Controversial Regulation of Gene Expression and Protein Transduction of Aquaporins under Drought and Salinity Stress
Abstract
1. Introduction
2. Aquaporins under Salinity and Drought
2.1. General Features
2.2. Discrepancies between the mRNA and Protein Levels of PIP Aquaporins
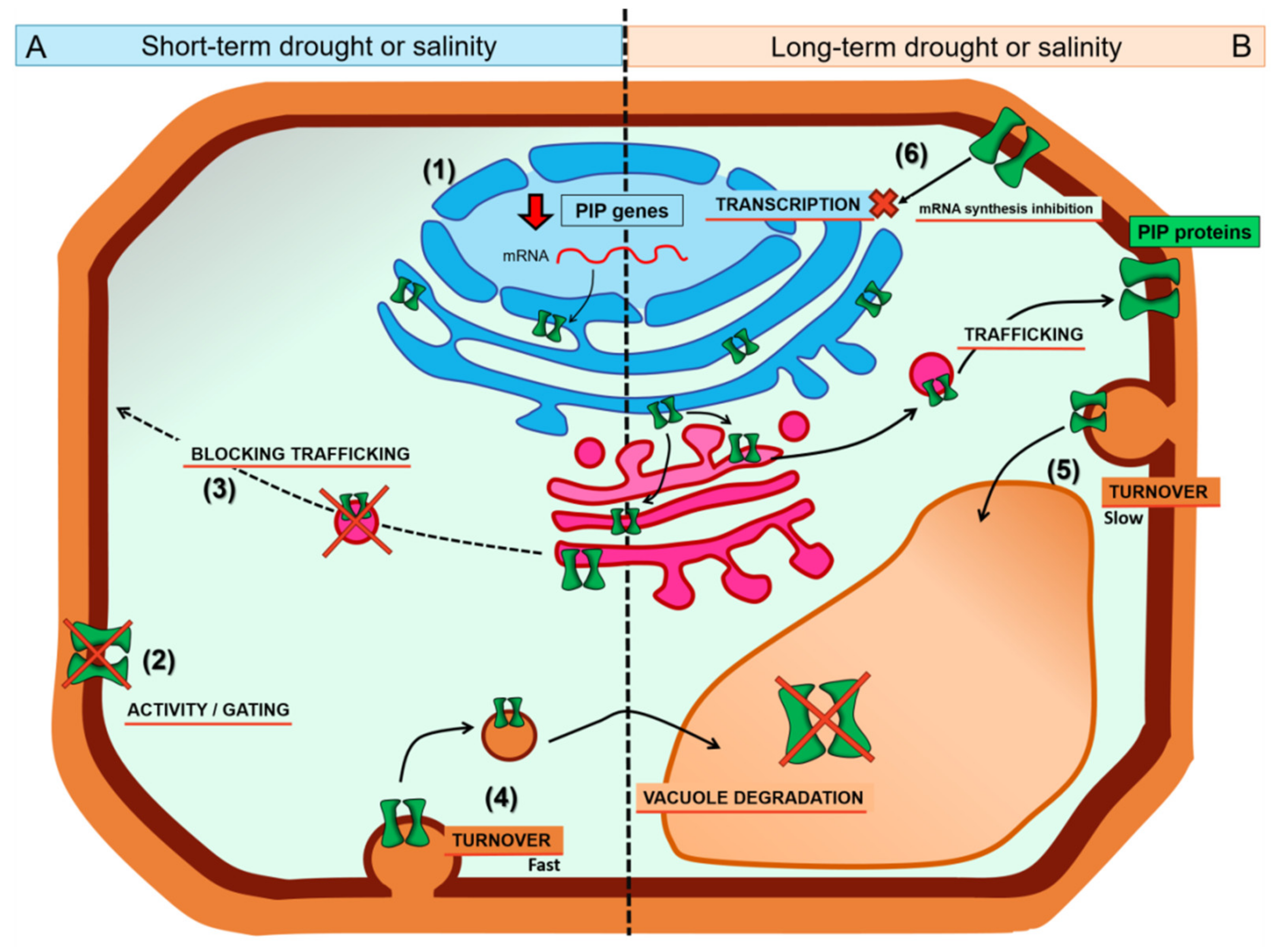
3. PIP Trafficking and Turnover under Drought and Salt Stresses
3.1. Aquaporins En Route (Secretory Pathway)
3.2. Aquaporins Already in the Plasma Membrane (Endocytic Pathway)
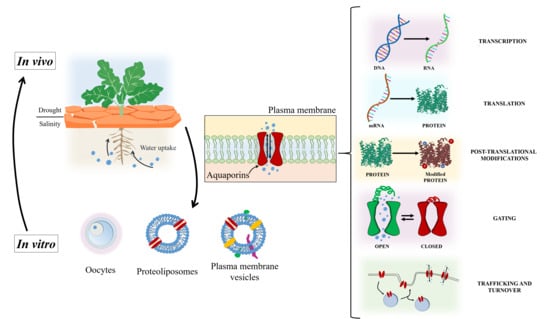
4. In Vitro Studies to Determine the In Vivo Physiology of Plant Aquaporins
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358. [Google Scholar] [CrossRef]
- Chaumont, F.; Tyerman, S.D. Aquaporins: Highly regulated channels controlling plant water relations. Plant Physiol. 2014, 164, 1600–1618. [Google Scholar] [CrossRef] [PubMed]
- Santoni, V. Plant Aquaporin Posttranslational Regulation. In Plant Aquaporins: From Transport to Signaling; Chaumont, F., Tyerman, S.D., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 83–105. ISBN 978-3-319-49395-4. [Google Scholar]
- Preston, G.M.; Agre, P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: Member of an ancient channel family. Proc. Natl. Acad. Sci. USA 1991, 88, 11110–11114. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Fotiadis, D.; Sjövall, S.; Johansson, I.; Hedfalk, K.; Engel, A.; Kjellbom, P. Reconstitution of water channel function of an aquaporin overexpressed and purified from Pichia pastoris. FEBS Lett. 2003, 537, 68–72. [Google Scholar] [CrossRef]
- López-Pérez, L.; Martínez-Ballesta, M.d.C.; Maurel, C.; Carvajal, M. Changes in plasma membrane lipids, aquaporins and proton pump of broccoli roots, as an adaptation mechanism to salinity. Phytochemistry 2009, 70, 492–500. [Google Scholar] [CrossRef]
- Jensen, M.; Mouritsen, O.G. Lipids do influence protein function-The hydrophobic matching hypothesis revisited. Biochim. Biophys. Acta-Biomembr. 2004, 1666, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Calanca, P.P. Effects of Abiotic Stress in Crop Production. In Quantification of Climate Variability, Adaptation and Mitigation for Agricultural Sustainability; Springer: Berlin/Heidelberg, Germany, 2017; pp. 165–180. [Google Scholar]
- Kapilan, R.; Vaziri, M.; Zwiazek, J.J. Regulation of aquaporins in plants under stress. Biol. Res. 2018, 51. [Google Scholar] [CrossRef]
- Afzal, Z.; Howton, T.C.; Sun, Y.; Mukhtar, M.S. The roles of aquaporins in plant stress responses. J. Dev. Biol. 2016, 4, 9. [Google Scholar] [CrossRef]
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef]
- Dresselhaus, T.; Hückelhoven, R. Biotic and abiotic stress responses in crop plants. Agronomy 2018, 8, 267. [Google Scholar] [CrossRef]
- Lisar, S.Y.S.; Motafakkerazad, R.; Hossain, M.M.; Rahm, I.M.M. Water Stress in Plants: Causes, Effects and Responses. In Water Stress; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Mcelrone, A.J.; Choat, B.; Gambetta, G.A.; Brodersen, C.R. Water Uptake and Transport in Vascular Plants. Nat. Educ. Knowl. 2013, 4, 6. [Google Scholar]
- Pawłowicz, I.; Masajada, K. Aquaporins as a link between water relations and photosynthetic pathway in abiotic stress tolerance in plants. Gene 2019, 687, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Pawłowicz, I.; Rapacz, M.; Perlikowski, D.; Gondek, K.; Kosmala, A. Abiotic stresses influence the transcript abundance of PIP and TIP aquaporins in Festuca species. J. Appl. Genet. 2017, 58, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Wallace, I.S.; Choi, W.G.; Roberts, D.M. The structure, function and regulation of the nodulin 26-like intrinsic protein family of plant aquaglyceroporins. Biochim. Biophys. Acta-Biomembr. 2006, 1758, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- López-Berenguer, C.; García-Viguera, C.; Carvajal, M. Are root hydraulic conductivity responses to salinity controlled by aquaporins in broccoli plants? Plant Soil 2006, 279, 13–23. [Google Scholar] [CrossRef]
- Sade, N.; Gebretsadik, M.; Seligmann, R.; Schwartz, A.; Wallach, R.; Moshelion, M. The role of tobacco Aquaporin1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress. Plant Physiol. 2010, 152, 245–254. [Google Scholar] [CrossRef]
- Maurel, C.; Verdoucq, L.; Rodrigues, O. Aquaporins and plant transpiration. Plant Cell Environ. 2016, 39, 2580–2587. [Google Scholar] [CrossRef]
- Maurel, C. Plant aquaporins: Novel functions and regulation properties. FEBS Lett. 2007, 581, 2227–2236. [Google Scholar] [CrossRef]
- Forrest, K.L.; Bhave, M. Major intrinsic proteins (MIPs) in plants: A complex gene family with major impacts on plant phenotype. Funct. Integr. Genom. 2007, 7, 263–289. [Google Scholar] [CrossRef]
- Boursiac, Y.; Chen, S.; Luu, D.T.; Sorieul, M.; Van Den Dries, N.; Maurel, C. Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 2005, 139, 790–805. [Google Scholar] [CrossRef]
- Muries, B.; Mohamed, F.; Carvajal, M.; Martínez-Ballesta, M.C. Identification and differential induction of the expression of aquaporins by salinity in broccoli plants. Mol. Biosyst. 2011, 7, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Patankar, H.V.; Al-Harrasi, I.; Al-Yahyai, R.; Yaish, M.W. Functional characterization of date palm aquaporin gene pdpip1;2 confers drought and salinity tolerance to yeast and arabidopsis. Genes 2019, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, W.; Liu, J.; Song, S.; Hou, X.; Jia, C.; Li, J.; Miao, H.; Wang, Z.; Tie, W.; et al. An aquaporin gene MaPIP2-7 is involved in tolerance to drought, cold and salt stresses in transgenic banana (Musa acuminata L.). Plant Physiol. Biochem. 2020, 147, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, F.; Bing, J.; Sun, W.; Feng, X.; Ma, X.; Zhou, Y.; Zhang, G. Overexpression of the Jojoba aquaporin gene, ScPIP1, enhances drought and salt tolerance in transgenic Arabidopsis. Int. J. Mol. Sci. 2019, 20, 153. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Knipfer, T.; Fricke, W.; McElrone, A.J. Aquaporins and Root Water Uptake. In Plant Aquaporins; Springer: Cham, Switzerland, 2017; pp. 133–153. [Google Scholar]
- Vandeleur, R.K.; Mayo, G.; Shelden, M.C.; Gilliham, M.; Kaiser, B.N.; Tyerman, S.D. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: Diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiol. 2009, 149, 445–460. [Google Scholar] [CrossRef]
- Martins, C.; Pedrosa, A.M.; Du, D.; Gonçalves, L.P.; Yu, Q.; Gmitter, F.G.; Costa, M.G.C. Genome-wide characterization and expression analysis of major intrinsic proteins during abiotic and biotic stresses in sweet orange (Citrus sinensis L. Osb.). PLoS ONE 2015, 10, e0138786. [Google Scholar] [CrossRef]
- Kammerloher, W.; Fischer, U.; Piechottka, G.P.; Schäffner, A.R. Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. Plant J. 1994, 6, 187–199. [Google Scholar] [CrossRef]
- Suga, S.; Komatsu, S.; Maeshima, M. Aquaporin isoforms responsive to salt and water stresses and phytohormones in radish seedlings. Plant Cell Physiol. 2002, 43, 1229–1237. [Google Scholar] [CrossRef]
- Bárzana, G.; Aroca, R.; Bienert, G.P.; Chaumont, F.; Ruiz-Lozano, J.M. New Insights into the Regulation of Aquaporins by the Arbuscular Mycorrhizal Symbiosis in Maize Plants under Drought Stress and Possible Implications for Plant Performance e-Xtra. Mol. Plant-Microbe Interact. 2014, 27, 349–363. [Google Scholar] [CrossRef]
- Pou, A.; Jeanguenin, L.; Milhiet, T.; Batoko, H.; Chaumont, F.; Hachez, C. Salinity-mediated transcriptional and post-translational regulation of the Arabidopsis aquaporin PIP2;7. Plant Mol. Biol. 2016, 92, 731–744. [Google Scholar] [CrossRef]
- Katsuhara, M.; Shibasaka, M. Barley root hydraulic conductivity and aquaporins expression in relation to salt tolerance. Soil Sci. Plant Nutr. 2007, 53, 466–470. [Google Scholar] [CrossRef]
- Katsuhara, M.; Akiyama, Y.; Koshio, K.; Shibasaka, M.; Kasamo, K. Functional analysis of water channels in barley roots. Plant Cell Physiol. 2002, 43, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Alexandersson, E.; Fraysse, L.; Sjövall-Larsen, S.; Gustavsson, S.; Fellert, M.; Karlsson, M.; Johanson, U.; Kjellbom, P. Whole gene family expression and drought stress regulation of aquaporins. Plant Mol. Biol. 2005, 59, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Johanson, U.; Karlsson, M.; Johansson, I.; Gustavsson, S.; Sjövall, S.; Fraysse, L.; Weig, A.R.; Kjellbom, P. The complete set of genes encoding major intrinsic proteins in arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 2001, 126, 1358–1369. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, D.G.; Kim, Y.O.; Kim, J.S.; Kang, H. An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Mol. Biol. 2004, 54, 713–725. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.; Moreno-Fernández, D.A.; Castejón, D.; Ochando, C.; Morandini, P.A.; Carvajal, M. The impact of the absence of aliphatic glucosinolates on water transport under salt stress in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 524. [Google Scholar] [CrossRef]
- Sutka, M.; Li, G.; Boudet, J.; Boursiac, Y.; Doumas, P.; Maurel, C. Natural variation of root hydraulics in Arabidopsis grown in normal and salt-stressed conditions. Plant Physiol. 2011, 155, 1264–1276. [Google Scholar] [CrossRef]
- Lee, S.H.; Zwiazek, J.J. Regulation of aquaporin-mediated water transport in arabidopsis roots exposed to NaCl. Plant Cell Physiol. 2015, 56, 750–758. [Google Scholar] [CrossRef]
- Alexandersson, E.; Danielson, J.Å.H.; Råde, J.; Moparthi, V.K.; Fontes, M.; Kjellbom, P.; Johanson, U. Transcriptional regulation of aquaporins in accessions of Arabidopsis in response to drought stress. Plant J. 2010, 61, 650–660. [Google Scholar] [CrossRef]
- Provart, N.J.; Alonso, J.; Assmann, S.M.; Bergmann, D.; Brady, S.M.; Brkljacic, J.; Browse, J.; Chapple, C.; Colot, V.; Cutler, S.; et al. 50 years of Arabidopsis research: Highlights and future directions. New Phytol. 2016, 209, 921–944. [Google Scholar] [CrossRef]
- Staiger, D.; Zecca, L.; Wieczorek Kirk, D.A.; Apel, K.; Eckstein, L. The circadian clock regulated RNA-binding protein AtGRP7 autoregulates its expression by influencing alternative splicing of its own pre-mRNA. Plant J. 2003, 33, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, A.S.; Chaumont, F. Trafficking of plant plasma membrane aquaporins: Multiple regulation levels and complex sorting signals. Plant Cell Physiol. 2015, 56, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Ueda, T. Exocytic trafficking pathways in plants: Why and how they are redirected. New Phytol. 2017, 215, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, M.; Gao, C.; Zeng, Y.; Cui, Y.; Shen, W.; Jiang, L. The roles of endomembrane trafficking in plant abiotic stress responses. J. Integr. Plant Biol. 2020, 62, 55–69. [Google Scholar] [CrossRef]
- Valencia, J.P.; Goodman, K.; Otegui, M.S. Endocytosis and Endosomal Trafficking in Plants. Annu. Rev. Plant Biol. 2016, 67, 309–335. [Google Scholar] [CrossRef]
- Otegui, M.S.; Spitzer, C. Endosomal Functions in Plants. Traffic 2008, 9, 1589–1598. [Google Scholar] [CrossRef]
- Inada, N.; Ueda, T. Membrane trafficking pathways and their roles in plant-microbe interactions. Plant Cell Physiol. 2014, 55, 672–686. [Google Scholar] [CrossRef]
- Rosquete, M.R.; Drakakaki, G. Plant TGN in the stress response: A compartmentalized overview. Curr. Opin. Plant Biol. 2018, 46, 122–129. [Google Scholar] [CrossRef]
- Törnroth-Horsefield, S.; Wang, Y.; Hedfalk, K.; Johanson, U.; Karlsson, M.; Tajkhorshid, E.; Neutze, R.; Kjellbom, P. Structural mechanism of plant aquaporin gating. Nature 2006, 439, 688–694. [Google Scholar] [CrossRef]
- Hachez, C.; Besserer, A.; Chevalier, A.S.; Chaumont, F. Insights into plant plasma membrane aquaporin trafficking. Trends Plant Sci. 2013, 18, 344–352. [Google Scholar] [CrossRef]
- Zelazny, E.; Borst, J.W.; Muylaert, M.; Batoko, H.; Hemminga, M.A.; Chaumont, F. FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proc. Natl. Acad. Sci. USA 2007, 104, 12359–12364. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Cavez, D.; Besserer, A.; Berny, M.C.; Gilis, D.; Rooman, M.; Chaumont, F. A conserved cysteine residue is involved in disulfide bond formation between plant plasma membrane aquaporin monomers. Biochem. J. 2012, 445, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Cho, S.K.; Son, O.; Xu, Z.; Hwang, I.; Kim, W.T. Drought stress-induced Rma1H1, a RING membrane-anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic arabidopsis plants. Plant Cell 2009, 21, 622–641. [Google Scholar] [CrossRef] [PubMed]
- Verdoucq, L.; Rodrigues, O.; Martinière, A.; Luu, D.T.; Maurel, C. Plant aquaporins on the move: Reversible phosphorylation, lateral motion and cycling. Curr. Opin. Plant Biol. 2014, 22C, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Zelazny, E.; Miecielica, U.; Borst, J.W.; Hemminga, M.A.; Chaumont, F. An N-terminal diacidic motif is required for the trafficking of maize aquaporins ZmPIP2;4 and ZmPIP2;5 to the plasma membrane. Plant J. 2009, 57, 346–355. [Google Scholar] [CrossRef]
- Sorieul, M.; Santoni, V.; Maurel, C.; Luu, D.T. Mechanisms and Effects of Retention of Over-Expressed Aquaporin AtPIP2;1 in the Endoplasmic Reticulum. Traffic 2011, 12, 473–482. [Google Scholar] [CrossRef]
- Prak, S.; Hem, S.; Boudet, J.; Viennois, G.; Sommerer, N.; Rossignol, M.; Maurel, C.; Santoni, V. Multiple Phosphorylations in the C-terminal Tail of Plant Plasma Membrane Aquaporins. Mol. Cell. Proteom. 2008, 7, 1019–1030. [Google Scholar] [CrossRef]
- Honsbein, A.; Sokolovski, S.; Grefen, C.; Campanoni, P.; Pratelli, R.; Paneque, M.; Chen, Z.; Johansson, I.; Blatt, M.R. A tripartite SNARE-K+ channel complex mediates in channel-dependent K+ nutrition in arabidopsis. Plant Cell 2009, 21, 2859–2877. [Google Scholar] [CrossRef]
- Besserer, A.; Burnotte, E.; Bienert, G.P.; Chevalier, A.S.; Errachid, A.; Grefen, C.; Blatt, M.R.; Chaumont, F. Selective Regulation of Maize Plasma Membrane Aquaporin Trafficking and Activity by the SNARE SYP121 W. Am. Soc. Plant Biol. 2012, 24, 3463–3481. [Google Scholar] [CrossRef]
- Sinclair, R.; Rosquete, M.R.; Drakakaki, G. Post-golgi trafficking and transport of cell wall components. Front. Plant Sci. 2018, 9, 1784. [Google Scholar] [CrossRef]
- Leyman, B.; Geelen, D.; Quintero, F.J.; Blatt, M.R. A Tobacco Syntaxin with a Role in Hormonal Control of Guard Cell Ion Channels. Science 1999, 283, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Zwiazek, J.J.; Xu, H.; Tan, X.; Navarro-Ródenas, A.; Morte, A. Significance of oxygen transport through aquaporins. Sci. Rep. 2017, 7, 40411. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Ribas-Carbó, M.; Hanson, D.T.; Bota, J.; Otto, B.; Cifre, J.; McDowell, N.; Medrano, H.; Kaldenhoff, R. Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2in vivo. Plant J. 2006, 48, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Hachez, C.; Laloux, T.; Reinhardt, H.; Cavez, D.; Degand, H.; Grefen, C.; De Rycke, R.; Inzé, D.; Blatt, M.R.; Russinova, E.; et al. Arabidopsis SNAREs SYP61 and SYP121 coordinate the trafficking of plasma membrane aquaporin PIP2;7 to modulate the cell membrane water permeability. Plant Cell 2014, 26, 3132–3147. [Google Scholar] [CrossRef]
- Baral, A.; Shruthi, K.S.; Mathew, M.K. Vesicular trafficking and salinity responses in plants. IUBMB Life 2015, 67, 677–686. [Google Scholar] [CrossRef]
- Mayor, S.; Pagano, R.E. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 603–612. [Google Scholar] [CrossRef]
- Vera-Estrella, R.; Barkla, B.J.; Bohnert, H.J.; Pantoja, O. Novel regulation of aquaporins during osmotic stress. Plant Physiol. 2004, 135, 2318–2329. [Google Scholar] [CrossRef]
- Boursiac, Y.; Prak, S.; Boudet, J.; Postaire, O.; Luu, D.T.; Tournaire-Roux, C.; Santoni, V.; Maurel, C. The response of Arabidopsis root water transport to a challenging environment implicates reactive oxygen species- and phosphorylation-dependent internalization of aquaporins. Plant Signal. Behav. 2008, 3, 1096–1098. [Google Scholar] [CrossRef]
- Luu, D.T.; Martiniãre, A.; Sorieul, M.; Runions, J.; Maurel, C. Fluorescence recovery after photobleaching reveals high cycling dynamics of plasma membrane aquaporins in Arabidopsis roots under salt stress. Plant J. 2012, 69, 894–905. [Google Scholar] [CrossRef]
- Hachez, C.; Veljanovski, V.; Reinhardt, H.; Guillaumot, D.; Vanhee, C.; Chaumont, F.; Batoko, H. The Arabidopsis Abiotic Stress-Induced Tspo-Related Protein Reduces Cell-Surface Expression of the Aquaporin PIP2;7 through Protein-Protein Interactions and Autophagic Degradation. Plant Cell 2014, 26, 4974–4990. [Google Scholar] [CrossRef]
- Kreps, J.A.; Wu, Y.; Chang, H.S.; Zhu, T.; Wang, X.; Harper, J.F. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 2002, 130, 2129–2141. [Google Scholar] [CrossRef]
- Bárzana, G.; Carvajal, M. Genetic regulation of water and nutrient transport in water stress tolerance in roots. J. Biotechnol. 2020, 324, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Tsutsumi, N.; Fujimoto, M. Salt stress induces internalization of plasma membrane aquaporin into the vacuole in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2016, 474, 742–746. [Google Scholar] [CrossRef]
- Jia, J.; Liang, Y.; Gou, T.; Hu, Y.; Zhu, Y.; Huo, H.; Guo, J.; Gong, H. The expression response of plasma membrane aquaporins to salt stress in tomato plants. Environ. Exp. Bot. 2020, 178, 104190. [Google Scholar] [CrossRef]
- Gerbeau, P.; Amodeo, G.; Henzler, T.; Santoni, V.; Ripoche, P.; Maurel, C. The water permeability of Arabidopsis plasma membrane is regulated by divalent cations and pH. Plant J. 2002, 30, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Verdoucq, L.; Grondin, A.; Maurel, C. Structure-function analysis of plant aquaporin AtPIP2;1 gating by divalent cations and protons. Biochem. J. 2008, 415, 409–416. [Google Scholar] [CrossRef]
- Niemietz, C.M.; Tyerman, S.D. Characterization of water channels in wheat root membrane vesicles. Plant Physiol. 1997, 115, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Martínez Ballesta, M.C.; García-Gomez, P.; Yepes-Molina, L.; Guarnizo, A.L.; Teruel, J.A.; Carvajal, M. Plasma membrane aquaporins mediates vesicle stability in broccoli. PLoS ONE 2018, 13, 1–19. [Google Scholar] [CrossRef]
- Polevoda, B.; Sherman, F. Nalpha-terminal acetylation of eukaryotic proteins. J. Biol. Chem. 2000, 275, 36479–36482. [Google Scholar] [CrossRef]
- Johansson, I.; Karlsson, M.; Shukla, V.K.; Chrispeels, M.J.; Larsson, C.; Kjellbom, P. Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell 1998, 10, 451–459. [Google Scholar] [CrossRef]
- Nyblom, M.; Frick, A.; Wang, Y.; Ekvall, M.; Hallgren, K.; Hedfalk, K.; Neutze, R.; Tajkhorshid, E.; Törnroth-Horsefield, S. Structural and Functional Analysis of SoPIP2;1 Mutants Adds Insight into Plant Aquaporin Gating. J. Mol. Biol. 2009, 387, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Alleva, K.; Niemietz, C.M.; Sutka, M.; Maurel, C.; Parisi, M.; Tyerman, S.D.; Amodeo, G. Plasma membrane of Beta vulgaris storage root shows high water channel activity regulated by cytoplasmic pH and a dual range of calcium concentrations. J. Exp. Bot. 2006, 57, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fukumoto, T.; Matsumoto, T.; Gena, P.; Frascaria, D.; Kaneko, T.; Katsuhara, M.; Zhong, S.; Sun, X.; Zhu, Y.; et al. Aquaporin OsPIP1;1 promotes rice salt resistance and seed germination. Plant Physiol. Biochem. 2013, 63, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Kirscht, A.; Survery, S.; Kjellbom, P.; Johanson, U. Increased permeability of the aquaporin SoPIP2;1 by mercury and mutations in loop a. Front. Plant Sci. 2016, 7, 1249. [Google Scholar] [CrossRef]
- Wang, H.; Schoebel, S.; Schmitz, F.; Dong, H.; Hedfalk, K. Characterization of aquaporin-driven hydrogen peroxide transport. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183065. [Google Scholar] [CrossRef]
- Wang, H.; Schoebel, S.; Schmitz, F.; Dong, H.; Hedfalk, K. Quantitative analysis of H2O2 transport through purified membrane proteins. MethodsX 2020, 7, 100816. [Google Scholar] [CrossRef]
- Tong, J.; Briggs, M.M.; McIntosh, T.J. Water permeability of aquaporin-4 channel depends on bilayer composition, thickness, and elasticity. Biophys. J. 2012, 103, 1899–1908. [Google Scholar] [CrossRef]
- Gandhavadi, M.; Allende, D.; Vidal, A.; Simon, S.A.; McIntosh, T.J. Structure, composition, and peptide binding properties of detergent soluble bilayers and detergent resistant rafts. Biophys. J. 2002, 82, 1469–1482. [Google Scholar] [CrossRef][Green Version]
- Jiang, J.; Daniels, B.V.; Fu, D. Crystal structure of AqpZ tetramer reveals two distinct Arg-189 conformations associated with water permeation through the narrowest constriction of the water-conducting channel. J. Biol. Chem. 2006, 281, 454–460. [Google Scholar] [CrossRef]
- Horsefield, R.; Nordén, K.; Fellert, M.; Backmark, A.; Törnroth-Horsefield, S.; Terwisscha Van Scheltinga, A.C.; Kvassman, J.; Kjellbom, P.; Johanson, U.; Neutze, R. High-resolution x-ray structure of human aquaporin 5. Proc. Natl. Acad. Sci. USA 2008, 105, 13327–13332. [Google Scholar] [CrossRef]
- Horie, T.; Kaneko, T.; Sugimoto, G.; Sasano, S.; Panda, S.K.; Shibasaka, M.; Katsuhara, M. Mechanisms of water transport mediated by PIP aquaporins and their regulation via phosphorylation events under salinity stress in barley roots. Plant Cell Physiol. 2011, 52, 663–675. [Google Scholar] [CrossRef]
- Besse, M.; Knipfer, T.; Miller, A.J.; Verdeil, J.L.; Jahn, T.P.; Fricke, W. Developmental pattern of aquaporin expression in barley (Hordeum vulgare L.) leaves. J. Exp. Bot. 2011, 62, 4127–4142. [Google Scholar] [CrossRef]
- Otto, B.; Uehlein, N.; Sdorra, S.; Fischer, M.; Ayaz, M.; Belastegui-Macadam, X.; Heckwolf, M.; Lachnit, M.; Pede, N.; Priem, N.; et al. Aquaporin tetramer composition modifies the function of tobacco aquaporins. J. Biol. Chem. 2010, 285, 31253–31260. [Google Scholar] [CrossRef] [PubMed]
- Uehlein, N.; Otto, B.; Eilingsfeld, A.; Itel, F.; Meier, W.; Kaldenhoff, R. Gas-tight triblock-copolymer membranes are converted to CO 2 permeable by insertion of plant aquaporins. Sci. Rep. 2012, 2, 538. [Google Scholar] [CrossRef] [PubMed]
- Chalbi, N.; Martínez-Ballesta, M.C.; Youssef, N.B.; Carvajal, M. Intrinsic stability of Brassicaceae plasma membrane in relation to changes in proteins and lipids as a response to salinity. J. Plant Physiol. 2015, 175, 148–156. [Google Scholar] [CrossRef] [PubMed]
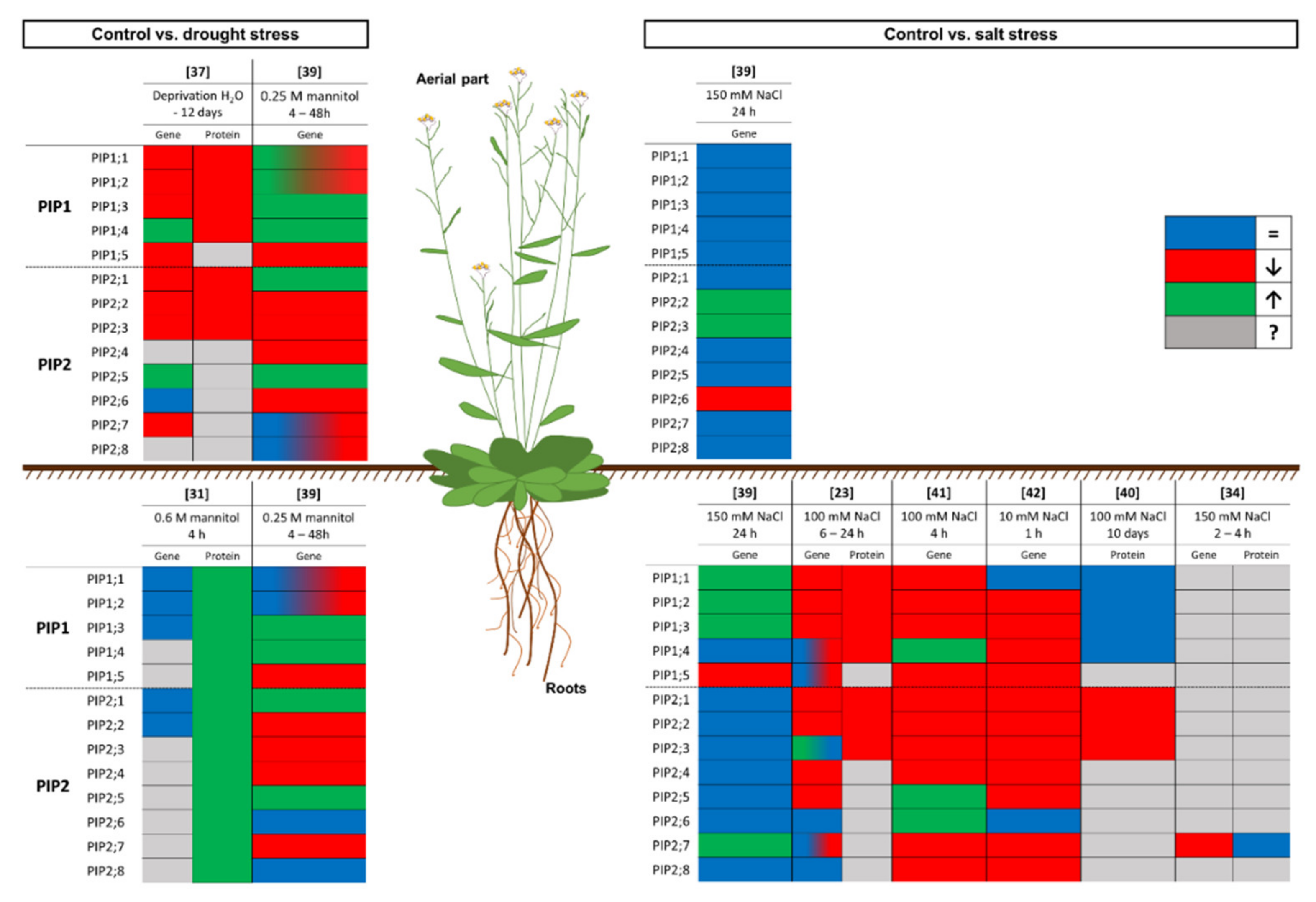
| Gene Expression | Protein Amount | References |
|---|---|---|
| = | = | |
| ↑ | [31,32] | |
| ↓ | [32] | |
| ↑ | = | |
| ↑ | ||
| ↓ | [32] | |
| ↓ | = | [33,34,35] |
| ↑ | [24] | |
| ↓ | [23,33,36,37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yepes-Molina, L.; Bárzana, G.; Carvajal, M. Controversial Regulation of Gene Expression and Protein Transduction of Aquaporins under Drought and Salinity Stress. Plants 2020, 9, 1662. https://doi.org/10.3390/plants9121662
Yepes-Molina L, Bárzana G, Carvajal M. Controversial Regulation of Gene Expression and Protein Transduction of Aquaporins under Drought and Salinity Stress. Plants. 2020; 9(12):1662. https://doi.org/10.3390/plants9121662
Chicago/Turabian StyleYepes-Molina, Lucía, Gloria Bárzana, and Micaela Carvajal. 2020. "Controversial Regulation of Gene Expression and Protein Transduction of Aquaporins under Drought and Salinity Stress" Plants 9, no. 12: 1662. https://doi.org/10.3390/plants9121662
APA StyleYepes-Molina, L., Bárzana, G., & Carvajal, M. (2020). Controversial Regulation of Gene Expression and Protein Transduction of Aquaporins under Drought and Salinity Stress. Plants, 9(12), 1662. https://doi.org/10.3390/plants9121662