Changes in Beneficial C-glycosylflavones and Policosanol Content in Wheat and Barley Sprouts Subjected to Differential LED Light Conditions
Abstract
1. Introduction
2. Results
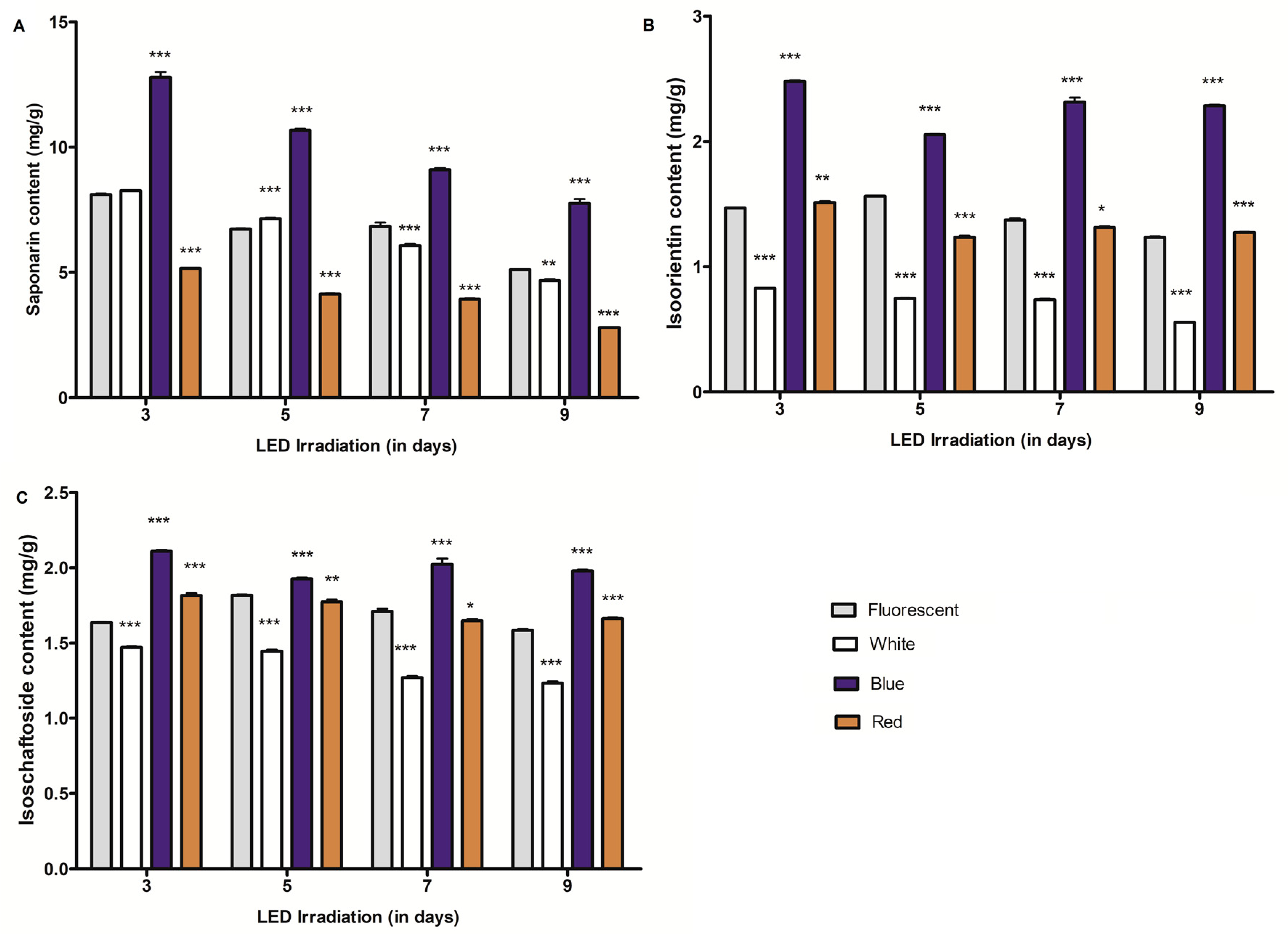
2.1. Changes of C-glycosylflavone Content in Barley and Wheat Seedlings Exposed to Differential LED Light Irradiation
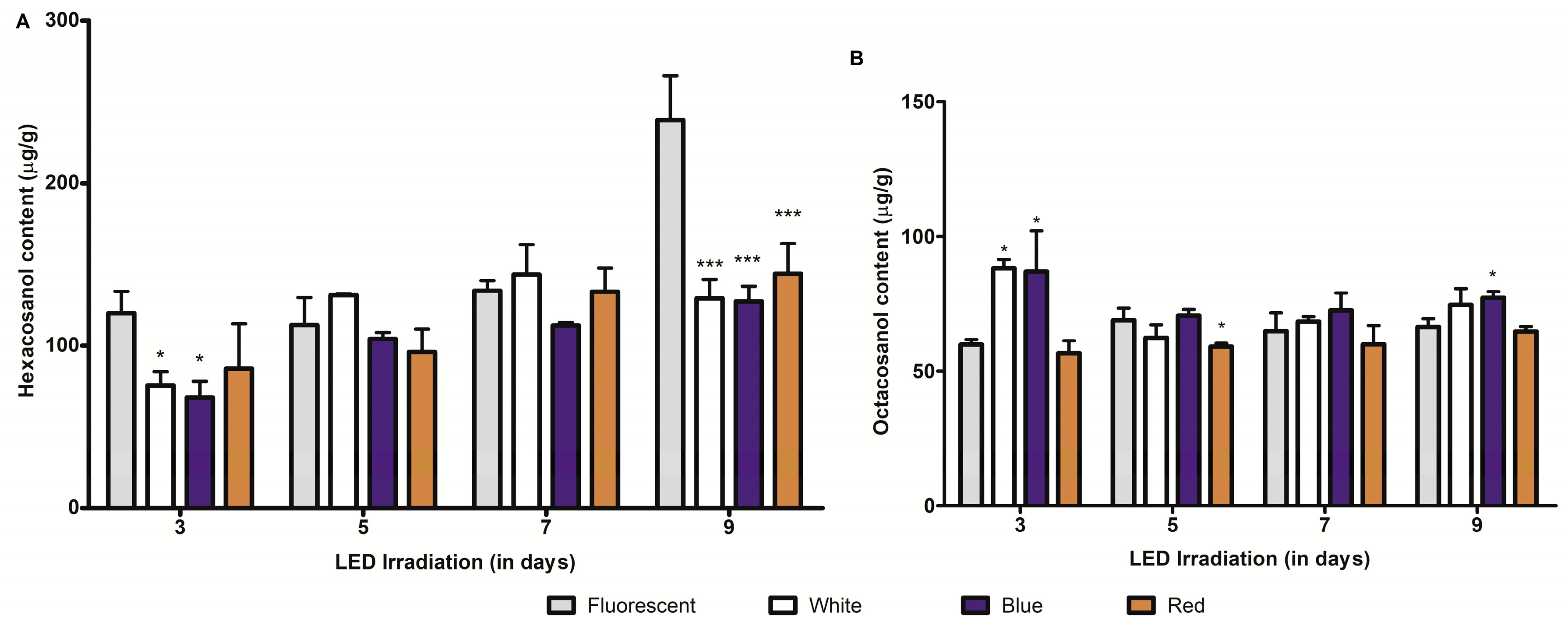
2.2. Effect of LED Light Irradiation on Major Long-Chain Fatty-Alcohol (Policosanol) Biosynthesis in Barley and Wheat Seedlings
2.3. Effect of Growth Periods on C-glycosylflavones and Policosanol Content in Young Barley and Wheat Seedlings
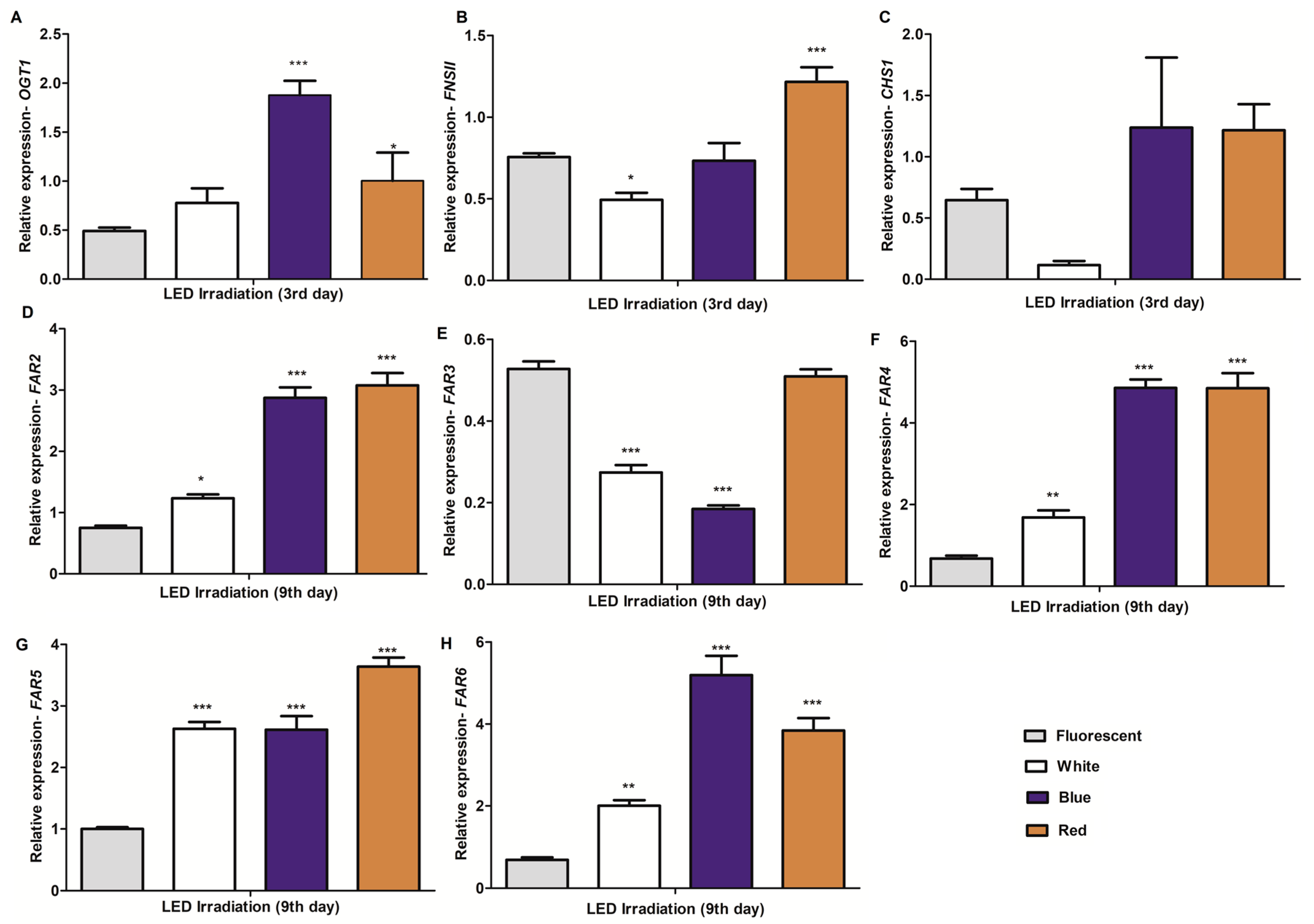
2.4. Expression Profiling of Potential Genes Involved in the Biosynthesis of C-glycosylflavones/Flavonoids and Policosanols in Barley Seedlings under Different Light Conditions and Growth Times
2.5. Influence of LED-Light Irradiation on Seedling Growth
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Growth Condition, and LED Treatment
4.2. Extraction of C-glycosylated Flavones and Measurement
4.3. Extraction and Quantification of Policosanols from Barley and Wheat Seedlings
4.4. Identification of Saponarin and Policosanol Biosynthetic-Related Genes
4.5. Analysis of Expression Pattern of Biosynthetic Related Genes in Seedlings Subjected to LED Light Irradiation
4.6. Phenotyping of LED-Treated Cereal Sprouts
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide research trends on wheat and barley: A bibliometric comparative analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- Byun, A.R.; Chun, H.; Lee, J.; Lee, S.W.; Lee, H.S.; Shim, K.W. Effects of a dietary supplement with barley sprout extract on blood cholesterol metabolism. Evid. Based Complement. Altern. Med. 2015, 473056, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Pu, X.; Yang, J.; Du, J.; Yang, X.; Li, X.; Li, L.; Zhou, Y.; Yang, T. Preventive and therapeutic role of functional ingredients of barley grass for chronic diseases in human beings. Oxid. Med. Cell. Longev. 2018, 2018, 3232080. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jia, Y.; Thach, T.T.; Han, Y.; Kim, B.; Wu, C.; Kim, Y.; Seo, W.D.; Lee, S.J. Hexacosanol reduces plasma and hepatic cholesterol by activation of AMP-activated protein kinase and suppression of sterol regulatory element-binding protein-2 in HepG2 and C57BL/6J mice. Nutr. Res. 2017, 43, 89–99. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, S.H.; Lee, S.; Kim, K.M.; Jung, J.C.; Son, T.G.; Ki, S.H.; Seo, W.D.; Kwak, J.H.; Hong, J.T.; et al. Antioxidant effect of barley sprout extract via enhancement of Nuclear Factor-Erythroid 2 Related Factor 2 activity and glutathione synthesis. Nutrients 2017, 9, 1252. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Hwang, S.H.; Jia, Y.; Seo, W.D.; Lee, S.J. Barley sprout extracts reduce hepatic lipid accumulation in ethanol-fed mice by activating hepatic AMP-activated protein kinase. Food Res. Int. 2017, 101, 209–217. [Google Scholar] [CrossRef]
- Seo, K.H.; Park, M.J.; Ra, J.E.; Han, S.I.; Nam, M.H.; Kim, J.H.; Lee, J.H.; Seo, W.D. Saponarin from barley sprouts inhibits NF-κB and MAPK on LPS-induced RAW 264.7 cells. Food Funct. 2014, 5, 3005–3013. [Google Scholar] [CrossRef]
- Park, M.J.; Seo, W.D.; Kang, Y.H. The antioxidant properties of four korean barley cultivars at different harvest times and profiling of major metabolites. J. Agric. Sci. 2015, 7, 94. [Google Scholar] [CrossRef]
- Seo, W.; Hae, J.; Jia, Y.; Wu, C.; Lee, S. Saponarin activates AMPK in a calcium-dependent manner and suppresses gluconeogenesis and increases glucose uptake via phosphorylation of CRTC2 and HDAC5. Bioorg. Med. Chem. Lett. 2015, 25, 5237–5242. [Google Scholar] [CrossRef]
- Lee, H.; Ra, S.W.J.; Woo, K.L.; Seo, D.; Hwan, J. Saponarin content and biosynthesis-related gene expression in young barley (Hordeum vulgare L.) seedlings. J. Plant Biotechnol. 2019, 2818, 247–254. [Google Scholar] [CrossRef]
- Ye, T.; Su, J.; Huang, C.; Yu, D.; Dai, S.; Huang, X.; Chen, B.; Zhou, M. Isoorientin induces apoptosis, decreases invasiveness, and downregulates VEGF secretion by activating AMPK signaling in pancreatic cancer cells. OncoTargets Targets 2016, 9, 7481–7492. [Google Scholar] [CrossRef]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the flavonoid C-glycosides and health benefits. Crit. Rev. Food Sci. Nutr. 2016, 56, S29–S45. [Google Scholar] [CrossRef]
- Nam, T.G.; Kim, D.-O.; Eom, S.H. Effects of light sources on major flavonoids and antioxidant activity in common buckwheat sprouts. Food Sci. Biotechnol. 2018, 27, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Bosko, P.; Živčák, M.; Brestic, M.; Smetanska, I. Bioactive phytochemicals and antioxidant properties of the grains and sprouts of colored wheat genotypes. Molecules 2018, 23, 2282. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted grains: A comprehensive review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Ra, J.; Woo, S.; Lee, K.; Ja, M.; Young, H.; Mi, H.; Chung, I.; Hyun, D.; Hwan, J.; Duck, W. Policosanol profiles and adenosine 5′-monophosphate-activated protein kinase (AMPK) activation potential of Korean wheat seedling extracts according to cultivar and growth time. Food Chem. 2020, 317, 126388. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Kim-Anne, L.; Van den Broeck, H.C.; Brouns, F.J.P.H.; De Brier, N.; et al. Impact of cereal seed sprouting on its nutritional and technological properties: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef]
- Rico, D.; Peñas, E.; del Carmen García, M.; Martínez-Villaluenga, C.; Rai, D.K.; Birsan, R.I.; Frias, J.; Martín-Diana, A.B. Sprouted barley flour as a nutritious and functional ingredient. Foods 2020, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Aborus, N.E.; Canadanovi, J.; Saponjac, V.T. Powdered barley sprouts: Composition, functionality and polyphenol digestibility. Int. J. Food Sci. Technol. 2017, 52, 231–238. [Google Scholar] [CrossRef]
- Wu, X.; Cai, K.; Zhang, G.; Zeng, F. Metabolite profiling of barley grains subjected to water stress: To explain the genotypic difference in drought-induced impacts on malting quality. Front. Plant. Sci. 2017, 8, 1547. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, X.; Xu, Q.; Mei, X.; Yuan, H.; Jiabu, D.; Sang, Z.; Nyima, T. Metabolite profiling in two contrasting Tibetan hulless barley cultivars revealed the core salt-responsive metabolome and key salt-tolerance biomarkers. AoB Plants 2019, 11, plz021. [Google Scholar] [CrossRef]
- Kang, C.H.; Yoon, E.K.; Muthusamy, M.; Kim, J.A.; Jeong, M.J.; Lee, S.I. Blue LED light irradiation enhances L-ascorbic acid content while reducing reactive oxygen species accumulation in Chinese cabbage seedlings. Sci. Hortic. 2020, 261, 108924. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, Y.; Fang, W.; Tao, J.; Yang, Z.; Yin, Y. iTRAQ-based proteomic and physiological analyses of mustard sprouts in response to heat stress. RSC Adv. 2020, 10, 6052–6062. [Google Scholar] [CrossRef]
- Muthusamy, M.; Hwang, J.E.; Kim, S.H.; Kim, J.A.; Jeong, M.J.; Park, H.C.; Lee, S.I. Elevated carbon dioxide significantly improves ascorbic acid content, antioxidative properties and restricted biomass production in cruciferous vegetable seedlings. Plant. Biotechnol. Rep. 2019, 13, 293–304. [Google Scholar] [CrossRef]
- Tuan, P.A.; Thwe, A.A.; Kim, Y.B.; Kim, J.K.; Kim, S.J.; Lee, S.; Chung, S.O.; Park, S.U. Effects of white, blue, and red light-emitting diodes on carotenoid biosynthetic gene expression levels and carotenoid accumulation in sprouts of tartary buckwheat (Fagopyrum tataricum gaertn.). J. Agric. Food Chem. 2013, 61, 12356–12361. [Google Scholar] [CrossRef]
- Meng, T.; Nakamura, E.; Irino, N.; Joshi, K.R.; Devkota, H.P.; Yahara, S.; Kondo, R. Effects of irradiation with light of different photon densities on the growth of young green barley plants. Agric. Sci. 2015, 06, 208–216. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bashir, T.; Bae, H. Use of utrasonication tchnology for the icreased poduction of pant scondary mtabolites. Molecules 2017, 22, 1046. [Google Scholar] [CrossRef]
- Kowalska, I.; Jedrejek, D.; Jonczyk, K.; Stochmal, A. UPLC-PDA-ESI-MS analysis and TLC-DPPH activity of wheat varieties. Acta Chromatogr. 2019, 31, 151–156. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E. Increases in shoot tissue pigments, glucosinolates, and mineral elements in sprouting broccoli after exposure to short-duration blue light from light emitting diodes. J. Am. Soc. Hortic. Sci. 2013, 138, 31–37. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Yu, C.Y.; Chung, I.M. Assessment of the phenolic profile, antimicrobial activity and oxidative stability of transgenic Perilla frutescens L.overexpressing tocopherol methyltransferase (γ-tmt) gene. Plant. Physiol. Biochem. 2017, 118, 77–87. [Google Scholar] [CrossRef]
- Brauch, D.; Porzel, A.; Schumann, E.; Pillen, K.; Mock, H.P. Changes in isovitexin-O-glycosylation during the development of young barley plants. Phytochemistry 2018, 148, 11–20. [Google Scholar] [CrossRef]
- Marinova, K.; Kleinschmidt, K.; Weissenböck, G.; Klein, M. Flavonoid biosynthesis in barley primary leaves requires the presence of the vacuole and controls the activity of vacuolar flavonoid transport. Plant. Physiol. 2007, 144, 432–444. [Google Scholar] [CrossRef]
- Kamiyama, M.; Shibamoto, T. Flavonoids with potent antioxidant activity found in young green barley leaves. J. Agric. Food Chem. 2012, 60, 6260–6267. [Google Scholar] [CrossRef]
- Irmak, S.; Dunford, N.T.; Milligan, J. Policosanol contents of beeswax, sugar cane and wheat extracts. Food Chem. 2006, 95, 312–318. [Google Scholar] [CrossRef]
- Seo, W.D.; Yuk, H.J.; Curtis-long, M.J.; Jang, K.C.; Lee, J.H.; Han, S.; Kang, H.W.; Nam, M.H.; Lee, S.; Lee, J.H.; et al. Effect of the growth stage and cultivar on policosanol profiles of barley sprouts and their adenosine 5′-monophosphate-activated protein kinase activation. J. Agric. Food Chem. 2013, 61, 1117–1123. [Google Scholar] [CrossRef]
- Sharma, R.; Matsuzaka, T.; Kaushik, M.K.; Sugasawa, T.; Ohno, H.; Wang, Y.; Motomura, K.; Shimura, T.; Okajima, Y.; Mizunoe, Y.; et al. Octacosanol and policosanol prevent high-fat diet-induced obesity and metabolic disorders by activating brown adipose tissue and improving liver metabolism. Sci. Rep. 2019, 9, 5169. [Google Scholar] [CrossRef]
- Richardson, A.; Franke, R.; Kerstiens, G.; Jarvis, M.; Schreiber, L.; Fricke, W. Cuticular wax deposition in growing barley (Hordeum vulgare) leaves commences in relation to the point of emergence of epidermal cells from the sheaths of older leaves. Planta 2005, 222, 472–483. [Google Scholar] [CrossRef]
- Gil, K.E.; Park, C.M. Thermal adaptation and plasticity of the plant circadian clock. New Phytol. 2019, 221, 1215–1229. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Sun, Y.; Wang, Y.; Li, T.; Chai, G.; Jiang, W.; Shan, L.; Li, C.; Xiao, E.; et al. FAR5, a fatty acyl-coenzyme A reductase, is involved in primary alcohol biosynthesis of the leaf blade cuticular wax in wheat (Triticum aestivum L.). J. Exp. Bot. 2015, 66, 1165–1178. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; You, Q.; Luo, W.; Wang, C.; Zhao, S.; Chai, G.; Li, T.; Shi, X.; Li, C.; et al. Three Fatty Acyl-Coenzyme A Reductases, BdFAR1, BdFAR2 and BdFAR3, are involved in cuticular wax primary alcohol biosynthesis in Brachypodium distachyon. Plant. Cell Physiol. 2018, 59, 527–543. [Google Scholar] [CrossRef]
- Wang, M.; Wu, H.; Xu, J.; Li, C.; Wang, Y.; Wang, Z. Five fatty acyl-coenzyme a reductases are involved in the biosynthesis of primary alcohols in Aegilops tauschii leaves. Front. Plant Sci. 2017, 8, 1012. [Google Scholar] [CrossRef]
- NCBI Gene. Available online: https://www.ncbi.nlm.nih.gov/gene (accessed on 7 August 2020).
- PlantsDB. Available online: http://pgsb.helmholtz-muenchen.de/plant/search.jsp (accessed on 7 August 2020).
- GrainGenes. Available online: https://wheat.pw.usda.gov/blast/ (accessed on 7 August 2020).
- NCBI Conserved Domains. Available online: https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi (accessed on 8 August 2020).
- Social Science Statistics. Available online: https://www.socscistatistics.com/tests/pearson/default.aspx. (accessed on 26 October 2020).


| Metabolite Content vs. Gene Expression | ||||||||
|---|---|---|---|---|---|---|---|---|
| HvOGT1 | HvFNSII | HvCHS1 | HvFAR2 | HvFAR3 | HvFAR4 | HvFAR5 | HvFAR6 | |
| Saponarin | 0.68 (p = 0.01) ** | −0.53 (p = 0.07) | 0.13 (p = 0.68) | -- | -- | -- | -- | -- |
| Hexacosanol | -- | -- | -- | −0.59 (p = 0.04) * | 0.67 ( p= 0.01) ** | −0.62 (p = 0.03) * | −0.76 (p = 0.004) ** | −0.69 (p = 0.01) ** |
| Growth periods (–9 days) vs. metabolite content | ||||||||
| Fluorescent | White | Blue | Red | |||||
| Saponarin | −0.93 (p = 0.00001) **** | −0.99 (p = 0.00001) **** | −0.99 (p = 0.00001) **** | −0.97 (p = 0.00001) **** | ||||
| Isoorientin | −0.82 (p = 0.001) *** | −0.92 (p = 0.00002) **** | −0.23 (p = 0.47) | −0.66 (p = 0.01) ** | ||||
| Isoschaftoside | −0.32 (p = 0.31) | −0.94 (p = 0.00001) **** | −0.47 (p = 0.12) | −0.90 (p = 0.00006) **** | ||||
| Hexacosanol | 0.79 (p = 0.001) *** | 0.69 (p = 0.01) ** | 0.91 (p = 0.00004) **** | 0.81 (p = 0.001) *** | ||||
| Octacosanol | −0.07 (p = 0.82) | −0.43 (p = 0.16) | −0.17 (p = 0.57) | −0.04 (p = 0.90) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthusamy, M.; Kim, J.H.; Kim, S.H.; Kim, J.Y.; Heo, J.W.; Lee, H.; Lee, K.-S.; Seo, W.D.; Park, S.; Kim, J.A.; et al. Changes in Beneficial C-glycosylflavones and Policosanol Content in Wheat and Barley Sprouts Subjected to Differential LED Light Conditions. Plants 2020, 9, 1502. https://doi.org/10.3390/plants9111502
Muthusamy M, Kim JH, Kim SH, Kim JY, Heo JW, Lee H, Lee K-S, Seo WD, Park S, Kim JA, et al. Changes in Beneficial C-glycosylflavones and Policosanol Content in Wheat and Barley Sprouts Subjected to Differential LED Light Conditions. Plants. 2020; 9(11):1502. https://doi.org/10.3390/plants9111502
Chicago/Turabian StyleMuthusamy, Muthusamy, Jong Hee Kim, Suk Hee Kim, Joo Yeol Kim, Jeong Wook Heo, HanGyeol Lee, Kwang-Sik Lee, Woo Duck Seo, Soyoung Park, Jin A Kim, and et al. 2020. "Changes in Beneficial C-glycosylflavones and Policosanol Content in Wheat and Barley Sprouts Subjected to Differential LED Light Conditions" Plants 9, no. 11: 1502. https://doi.org/10.3390/plants9111502
APA StyleMuthusamy, M., Kim, J. H., Kim, S. H., Kim, J. Y., Heo, J. W., Lee, H., Lee, K.-S., Seo, W. D., Park, S., Kim, J. A., & Lee, S. I. (2020). Changes in Beneficial C-glycosylflavones and Policosanol Content in Wheat and Barley Sprouts Subjected to Differential LED Light Conditions. Plants, 9(11), 1502. https://doi.org/10.3390/plants9111502







