Kim, S.; Kim, Y.G.; Kim, K.-B.; Choi, W.S.; Kacew, S.; Kim, K.S.; Jung, J.H.; Lee, B.M.; Kim, S.; et al. Discovery of urinary metabolomic biomarkers for early detection of acute kidney injury. Mol. BioSyst. 2016, 12, 133–144. [Google Scholar] [CrossRef]
- Tamura, Y.; Ikeda, O.; Nakasone, Y.; Iryo, Y.; Yamashita, Y. Effect of sodium thiosulfate on cisplatin removal after intra-arterial embolization with a lipiodol-platinum suspension for hepatocellular carcinoma. Acta Radiol. 2010, 51, 383–388. [Google Scholar] [CrossRef]
- Farrell, N.; Roberts, J.D.; Hacker, M.P. Shikimic acid complexes of platinum. Preparation, reactivity, and antitumor activity of (R,R-1,2-diaminocyclohexane) bis(shikimato)platinum(II). Evidence for a novel rearrangement involving platinum-carbon bond formation. J. Inorg. Biochem. 1991, 42, 237–246. [Google Scholar] [CrossRef]
- Lee, D.; Lee, D.S.; Jung, K.; Hwang, G.S.; Lee, H.L.; Yamabe, N.; Lee, H.J.; Eom, D.W.; Kim, K.H.; Kang, K.S. Protective effect of ginsenoside Rb1 against tacrolimus-induced apoptosis in renal proximal tubular LLC-PK1 cells. J. Ginseng Res. 2018, 42, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Ju, S.; Song, H. Dendropanax morbifera extract protects cardiomyocytes against hypoxia/reoxygenation injury by inhibition of reactive oxygen species generation and calcium perturbation. Nat. Prod. Sci. 2019, 25, 136–142. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, D.W.; Jung, B.H.; Lee, J.H.; Lee, H.; Hwang, G.S.; Kang, K.S.; Lee, J.W. Ginsenoside Rb2 suppresses the glutamate-mediated oxidative stress and neuronal cell death in HT22 cells. J. Ginseng Res. 2019, 43, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.A.; Park, E.-J.; Lee, D.; Song, J.H.; Lee, H.L.; Kim, K.H.; Kim, Y.; Jung, K.; Kang, K.S.; Yoo, J.-E. Estrogenic activity of sanguiin H-6 through activation of estrogen receptor α coactivator-binding site. Nat. Prod. Sci. 2019, 25, 28–33. [Google Scholar] [CrossRef][Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Nguyen, Q.N.; Park, J.Y.; Lee, S.; Hwang, G.S.; Yamabe, N.; Choi, S.; Kang, K.S. Protective Effect of Shikimic Acid against Cisplatin-Induced Renal Injury: In Vitro and In Vivo Studies. Plants 2020, 9, 1681. https://doi.org/10.3390/plants9121681
Lee J, Nguyen QN, Park JY, Lee S, Hwang GS, Yamabe N, Choi S, Kang KS. Protective Effect of Shikimic Acid against Cisplatin-Induced Renal Injury: In Vitro and In Vivo Studies. Plants. 2020; 9(12):1681. https://doi.org/10.3390/plants9121681
Chicago/Turabian StyleLee, Jinkyung, Quynh Nhu Nguyen, Jun Yeon Park, Sullim Lee, Gwi Seo Hwang, Noriko Yamabe, Sungyoul Choi, and Ki Sung Kang. 2020. "Protective Effect of Shikimic Acid against Cisplatin-Induced Renal Injury: In Vitro and In Vivo Studies" Plants 9, no. 12: 1681. https://doi.org/10.3390/plants9121681
APA StyleLee, J., Nguyen, Q. N., Park, J. Y., Lee, S., Hwang, G. S., Yamabe, N., Choi, S., & Kang, K. S. (2020). Protective Effect of Shikimic Acid against Cisplatin-Induced Renal Injury: In Vitro and In Vivo Studies. Plants, 9(12), 1681. https://doi.org/10.3390/plants9121681