Metabolic Profiling of Primary Metabolites and Galantamine Biosynthesis in Wounded Lycoris radiata Callus
Abstract
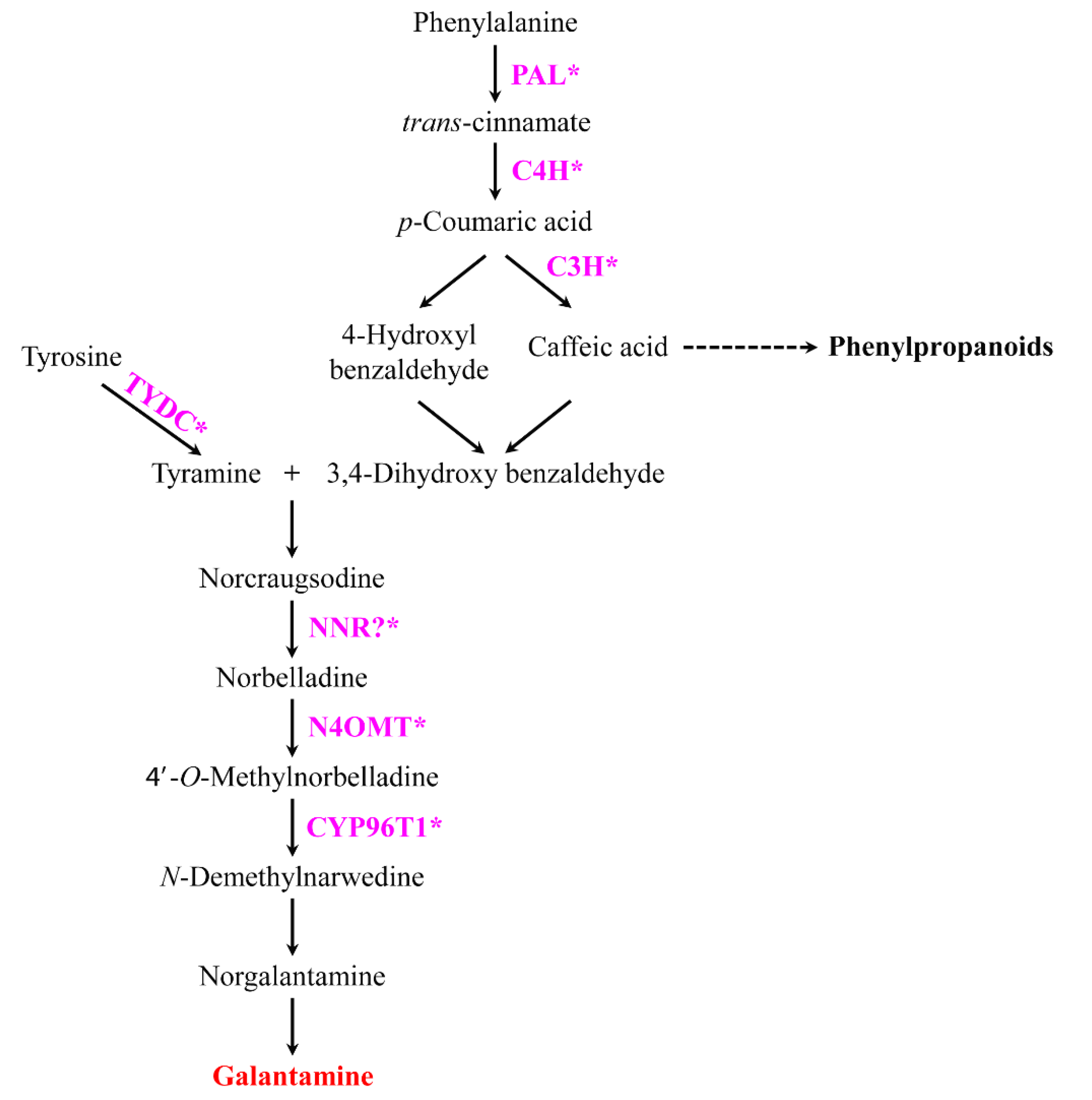
1. Introduction
2. Results
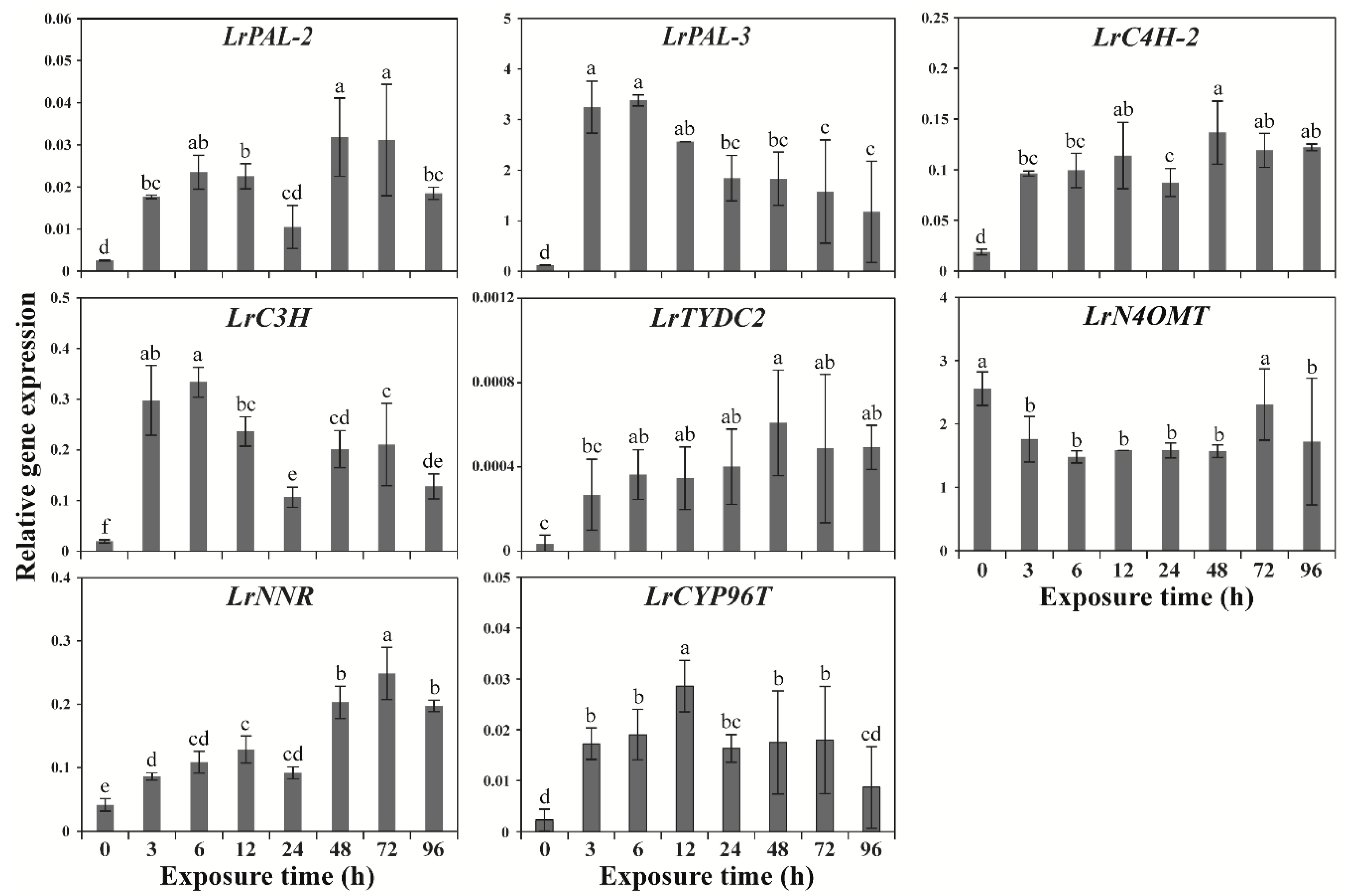
2.1. Effects of Wounding Stress on GP Gene Expression
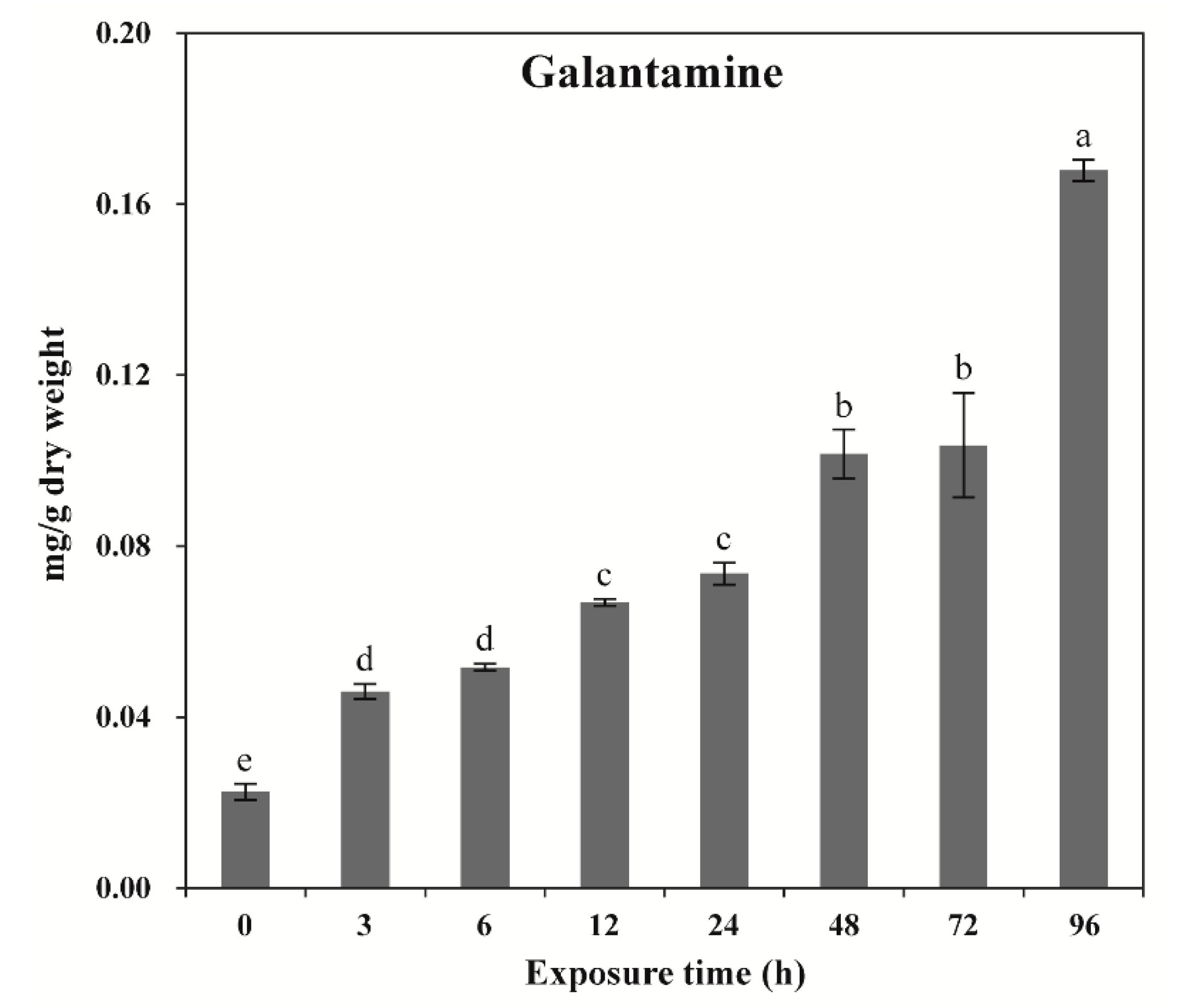
2.2. Effects of Wounding Stress on GAL Content
2.3. Metabolic Profiling
3. Discussion
4. Materials and Methods
4.1. Callus Induction
4.2. Wounding Treatment
4.3. RNA Extraction, cDNA Synthesis, and qRT PCR
4.4. High-Performance Liquid Chromatography (HPLC)
4.5. Gas Chromatography–Time-of-Flight Mass Spectrometry (GC–TOFMS)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Moreira, R.; Pereira, D.M.; Valentao, P.; Andrade, P.B. Pyrrolizidine alkaloids: Chemistry, pharmacology, toxicology and food safety. Int. J. Mol. Sci. 2018, 19, 1668. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zheng, T.T.; Li, X.; Liang, Y.; Wang, L.J.; Huang, Y.C.; Xiao, H.T. Plant-derived alkaloids: The promising disease-modifying agents for inflammatory bowel disease. Front. Pharmacol. 2019, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.Q.; Zhang, C.Y.; Guo, M.Q. Comparative analysis of Amaryllidaceae alkaloids from three Lycoris species. Molecules 2015, 20, 21854–21869. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yeo, H.J.; Park, Y.E.; Baek, S.A.; Kim, J.K.; Park, S.U. Transcriptome analysis and metabolic profiling of Lycoris radiata. Biology 2019, 8, 63. [Google Scholar] [CrossRef]
- Hao, B.; Shen, S.F.; Zhao, Q.J. Cytotoxic and antimalarial Amaryllidaceae alkaloids from the bulbs of Lycoris radiata. Molecules 2013, 18, 2458–2468. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.Y.; Wang, Z.Y.; Pi, H.F.; Zhang, P.; Ruan, H.L. Neuroprotective compounds from the bulbs of Lycoris radiata. Fitoterapia 2013, 88, 82–90. [Google Scholar] [CrossRef]
- Miyasaka, K.; Hiramatsu, Y. Pharmacological studies of lycorenine, an alkaloid of Lycoris radiata Herb.: II. Effects of blood pressure in rats and dogs and the mechanism of tachyphylaxis to the vasodepressor action of lycorenine in rats. JPN J. Pharmacol. 1980, 30, 655–664. [Google Scholar] [CrossRef]
- Son, M.; Kim, A.; Lee, J.; Park, C.H.; Heo, J.C.; Lee, H.J.; Lee, S.H. Ethanol extract of Lycoris radiata induces cell death in B16F10 melanoma via p38-mediated AP-1 activation. Oncol. Rep. 2010, 24, 473–478. [Google Scholar]
- Almanza, G.R.; Fernandez, J.M.; Wakori, E.W.T.; Viladomat, F.; Codina, C.; Bastida, J. Alkaloids from Narcissus cv Salome. Phytochemistry 1996, 43, 1375–1378. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Xia, N.; Li, X.D.; Shen, W.B.; Liang, L.J.; Wang, C.Y.; Wang, R.; Peng, F.; Xia, B. Molecular cloning and characterization of a phenylalanine ammonia-lyase gene (LrPAL) from Lycoris radiata. Mol. Biol. Rep. 2011, 38, 1935–1940. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Wilson, S.A.; Roberts, S.C. Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol. J. 2012, 10, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Emans, N.; Schuster, F.; Hellwig, S.; Drossard, J. Towards molecular farming in the future: Using plant-cell-suspension cultures as bioreactors. Biotechnol. Appl. Biochem. 1999, 30, 109–112. [Google Scholar] [PubMed]
- Dong, Y.S.; Duan, W.L.; He, H.X.; Su, P.; Zhang, M.; Song, G.H.; Fu, C.H.; Yu, L.J. Enhancing taxane biosynthesis in cell suspension culture of Taxus chinensis by overexpressing the neutral/alkaline invertase gene. Process Biochem. 2015, 50, 651–660. [Google Scholar] [CrossRef]
- Murthy, H.N.; Paek, K.Y. Panax ginseng adventitious root suspension culture: Protocol for biomass production and analysis of ginsenosides by high pressure liquid chromatography. Methods Mol. Biol. 2016, 1391, 125–139. [Google Scholar]
- Pan, Y.C.; Li, L.; Xiao, S.J.; Chen, Z.J.; Sarsaiya, S.; Zhang, S.B.; Yanni, S.G.; Liu, H.B.; Xu, D.L. Callus growth kinetics and accumulation of secondary metabolites of Bletilla striata Rchb. f. using a callus suspension culture. PLoS ONE 2020, 15, e0220084. [Google Scholar] [CrossRef]
- Berkov, S.; Georgieva, L.; Kondakova, V.; Atanassov, A.; Viladomat, F.; Bastida, J.; Codina, C. Plant sources of galanthamine: Phytochemical and biotechnological aspects. Biotechnol. Biotechnol. Equip. 2009, 23, 1170–1176. [Google Scholar] [CrossRef]
- Bulduk, I.; Karafakıoğlu, Y.S. Evaluation of galantamine, phenolics, flavonoids and antioxidant content of Galanthus species in Turkey. Int. J. Biochem. Res. Rev. 2019, 25, 1–12. [Google Scholar] [CrossRef]
- Klosi, R.; Mersinllari, M.; Gavani, E. Galantamine content in Leucojum aestivum populations grown in northwest Albania. Albanian J. Pharm. Sci. 2016, 3, 1–3. [Google Scholar]
- Kilgore, M.B.; Holland, C.K.; Jez, J.M.; Kutchan, T.M. Identification of a noroxomaritidine reductase with Amaryllidaceae alkaloid biosynthesis related activities. J. Biol. Chem. 2016, 291, 16740–16752. [Google Scholar] [CrossRef]
- Suhadolnik, R.J.; Fischer, A.G.; Zulalian, J. Biogenesis of the Amaryllidaceae alkaloids. II. Studies with whole plants, floral primordia and cell free extracts. Biochem. Biophys. Res. Commun. 1963, 11, 208–212. [Google Scholar] [CrossRef]
- Franke, R.; Humphreys, J.M.; Hemm, M.R.; Denault, J.W.; Ruegger, M.O.; Cusumano, J.C.; Chapple, C. The Arabidopsis REF8 gene encodes the 3-hydroxylase of phenylpropanoid metabolism. Plant J. 2002, 30, 33–45. [Google Scholar] [CrossRef]
- Lehmann, T.; Pollmann, S. Gene expression and characterization of a stress-induced tyrosine decarboxylase from Arabidopsis thaliana. FEBS Lett. 2009, 583, 1895–1900. [Google Scholar] [CrossRef]
- Kilgore, M.B.; Augustin, M.M.; Starks, C.M.; O’Neil-Johnson, M.; May, G.D.; Crow, J.A.; Kutchan, T.M. Cloning and characterization of a norbelladine 4′-O-methyltransferase involved in the biosynthesis of the Alzheimer’s drug galanthamine in Narcissus sp. aff. pseudonarcissus. PLoS ONE 2014, 9, e103223. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Zevallos, L. The use of controlled postharvest abiotic stresses as a tool for enhancing the nutraceutical content and adding-value of fresh fruits and vegetables. J. Food Sci. 2003, 68, 1560–1565. [Google Scholar] [CrossRef]
- Schreiner, M.; Huyskens-Keil, S. Phytochemicals in fruit and vegetables: Health promotion and postharvest elicitors. Crit. Rev. Plant Sci. 2006, 25, 267–278. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. An alternative use of horticultural crops: Stressed plants as biofactories of bioactive phenolic compounds. Agriculture 2012, 2, 259–271. [Google Scholar] [CrossRef]
- Heredia, J.B.; Cisneros-Zevallos, L. The effect of exogenous ethylene and methyl jasmonate on pal activity, phenolic profiles and antioxidant capacity of carrots (Daucus carota) under different wounding intensities. Postharvest Biol. Technol. 2009, 51, 242–249. [Google Scholar] [CrossRef]
- Jacobo-Velazquez, D.A.; Martinez-Hernandez, G.B.; Rodriguez, S.D.; Cao, C.M.; Cisneros-Zevallos, L. Plants as biofactories: Physiological role of reactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. J. Agric. Food Chem. 2011, 59, 6583–6593. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lu, X.Y.; Guo, X.R.; Liu, J.; Liu, Y.; Guo, Q.X.; Tang, Z.H. The specific responses to mechanical wound in leaves and roots of Catharanthus roseus seedlings by metabolomics. J. Plant Interact. 2018, 13, 450–460. [Google Scholar] [CrossRef]
- Corey-Bloom, J. Galantamine: A review of its use in Alzheimer’s disease and vascular dementia. Int. J. Clin. Pract. 2003, 57, 219–223. [Google Scholar] [PubMed]
- Jiang, Y.M.; Xia, B.; Liang, L.J.; Li, X.D.; Xu, S.; Peng, F.; Wang, R. Molecular and analysis of a phenylalanine ammonia-lyase gene (LrPAL2) from Lycoris radiata. Mol. Biol. Rep. 2013, 40, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Marco-Contelles, J.; Carreiras, M.D.; Rodriguez, C.; Villarroya, M.; Garcia, A.G. Synthesis and pharmacology of galantamine. Chem. Rev. 2006, 106, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, K.; Hiramatsu, Y.; Takezaki, T. Pharmacological studies of lycorenine, an alkaloid of Lycoris radiata Herb.: Vasodepressor mechanism in rats. JPN J. Pharmacol. 1979, 29, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Luo, Q.; Li, J.; Wang, Y. Comparative anatomy of leaves in 12 species of Lycoris (Amaryllidaceae). Acta Bot. Yunnanica 2006, 28, 473–480. [Google Scholar]
- Zhou, S.B.; Liu, K.; Zhang, D.; Li, Q.F.; Zhu, G.P. Photosynthetic performance of Lycoris radiata var. radiata to shade treatments. Photosynthetica 2010, 48, 241–248. [Google Scholar] [CrossRef]
- Shoubiao, Z.; Benqi, Y.; Qi, L.; Weihua, Q.; Ying, W. Pollen morphology of Lycoris Herb. and its taxonomic significance. Acta Hortic. Sin. 2005, 32, 914. [Google Scholar]
- Lee, D.; Ellard, M.; Wanner, L.A.; Davis, K.R.; Douglas, C.J. The Arabidopsis thaliana 4-Coumarate-Coa Ligase (4CL) gene: Stress and developmentally regulated expression and nucleotide sequence of its cDNA. Plant Mol. Biol. 1995, 28, 871–884. [Google Scholar] [CrossRef]
- Soltani, B.M.; Ehlting, J.; Hamberger, B.; Douglas, C.J. Multiple cis-regulatory elements regulate distinct and complex patterns of developmental and wound-induced expression of Arabidopsis thaliana 4CL gene family members. Planta 2006, 224, 1226–1238. [Google Scholar] [CrossRef]
- Jacobo-Velazquez, D.A.; Gonzalez-Aguero, M.; Cisneros-Zevallos, L. Cross-talk between signaling pathways: The link between plant secondary metabolite production and wounding stress response. Sci. Rep. 2015, 5, 8608. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.; Nonogaki, H.; Suslow, T.; Saltveit, M.E. Isolation and characterization of a wound inducible phenylalanine ammonia-lyase gene (LsPAL1) from Romaine lettuce leaves. Physiol. Plant. 2004, 121, 429–438. [Google Scholar] [CrossRef]
- Chen, C.Y.; Meyermans, H.; Burggraeve, B.; De Rycke, R.M.; Inoue, K.; De Vleesschauwer, V.; Steenackers, M.; Van Montagu, M.C.; Engler, G.J.; Boerjan, W.A. Cell-specific and conditional expression of caffeoyl-coenzyme A-3-O-methyltransferase in poplar. Plant Physiol. 2000, 123, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Mishra, S.; Triptahi, V.; Singh, S.; Phukan, U.J.; Gupta, M.M.; Shanker, K.; Shukla, R.K. Wound induced tanscriptional regulation of benzylisoquinoline pathway and characterization of wound inducible PsWRKY transcription factor from Papaver somniferum. PLoS ONE 2013, 8, e52784. [Google Scholar] [CrossRef]
- Becerra-Moreno, A.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velazquez, D.A. Plants as biofactories: Glyphosate-induced production of shikimic acid and phenolic antioxidants in wounded carrot tissue. J. Agric. Food Chem. 2012, 60, 11378–11386. [Google Scholar] [CrossRef]
- Sakuta, M.; Hirano, H.; Kakegawa, K.; Suda, J.; Hirose, M.; Joy, R.W.; Sugiyama, M.; Komamine, A. Regulatory mechanisms of biosynthesis of betacyanin and anthocyanin in relation to cell-division activity in suspension-cultures. Plant Cell Tissue. Org. 1994, 38, 167–169. [Google Scholar] [CrossRef]
- Singh, D.P.; Bahadur, A.; Sarma, B.K.; Maurya, S.; Singh, H.B.; Singh, U.P. Exogenous application of L-phenylalanine and ferulic acid enhance phenylalanine ammonia lyase activity and accumulation of phenolic acids in pea (Pisum sativum) to offer protection against Erysiphe pisi. Arch. Phytopathol. Plant Prot. 2010, 43, 1454–1462. [Google Scholar] [CrossRef]
- Feduraev, P.; Skrypnik, L.; Riabova, A.; Pungin, A.; Tokupova, E.; Maslennikov, P.; Chupakhina, G. Phenylalanine and tyrosine as exogenous precursors of wheat (Triticum aestivum L.) secondary metabolism through PAL-associated pathways. Plants 2020, 9, 476. [Google Scholar] [CrossRef]
- Hu, G.S.; Jia, J.M.; Doh Hoon, K. Effects of feeding tyrosine and phenylalanine on the accumulation of phenylethanoid glycosides to Cistanche deserticola cell suspension culture. Chin. J. Nat. Med. 2014, 12, 367–372. [Google Scholar] [CrossRef]
- Palacio, L.; Cantero, J.J.; Cusido, R.; Goleniowski, M. Phenolic compound production by Larrea divaricata Cav. plant cell cultures and effect of precursor feeding. Process Biochem. 2011, 46, 418–422. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Park, S.Y.; Kim, J.K.; Park, S.U. Comparative phytochemical analyses and metabolic profiling of different phenotypes of Chinese cabbage (Brassica rapa ssp. pekinensis). Foods 2019, 8, 587. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Park, S.Y.; Lee, S.Y.; Kim, J.K.; Park, S.U. Analysis of metabolites in white flowers of Magnolia denudata Desr. and violet flowers of Magnolia liliiflora Desr. Molecules 2018, 23, 1558. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.H.; Sathasivam, R.; Nguyen, B.V.; Baek, S.-A.; Yeo, H.J.; Park, Y.E.; Kim, H.H.; Kim, J.K.; Park, S.U. Metabolic Profiling of Primary Metabolites and Galantamine Biosynthesis in Wounded Lycoris radiata Callus. Plants 2020, 9, 1616. https://doi.org/10.3390/plants9111616
Park CH, Sathasivam R, Nguyen BV, Baek S-A, Yeo HJ, Park YE, Kim HH, Kim JK, Park SU. Metabolic Profiling of Primary Metabolites and Galantamine Biosynthesis in Wounded Lycoris radiata Callus. Plants. 2020; 9(11):1616. https://doi.org/10.3390/plants9111616
Chicago/Turabian StylePark, Chang Ha, Ramaraj Sathasivam, Bao Van Nguyen, Seung-A Baek, Hyeon Ji Yeo, Ye Eun Park, Haeng Hoon Kim, Jae Kwang Kim, and Sang Un Park. 2020. "Metabolic Profiling of Primary Metabolites and Galantamine Biosynthesis in Wounded Lycoris radiata Callus" Plants 9, no. 11: 1616. https://doi.org/10.3390/plants9111616
APA StylePark, C. H., Sathasivam, R., Nguyen, B. V., Baek, S.-A., Yeo, H. J., Park, Y. E., Kim, H. H., Kim, J. K., & Park, S. U. (2020). Metabolic Profiling of Primary Metabolites and Galantamine Biosynthesis in Wounded Lycoris radiata Callus. Plants, 9(11), 1616. https://doi.org/10.3390/plants9111616






