1. Introduction
Melanin is a mixture of pigmented biopolymers synthesized by specialized cells determinant of skin, hair and eye color. Although pigmentation play a crucial photo-protective role against the carcinogenetic effects of ultra violet (UV) irradiation through direct UV absorption, excessive production of melanin causes hyperpigmentary skin disorders, including melisma, Riehl melanosis, erythromelanosis, follicularis faciei, poikiloderma of civatte, porphyria cutanea and melanoma [
1]. Melanin biosynthesis in melanocytes is a complex process involved many factors, including UV irradiation, stem cell factors (SCF), α-melanocyte stimulating hormone (α-MSH), cAMP and transcription factors [
2]. For example, α-MSH binds to melanocortin receptor-1 (MC1R), a member of G-protein receptors in melanocytes induces the activation of adenylyl cyclase enzyme, followed by increased cyclic adenosine monophosphate (cAMP) production. cAMP leads to transcriptional activation of cAMP response element-binding protein (CREB), which in turn stimulate microphthalmia transcription factor (MITF) promoter activity [
3]. MITF, a basic leucine zipper transcription factor directly binds to the promoter regions of melanin production genes and positively regulates their genes, including tyrosinase (
TYR), tyrosinase related protein-1 (
TRP-1) and dopachrome tautomerase (
DCT) [
3]. Among these enzymes, tyrosinase is the rate-limiting enzyme and catalyzes the hydroxylation of tyrosinase to
L-3,4-dihydroxyphenylalanine (
L-DOPA) and the oxidation of
L-DOPA to dopaquinone. DCT and TRP-1 were involved in conversion of DOPA-chrome into 5,6-dihydroxyindole-2-carboxilic acid (DHICA) and DHICA into eumelanin, respectively. There are several factors negatively regulate MITF activity in melanocytes. It was reported that the inhibition of the ERK signaling pathway induces melanoma cell proliferation and MITF activity in vitro suggesting that the ERK signaling pathway negatively regulate MITF-mediated melanogenesis [
4]. Further studies have revealed that ERK phosphorylates MITF at serine 73 and that the phosphorylation of MITF at serine 73 is responsible for MITF ubiquitination and degradation [
5]. Likewise, inhibition of phosphoinositide 3-kinase (PI3K) increases MITF transcriptional activity, leading to increase tyrosinase and TRP-1 expression and melanogenesis [
6]. However, UV-irradiation-induced p38 MAPK activation has been shown to be involved in melanogenesis through the increase of MITF transcriptional activity and subsequent increase of tyrosinase [
7].
Zingiberaceae is one of the largest dietary/spice families in the plant kingdom, which comprises 53 genera along with 1600 know species. Among them genus
Alpinia is the largest genus in Zingiberaceae with about 230 species, which are widely distributed in tropical and sub-tropical regions of Asia, Australia and the Pacific Islands [
8].
Alpinia, also popularly known as “shell ginger” plants were economically important and used as food, food adjunct, spices, folk medicine and cultivated ornamentals [
9].
A. nantoensis F.Y. Lu & Y.W. Kuo is a newly identified species, which is native to the mountain regions of Taiwan. Previously,
A. nantoensis was recognized as
A. pricei due to its identical leaf size and flowers to those of
A. pricei. However, its flowers has a bracteole and an oblong labellum, which differs from its congeneric native species of
A. pricei, which has no bracteole and has a rhombic labellum (Kuo et al., 2008). Traditionally, the leaves of
A. pricei were used to wrap zongzi (a glutinous rice dumplings) and the aromatic rhizome was used to treat abdominal discomfort and to increase stomach secretion and peristalsis (Tsao et al., 2019). While, leaves and rhizomes of
A. zerumbet traditionally used for skin care and insect repellent (Chompoo et al., 2012). Since, there undistinguishable similarity between
A. pricei and
A. nantoensis, the leaves
A. nantoensis was used to prepare zongzi and the rhizomes were used for traditional Chinese medicine preparation [
10,
11]. We recently reported that leaf, stem and rhizome extracts of
A. nantoensis exhibited strong anti-metastatic properties in human breast cancer cells [
11]. Another study also shown that
trans-3-methoxy-5-hydroxystilbene isolated from the rhizome of
A. nantoensis inhibited lung cancer cell metastasis in vitro [
10]. However, other biological activities of this newly identified species was poorly investigated. Therefore, in the present study, we aimed to investigate the anti-melanogenic properties of essential oils obtained from leaf and rhizomes of
A. nantoensis.
3. Discussion
Several synthetic agents are use in the cosmetic industry as skin whitening agents. Most of these agents are direct tyrosinase inhibitors but the development of natural agents is becoming more important due to the disadvantages of synthetics such as high cytotoxicity, insufficient penetrating power and low activity. For example, kojic acid, a synthetic tyrosinase inhibitor is widely used as a skin-whitening agent in cosmetics, later it was reported to cause several serious side effects, including erythema and contact dermatitis [
16]. Therefore, the identification of safe, bio-combatable as well as natural derived skin-whitening agents represents the hour of need.
Essential oils has been widely used in the preparation of pharmaceutical and cosmetic products as they offer broad-spectrum of health benefits as well as preservatives. Their biological activities are ranging from anti-bacterial, anti-fungal, anti-viral, anti-septic, analgesic, anti-inflammatory and dermato-protection [
17]. In addition to their putative bioactivities, their unique pleasant aroma enables to use in cosmetic products. Currently, essential oils are one of the subjects of intensive scientific research and also received much attention from cosmetic and pharmaceutical industries. Particularly, natural product like essential oils were reported to have direct tyrosinase inhibition, which enable them to use as skin-lightening agents [
18].
Alpinia plants are generally aromatic due to their rich content of essential oils. Previous studies have reported that aqueous extract of rhizome of
A. officinarum and fruit extracts of
A. galangal exhibited strong anti-melanogenic activity in cultured melanoma cells [
19,
20]. Therefore, we hypothesis that the essential oils of
A. nantoensis may possess anti-melanogenic properties.
Melanin producing murine melanoma B16-F10 cell line is widely used to investigate anti-melanogenic properties of synthetic or natural agents. There are several agents induce melanogenesis in melanoma cells such as α-MSH, β-MSH, 3-isobutyl-1-methylxanthine (IBMX), forskolin (FRK), α-lipotropin (β-LPH), β-endorphin (β-END) and so forth. Among them, α-MSH, β-MSH, IBMX and forskolin (FRK) were recognized as cAMP activators, which trigger melanogenesis [
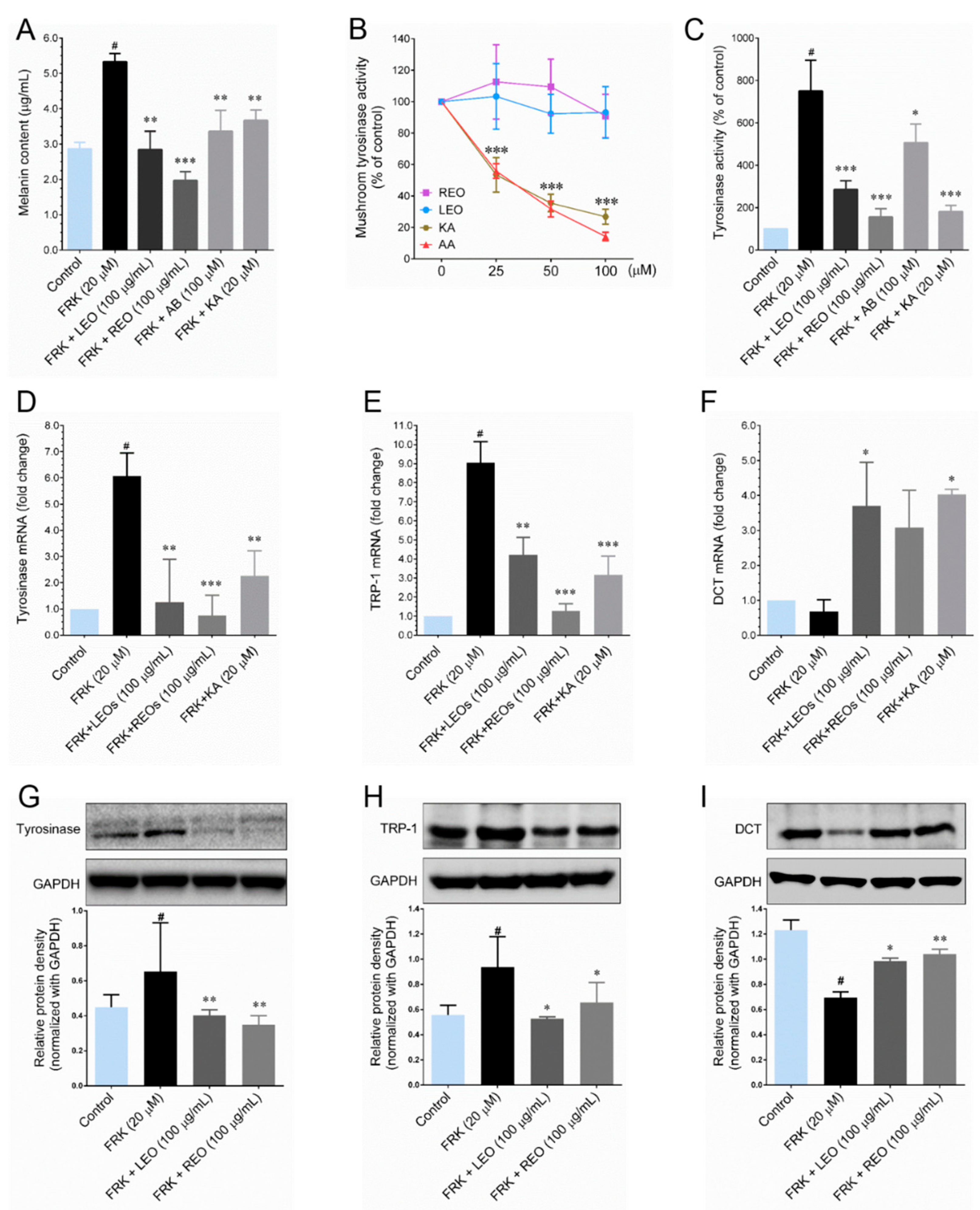
21]. In the present study, we subjected FRK to induce melanin production in B16-F10 cells and determined the inhibitory effect of LEO and REO. Our results showed that co-treatment with LEO and REO significantly inhibited FRK-induced melanin production. Melanin biosynthesis is critically regulated by melanogenic enzymes, including tyrosinase, TRP-1 and DCT [
21]. Therefore, we sought to determine whether LEO and REO could modulate tyrosinase enzyme activity. Since, the mushroom tyrosinase inhibitory assay is a widely used tool for determining the skin whitening effect of candidate agents in cell-free system, because tyrosinase is the limiting enzyme in melanin formation in skin. Utilizing this assay, we determined the tyrosinase inhibitory effect of LEO and REO where
L-DOPA was used as substrate. We found that either LEO or REO failed to inhibit mushroom tyrosinase activity in cell-free system. Indeed, treatment with LEO and REO exhibited strong cellular tyrosinase activity in FRK-stimulated cells, which is consistent with other’s observation that several herbal extracts inhibits cellular tyrosinase activity without affecting mushroom tyrosinase activity [
22]. Our results show that LEO and REO down-regulated the expression levels of TYR and TRP-1 possibility by suppressing MITF transcriptional activity. In contrast, a reduced expression of DCT was observed in FRK-treated cells, while LEO and REO significantly provoked FRK-mediated decrease of DCT. It has been explained by transient transfection assays that MITF isoforms regulate the transcription of DCT gene in a different manner as those of TYR and TRP-1 [
23]. Thus, we speculate that LEO/REO may regulate the expression of
DCT gene in a different manner then that of
TYR and
TRP-1 genes, suggesting that the anti-melanogenic effect driven by LEO and REO are mediated via TYR and TRP-1, since TYR is necessary for melanogenesis as the critical rate-limiting enzyme.
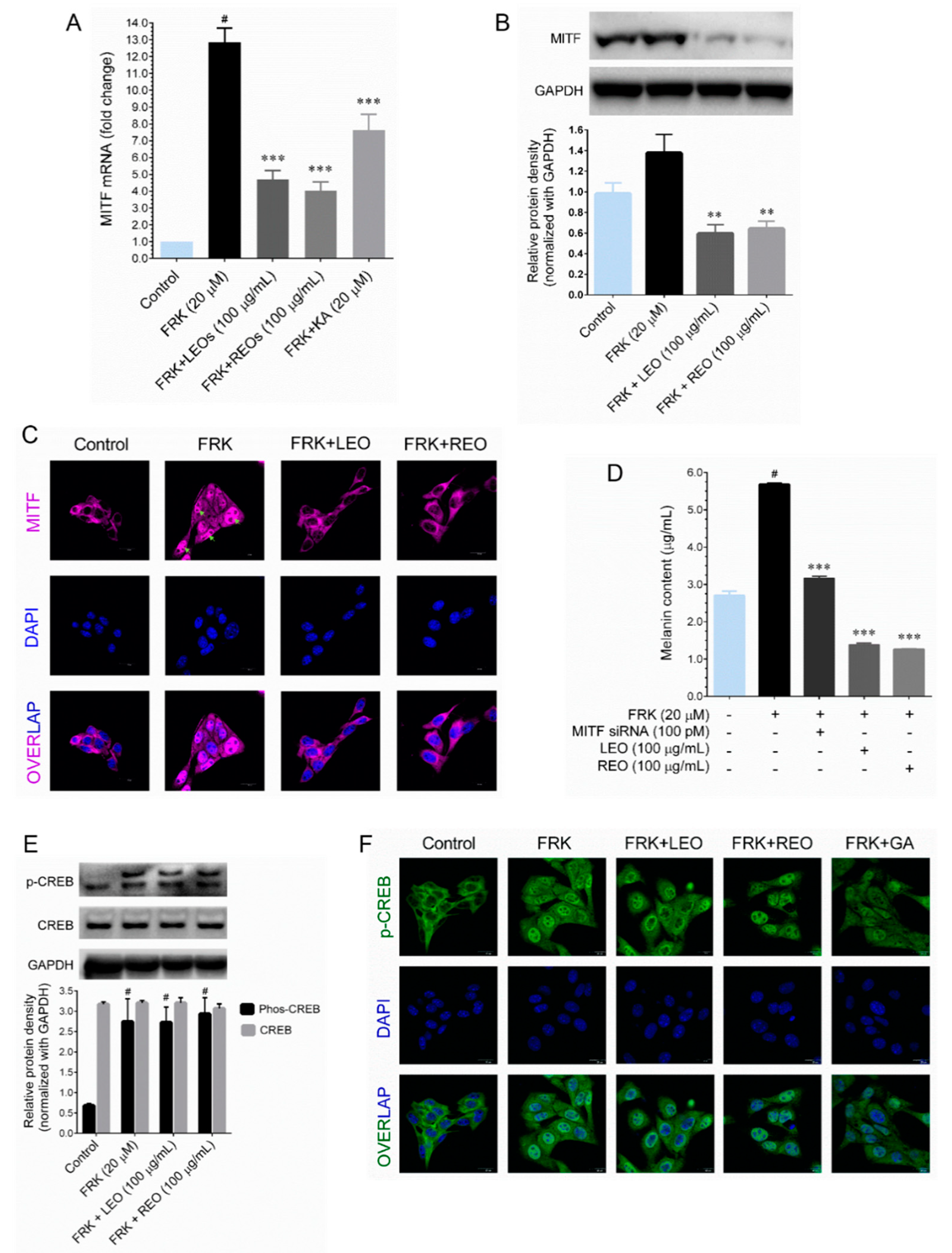
During the melanogenesis, MITF is regulated at both transcriptionally and post-translationally. At the transcription level, MITF is regulated by CREB, a cAMP-dependent transcription factor transcribed MITF upon stimulation [
2]. We found that LEO and REO treatment significantly down-regulated MITF protein and mRNA expression in FRK-stimulated cells, which allowed us to determine the involvement of CREB in MITF regulation. Treatment with either LEO or REO does not modulate the FRK-induced CREB phosphorylation as well as nuclear translocation. Therefore, we hypothesis that LEO/REO regulate MITF at post-translationally. In such conditions, MITF may undergo various post-translational modifications, including phosphorylation, sumoylation and ubiquitination [
2]. A previous study reported that most melanoma cells contain hyper-activated MAPKs, which triggers MITF phosphorylation at serine 73, leads ubiquitin-mediated proteasomal degradation [
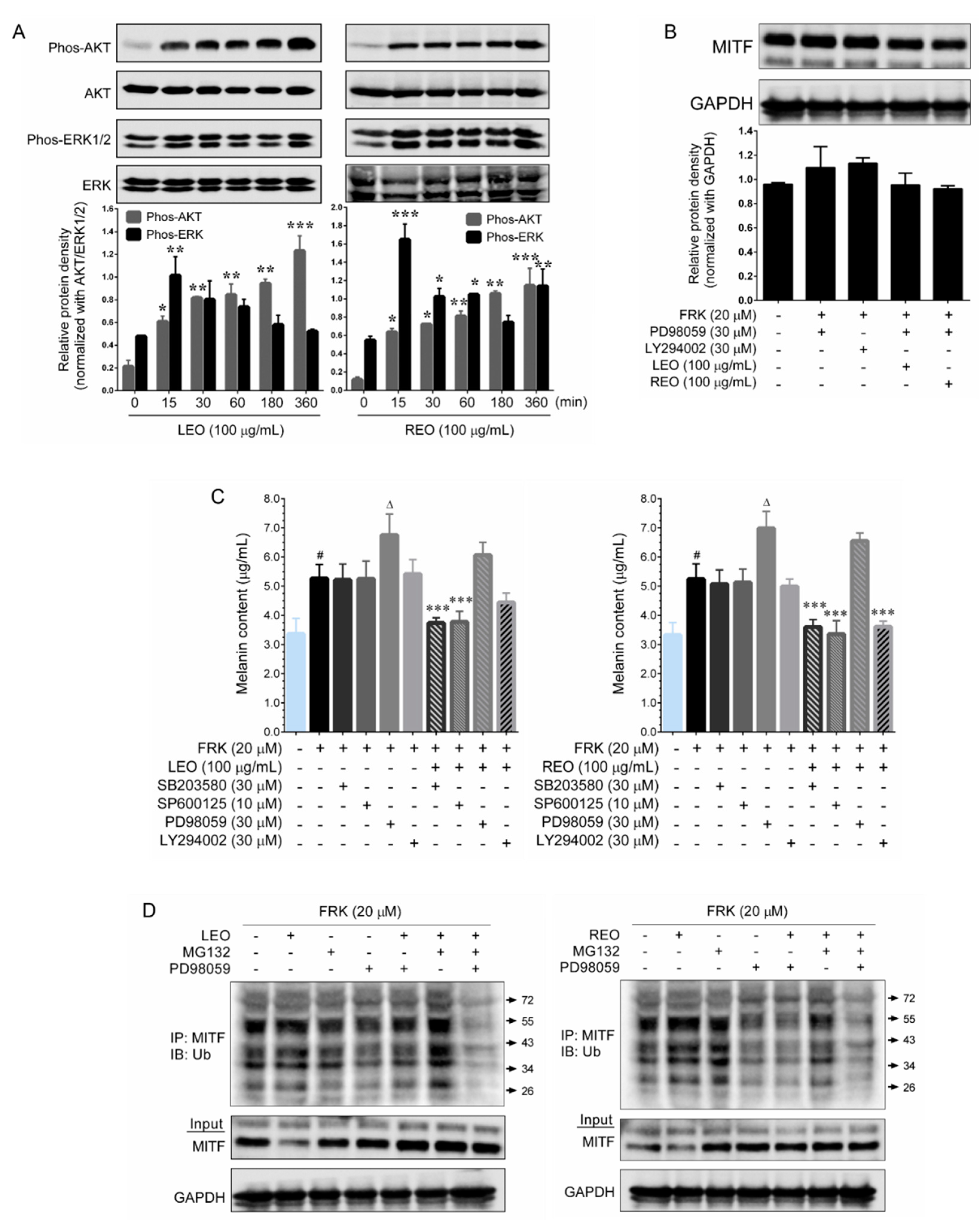
5]. To validate our hypothesis, cells were pre-treated with pharmacological inhibitors of p38 MAPK, ERK1/2, JNK/SAPK and AKT and then exposed to FRK in the presence or absence of LEO and REO and the melanin production was determined. Interestingly, treatment with LEO and REO failed to inhibit FRK-induced melanin production in ERK1/2 inhibitor treated cells, while significant reduction in melanin production were observed in p38 MAPK, JNK/SAPK and AKT inhibitors treated cells. This data is consistent with a significant increase of ERK1/2 phosphorylation by LEO and REO. In this study, we also noted a significant and time-dependent increase of AKT phosphorylation by LEO and REO. It has been well characterized that PI3K/AKT pathway plays a key role in cellular metabolism, proliferation, survival and chemo-resistance [
24]. Therefore, we speculate that the LEO and REO-mediated AKT phosphorylation may involve in cellular metabolism or survival and it is very clear that LEO/REO-mediated AKT activation does not have any functional role in MITF regulation. Furthermore, we found that treatment with LEO/REO, ERK1/2 inhibitor (PD98059) and proteasome inhibitor (MG132) were accelerated MITF degradation via ubiquitin-proteasome system. A similar anti-melanogenic mechanism was also reported by other researchers [
25].
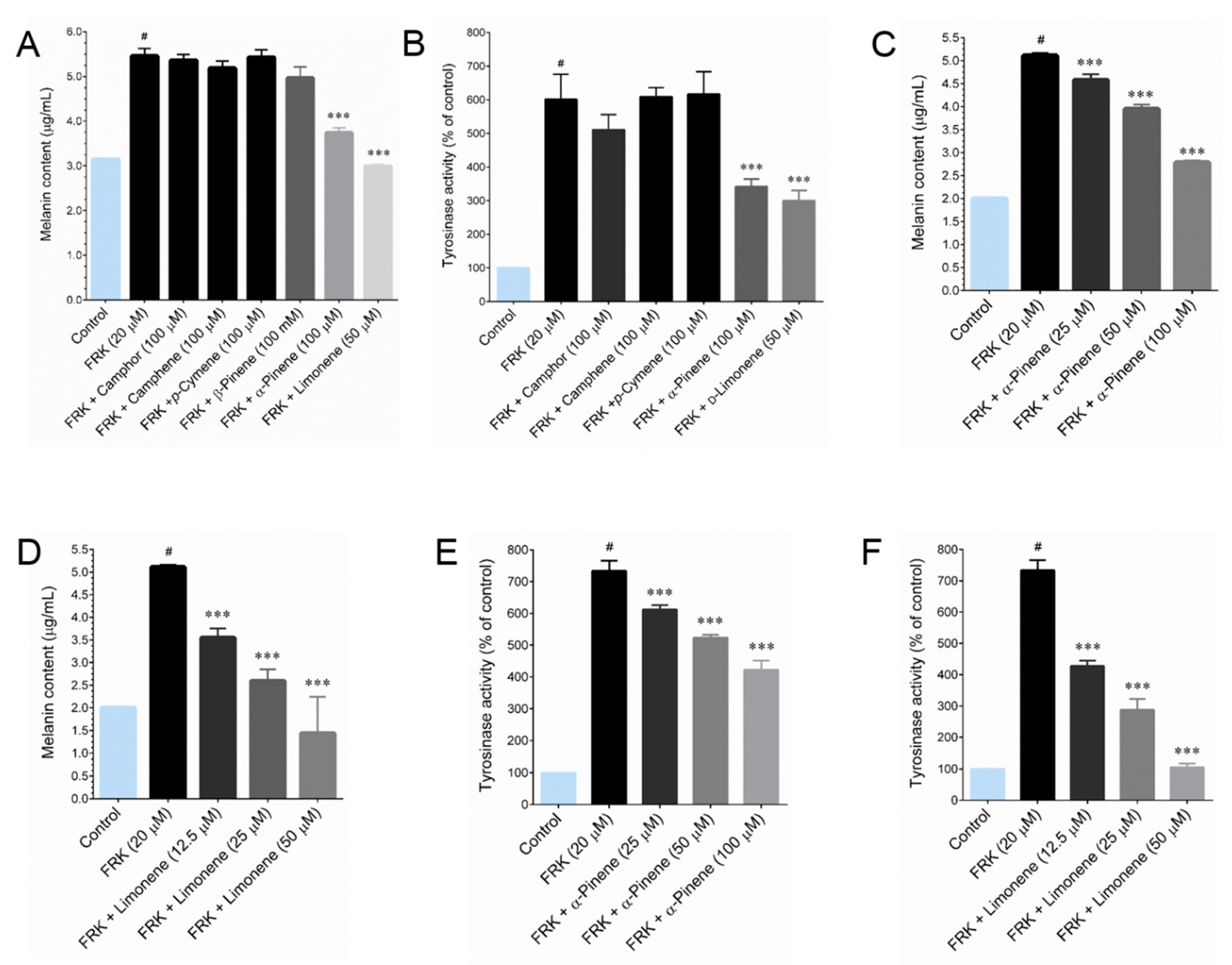
In the present study, we also investigated the chemical composition and their melanin inhibitory effect in vitro. GC-MS analyses resulted with 18 identified compound in both LEO and REO, most of these constituents are monoterpenoids and sesquiterpenoids. LEO and REO exhibited the most identical compositions, among the 18 identified compounds 12 compounds are present in both essential oils. That may be a reason for the similar effects observed in both essential oils. Further studies revealed that the major compounds, that is, camphor, camphene and
p-cymene were not effect in the context of melanin inhibition, which is in agreements with a previous study that camphor isolated from the essential oil of
Achillea millefolium does not showed significant melanin inhibition up to a concentration of 400 μM [
26]. Indeed, the minor compounds in LEO and REO, α-pinene and
D-limonene, exhibited strong melanin inhibition. A previous study support our notion that α-pinene is a potential anti-melanogenic agent [
27]. However, to the best of our knowledge, there is no specific record exist on the anti-melanogenic action of
D-limonene, which resulted in as a potent melanin inhibitor then that of α-pinene.
4. Materials and Methods
4.1. A. nantoensis and Essential Oil Preparation
A. nantonensis was collected in March 2019 from Nantou County, Taiwan and was identified by Prof Yen- Hsueh Tseng (Department of Forestry, National Chung Hsing University, Taichung, Taiwan). The voucher specimen (TCF Tseng4590) was deposited in the herbarium of the same university. Air-dried rhizome and leaves of A. nantonensis were subjected to hydrodistillation in a Clevenger-type apparatus for 6 h, followed by determination of oil contents. Leaf (LEO) or rhizome (REO) essential oils were stored in airtight sample vial prior to analysis by gas chromatography–mass spectrometry (GC–MS) and bioactivity evaluation.
4.2. Chemicals and Reagents
Roswell Park Memorial Institute
(RPMI) 1640 medium, fetal bovine serum (FBS), penicillin and streptomycin were obtained from Life Technologies, Grand Island, NY. Melanin, 2’,7’-dichlorofluorescein diacetate (DCFH
2-DA), 4′-6-diamidino-2-phenylindole (DAPI), tyrosinase (EC 1.14.18.1, activity of 6680 units/mg) and 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St Louis, CA, USA). Specific pharmacological inhibitors for ERK1/2 (PD98059), p38 MAPK (SB203580), JNK/SAPK (SP600125) and PI3K/AKT (LY294002) were obtained from Calbiochem (La Jolla, CA, USA). Forskolin was obtained from Selleckchem (Houston, TX, USA). Primary and secondary antibodies used in this study were listed in
Supplementary Table S1. All other chemicals were reagent grade or HPLC grade and supplied by either Merck (Darmstadt, Germany) or Sigma-Aldrich.
4.3. Cell Culture and Cell Viability Assay
Murine melanoma (B16-F10), human skin fibroblast (CCD966SK) and human skin keratinocytes (HaCaT) cell lines were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Human epidermal melanocytes-adult (HEM-a) was purchased from ScienCell Research Laboratories, Caralsbad, CA, USA. Cells were cultured in Dulbecco’s Modified Eagle medium (DMEM) or Roswell Park Memorial Institute (RPMI) medium or melanocyte medium (MelM), supplemented with 10% fetal bovine serum (FBS), glucose, penicillin and streptomycin. Cells were grown in 10 cm culture dish and incubated in a humidified atmosphere containing 5% CO
2 at 37 °C. Cell viability was assessed by MTT colorimetric assay as described previously [
28].
4.4. Determination of Melanin Content and Tyrosinase Activity
Melanin content and tyrosinase activity was determined as described previously [
29]. Briefly, B16F10 cells were seeded in 10 cm cell culture dish at a density of 5 × 10
5 cells/dish. The 50% confluent cells were treated with forskolin (20 μM) in the presence or absence of LEO (25–100 μg/mL) or REO (25–100 μg/mL) or arbutin (100 μM) or kojic acid (20 μM) for 48 h. After treatment, the cells were harvested and washed twice with PBS and the intercellular melanin was solubilized in 1 N NaOH. The melanin content was determined by measuring the absorbance at 475 nm using an enzyme-linked immunosorbent assay (ELISA) micro-plate reader. On the other hand, cells were treated with similar condition for 48 h. The cultured cells were lysed using lysis buffer and clarified by centrifugation at 11,000×
g for 10 min. 90 µL of each lysate containing an equal amount of protein (100 µg) was placed into a 96-well plate and 10 µL of 15 mM
L-DOPA was added per well. After incubation at 37 °C for 20 min, the dopachrome formation was measured at 475 nm using an ELISA micro-plate reader. Mushroom tyrosinase activity was determined by ELISA micro-plate reader as described previously [
29].
4.5. RNA Extraction and Q-PCR Analyses
Total RNA was extracted from cultured B16-F10 cells using the Trizol Reagent (Thermo Fisher Scientific, Waltham, MA, USA). Total RNA concentration was quantified with a NanoVue Plus spectrophotometer (GE Health Care Life Sciences, Chicago, IL, USA). Real-time PCR was performed on a real-time PCR detection system and software (Applied Biosystems, Foster City, CA, USA). First-strand cDNA was generated by SuperScript III reverse transcriptase kit (Invitrogen). Quantification of mRNA expression for genes of interest was performed by qPCR reactions were performed with equal volume of cDNA, forward and reverse primers (10 µM), power SYBR Green Master Mix (Applied Biosystems). The mRNA levels were normalized with GAPDH, while miRNA levels were normalized with U6. The primer sequences of each gene for qPCR were designed by TRIbioteck (Hsinchu, Taiwan) and summarized in
Supplementary Table S2.
4.6. Protein Extraction and Western Blot Analysis
Cells were lysed by either mammalian protein extraction reagent or radio-immunoprecipitation assay (RIPA) buffer (Pierce Biotechnology, Rockford, IL, USA). Protein concentrations were determined using Bio-Rad protein assay reagent (Bio-Rad Laboratories, Hercules, CA, USA). Equal amount of protein samples (60–100 µg) were separated by 8–12% SDS-PAGE and separated proteins were transferred onto polyvinylidene fluoride (PVDF) membrane for overnight. The transferred protein membranes were blocked with 5% non-fat skim milk for 30 min, followed by incubation with specific primary antibodies for overnight and either horseradish peroxidase-conjugated anti-rabbit or anti-mouse or anti-goat antibodies for 2 h. Immunoblots were developed with the enhanced chemiluminescence (ECL) reagents (Advansta Inc., San Jose, CA, USA), images were captured by ChemiDoc XRS+ docking system and the protein bands were quantified by using Imagelab software (Bio-Rad laboratories, Hercules, CA, USA).
4.7. Immunofluorescence and Confocal Microscopy
B16-F10 cells at a density of 2 × 104 cells/well were seeded in eight-well glass Nunc Lab-Tek® chamber (ThermoFisher Scientific, Waltham, MA, USA) and treated FRK with or without LEO or REO for indicated time points. After treatment, culture media was removed and the cells were fixed in 2% paraformaldehyde for 15 min, cells were permeabilized with 0.1% Triton X-100 for 10 min, washed and blocked with 10% FBS in PBS and then incubated for 2 h with the anti-phospho-CREB or anti-MITF primary antibodies in 1.5% FBS. The cells were then incubated with the fluorescein isothiocyanate (FITC)-conjugated secondary antibody for another 1 h in 6% bovine serum albumin (BSA). Next, the cells were stained with 1 μg/mL DAPI for 5 min, washed with PBS and visualized using a laser scanning confocal microscope (Leika Microsystems, Buffalo Grove, IL, USA) at a 20 × magnification.
4.8. siRNA Transfection
siRNA was transfected with Lipofectamine RNAiMax (Invitrogen) according to the manufacturer’s instructions. For transfection, B16-F10 cells were plated in 6-well plates to give 40–60% confluence at the time of transfection. The next day, the culture medium was replaced with 500 μL of Opti-MEM (GIBCOBRL/Invitrogen) and the cells were transfected using the RNAiMAX transfection reagent. In a separate tube, 100 pM of siRNA was added to 500 μL of an Opti-MEM and RNAiMAX reagent mixture. The resulting siRNA/RNAiMAX mixture was incubated for an additional 25 min at room temperature to allow complex formation. Subsequently, the solution was added to the cells in the 6-well plates, giving a final transfection volume of 1 mL. After 6 h incubation, the transfection medium was replaced with 2 mL of standard growth medium and the cells were cultured at 37 °C. After treatment with LEO or REO for 2 or 48 h, the cells were then subjected to Western blot analysis or melanin quantification.
4.9. Immunoprecipitation
B16-F10 cells (1 × 106 cells/dish) were seeded in 10 cm dish and treated with FRK in the presence or absence of LEO or REO or MG132 for 12 h. Cells were collected and centrifuged at 1000× g for 5 min and the pellet was resolved in RIPA buffer and then homogenized in an ultrasonicator for 5 s for five times and incubated on ice for 30 min. The lysates were centrifuged at 16,000× g for 10 min at 4 °C and the supernatant was recovered. After pre-cleaning of protein A agarose (PAA) with RIPA buffer, protein samples were incubated with PAA for 1 h at 4 °C. Then 3 μg of primary antibody against MITF in antibody diluent (1% BSA, 1% NaN3 in PBS) was added to PAA protein complex and incubated for overnight. The PAA, protein and antibody complex was collected by brief centrifugation at 2000× g for 5 min and subjected to 3 rounds of washing with 1 mL of RIPA buffer. After adding 50 μL of 3 × sample dye (125 mM Tris-HCL (pH 6.8), 2% SDS, 5% Glycerol, 0.03% bromophenol blue, 1% β-meanptoethanol), samples were resolved by 10% SDS-PAGE and transferred into a PVDF membrane for overnight. The membranes were blocked with 5% skim milk for 30 min and the blots were incubated with antibodies against MITF or ubiquitin for 2 h and then the blots were incubated with horse-radish peroxide (HRP)-conjugated anti-goat IgG or anti-rabbit IgG for 1 h. Immunoblots were developed with the enhanced chemiluminescence (ECL) reagents (Advansta Inc., San Jose, CA, USA), images were captured by ChemiDoc XRS+ docking system and the protein bands were quantified by using Imagelab software (Bio-Rad laboratories, Hercules, CA, USA).
4.10. GC–MS Analysis
To determine the chemical composition of leaf and rhizome essential oils of
A. nantoensis, we carried out GC-MS analyses using an ITQ 900 mass spectrometer coupled with a DB-5MS column as described previously [
30]. The temperature program was as follows: 45 °C for 3 min, then increased to 3 °C/min to 180 °C and then increased to 10 °C/min to 280 °C hold for 5 min. The other parameters were injection temperature, 240 °C; ion source temperature, 200 °C; EI, 70 eV; carrier gas, He 1 mL/min; and mass scan range, 40–600
m/z. The volatile compounds were identified by Wiley/NBS Registry of mass spectral databases (V. 8.0, Hoboken, NJ, USA), National Institute of Standards and Technology (NIST) Ver. 2.0 GC/MS libraries and the Kovats indices were calculated for all volatile constituents using a homologous series of
n-alkanes C
9–C
24. The major components were identified by co-injection with standards (wherever possible).
4.11. Statistical analysis
Data are expressed as mean ± SD. All data were analyzed using the statistical software GraphPad Prism version 6.0 for Windows (GraphPad Software, La Jolla, CA, USA). Statistical analysis was performed using one-way ANOVA followed by Dunnett’s test for multiple comparison. p values of less than 0.05 *, 0.01 ** and 0.001 *** were considered statistically significant for the FRK treatment vs. LEO/REO treatment groups. p values of less than 0.01 # was considered statistically significant for the FRK treatment vs. the control group. p values of less than 0.05 Δ was considered statistically significant for the FRK treatment vs. the ERK1/2 inhibitor treatment group.