Physiological Performance of Pyrus pyraster L. (Burgsd.) and Sorbus torminalis (L.) Crantz Seedlings under Drought Treatment
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Experimental Design
- Mn = moisture content (%) of material n
- Ww = wet weight of the sample
- Wd = weight of the sample after drying
2.3. Measurement and Analysis of Plant Parameters
2.4. Leaf Gas Exchange
2.5. Leaf Water Potential and Relative Water Content
2.6. Statistical Analysis
3. Results
3.1. Growth and Biomass Allocation in the Plant Organs
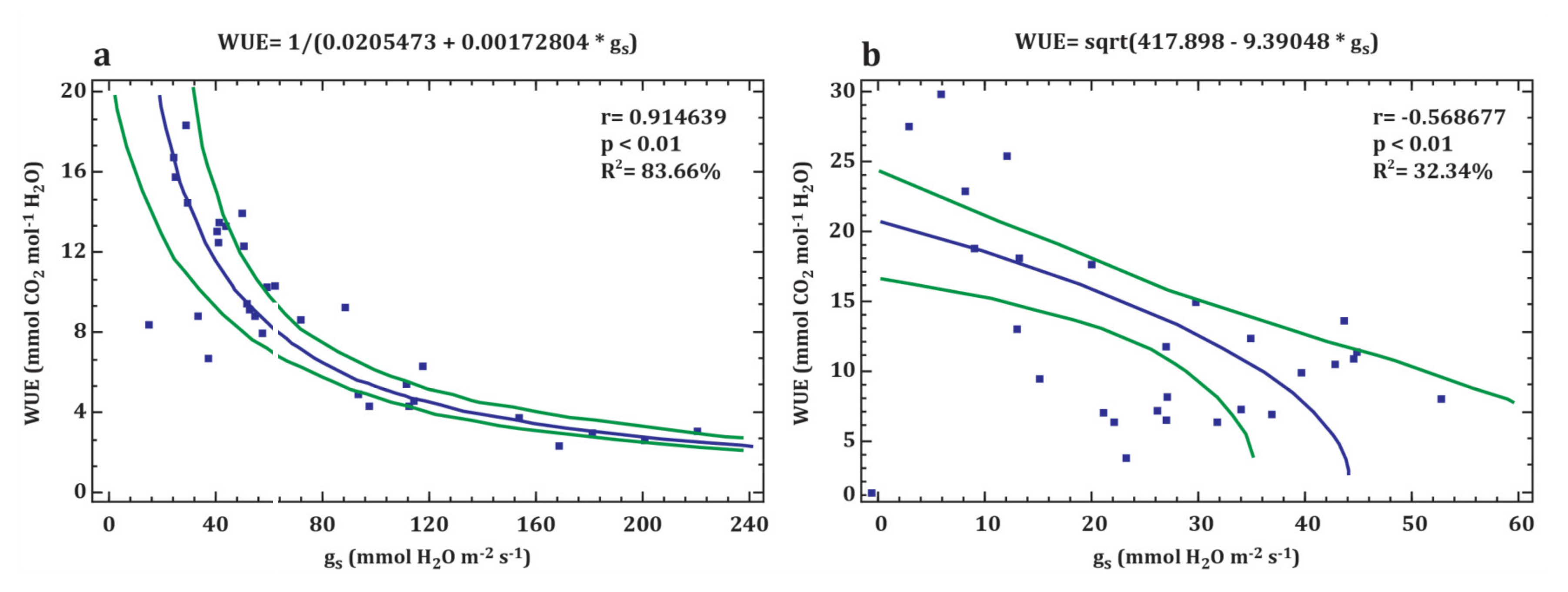
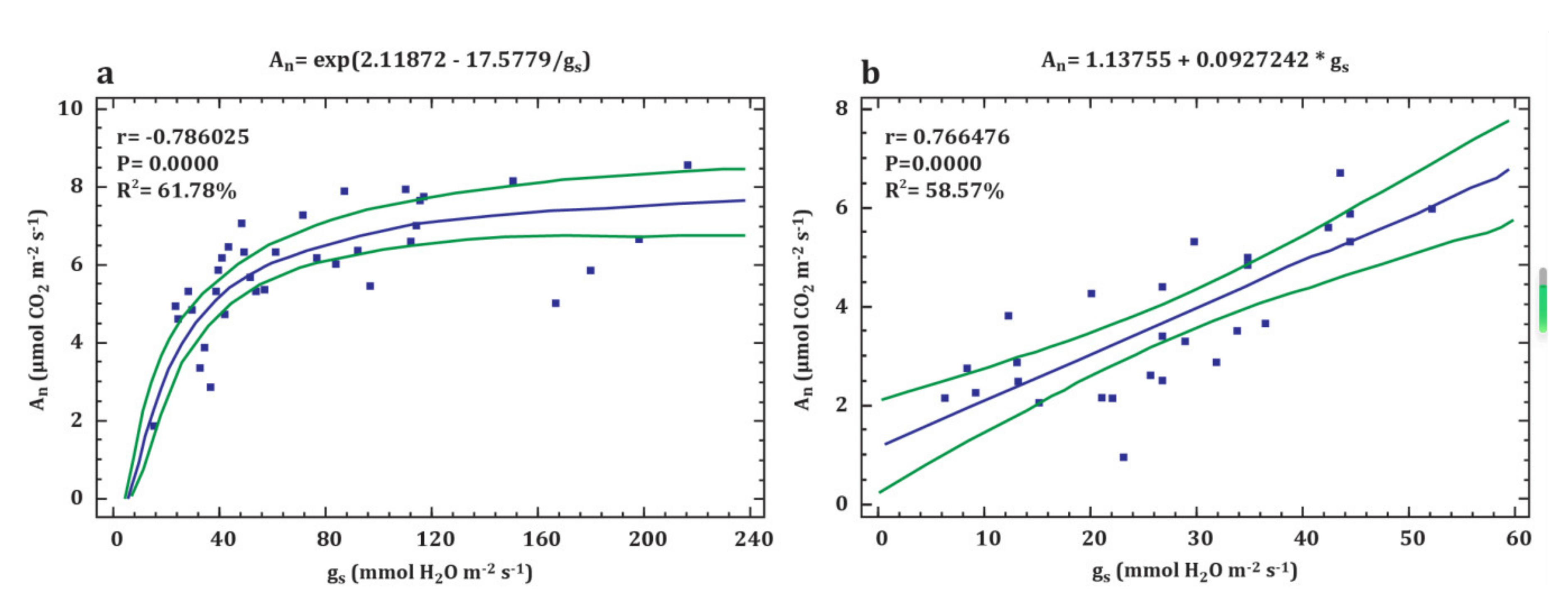
3.2. Leaf Gas Exchange
3.3. Leaf Water Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Donovan, L.; Ehleringer, J.R. Contrasting water-use patterns among size and life-history classes of a semi-arid shrub. Funct. Ecol. 1992, 6, 482–488. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Yepez, E.A.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Yang, W.; Wang, C.; Gu, C.; Niu, D.D.; Liu, H.X.; Wang, Y.P.; Guo, J.H. Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS ONE 2012, 7, e52565. [Google Scholar] [CrossRef]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Huang, Y.; Gerber, S.; Huang, T.; Lichstein, J.W. Evaluating the drought response of CMIP5 models using global gross primary productivity, leaf area, precipitation, and soil moisture data. Glob. Biogeochem. Cycles 2016, 30, 1827–1846. [Google Scholar] [CrossRef]
- Zhao, M.; Running, S.W. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science 2010, 329, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Kunz, J.; Räder, A.; Bauhus, J. Effects of drought and rewetting on growth and gas exchange of minor European broadleaved tree species. Forests 2016, 7, 239. [Google Scholar] [CrossRef]
- Gregory, P.J.; Simmonds, L.P.; Pilbeam, C.J. Soil type, climatic regime, and the response of water use efficiency to crop management. Agron. J. 2000, 92, 814–820. [Google Scholar] [CrossRef]
- Gebrekirstos, A.; Van Noordwijk, M.; Neufeldt, H.; Mitlöhner, R. Relationships of stable carbon isotopes, plant water potential and growth: An approach to assess water use efficiency and growth strategies of dry land agroforestry species. Trees 2011, 25, 95–102. [Google Scholar] [CrossRef]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Tardieu, F.; Davies, W.J. Integration of hydraulic and chemical signalling in the control of stomatal conductance and water status of droughted plants. Plant Cell Environ. 1993, 16, 341–349. [Google Scholar] [CrossRef]
- Hammer, G.; Cooper, M.; Tardieu, F.; Welch, S.; Walsh, B.; Eeuwijk, F.; Chapman, S.; Podlich, D. Models for navigating biological complexity in breeding improved crop plants. Trends Plant Sci. 2006, 11, 587–593. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups; Springer Science & Business Media: Berlin, Germany, 2003. [Google Scholar]
- Roper, P. The distribution of the wild service tree, Sorbus torminalis (L.) Crantz, in the British Isles. Watsonia 1993, 19, 209–229. [Google Scholar]
- Kausch-Blecken Von Schmeling, W. Die Elsbeere (Sorbus torminalis (L.) Crantz; Selbstverlag: Bovenden, Germany, 1994; p. 253. [Google Scholar]
- Wagner, I. Identifikation von Wildapfel (Malus sylvestris (L.) Mill.) und Wildbirne (Pyrus pyraster (L.) Burgsd.). Forstarchiv 1995, 66, 39–47. [Google Scholar]
- Paganová, V. Wild pear Pyrus pyraster (L.) Burgsd. requirements on environmental conditions. Ekológia (Bratislava) 2003, 22, 225–241. [Google Scholar]
- Paganová, V. Ecology and distribution of Sorbus torminalis (L.) Crantz. in Slovakia. Hortic. Sci. 2007, 34, 138–151. [Google Scholar] [CrossRef]
- Paganová, V. Ecological requirements of wild service tree (Sorbus torminalis [L.] Crantz.) and service tree (Sorbus domestica L.) in relation with their utilization in forestry and landscape. J. For. Sci. 2008, 54, 216–226. [Google Scholar] [CrossRef]
- Rameau, J.C.; Mansion, D.; Dumé, G.; Timbal, J.; Lecointe, A.; Dupont, P.; Keller, R. Flore Forestière Française. Plaines et Collines; IDF: Paris, France; ENGREF: Nancy, France, 1989; Volume 1. [Google Scholar]
- Májovský, J.; Crantz, S.L. Flóra Slovenska IV/3; Berthová, L., Ed.; Vydavateľstvo SAV: Bratislava, Slovakia, 1992; pp. 401–446. [Google Scholar]
- Drapier, N. Ecologie de l‘alisier torminal Sorbus torminalis (L.) crantz. Rev. For. Fr. 1993, 45, 229–242. [Google Scholar] [CrossRef]
- Wilhelm, G.J. Im vergleich mit elsbeere und speierling beobachtungen zur wildbirne. Allg. Forstz. Wald 1998, 16, 856–859. [Google Scholar]
- Wagner, I.; Büttner, R. Hybridization in wild pear (Pyrus pyraster) from various regions in Germany and from Luxembourg with respect to Pyrus × communis. In III International Symposium on Horticulture in Europe-SHE2016; International Society for Horticultural Science: Leuven, Belgium, 2016; Volume 1242, pp. 427–434. [Google Scholar]
- Stephan, B.R.; Wagner, I.; Kleinschmit, J. EUFORGEN Technical Guidelines for Genetic Conservation and Use for Wild Apple and Pear (Malus sylvestris and Pyrus pyraster); International Plant Genetic Resources Institute: Rome, Italy, 2003; p. 6. Available online: http://www.euforgen.org/publications/publication/imalus-sylvestrisi-and-ipyrus-pyrasteri-technical-guidelines-for-genetic-conservation-an/ (accessed on 18 August 2020).
- Zohary, D. Wild apples and wild pears. Bocconea 1997, 7, 409–416. [Google Scholar]
- Milner, E. Trees of Britain and Ireland; Natural History Museum: London, UK, 2011. [Google Scholar]
- Ellenberg, H. Vegetation Mitteleuropas Mit Den Alpen in Ökologischer Sicht, 3rd ed.; Ulmer Verlag: Stuttgart, Germany, 1983. [Google Scholar]
- Peniašteková, M.; Pyrus, L. Hruška. In Flóra Slovenska IV/3; Berthová, L., Ed.; Vydavateľstvo SAV: Bratislava, Slovakia, 1992; pp. 384–388. [Google Scholar]
- Machar, I. Historical development of floodplain forests in the Upper Moravian Vale (Vrapač National Nature Reserve, Czech Republic). J. For. Sci. 2008, 54, 426–437. [Google Scholar] [CrossRef]
- Ewald, C.; Zander, M.; Jander, A. Die elsbeere (Sorbus torminalis/L./crantz.) in brandenburg. Der Wald 1994, 44, 232–235. [Google Scholar]
- Dinca, L. Elsbeere in Rumänien. Corminaria 2000, 14, 28. [Google Scholar]
- Lapin, M.; Faško, P.; Melo, M.; Štastný, P.; Tomlain, J. Klimatické oblasti. In Atlas Krajiny Slovenskej Republiky; Ministerstvo Životného Prostredia SR: Bratislava, Slovakia, 2002; p. 344. [Google Scholar]
- Meier, U. Phenological growth stages. In Phenology: An Integrative Environmental Science; Tasks for Vegetation Science; Schwartz, M.D., Ed.; Springer: Dordrecht, The Netherlands, 2003; pp. 269–283. [Google Scholar]
- Poorter, H.; Bühler, J.; Van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef]
- Trautmann, N.; Richard, T. Moisture Content. Cornell Waste Management Institute. 1996. Available online: http://compost.css.cornell.edu/calc/moisture_content.html (accessed on 15 February 2019).
- Measures, M.; Weinberger, P.; Baer, H. Variability of plant growth within controlled-environment chambers as related to temperature and light distribution. Can. J. Plant Sci. 1973, 53, 215–220. [Google Scholar] [CrossRef]
- Porter, A.S.; Evans-FitzGerald, C.; McElwain, J.C.; Ziotis, C.; Elliott-Kingston, C. How well do you know your growth chambers? Testing for chamber effect using plant traits. Plant Methods 2015, 11, 44. [Google Scholar] [CrossRef]
- Evans, G.C. The Quantitative Analysis of Plant Growth; Blackwell Scientific Publications: Oxford, UK, 1972. [Google Scholar]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficit in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, H.; Song, X.; Jin, J.; Zhang, X. The responses of plant leaf CO2/H2O exchange and water use efficiency to drought: A meta-analysis. Sustainability 2018, 10, 551. [Google Scholar] [CrossRef]
- Buckley, T.N. The control of stomata by water balance. New Phytol. 2005, 168, 275–292. [Google Scholar] [CrossRef]
- Caldeira, C.F.; Bossio, M.; Parent, B.; Jeanguenin, L.; Chaumont, F.; Tardieu, F. A hydraulic model is compatible with rapid changes in leaf elongation under fluctuating evaporative demand and soil water status. Plant Physiol. 2014, 164, 1718–1730. [Google Scholar] [CrossRef]
- Pyttel, P.; Kunz, J.; Bauhus, J. Growth regeneration and shade tolerance of wild Service trees (Sorbus torminalis L.Cranz.) in aged oak coppice forests. Trees 2013, 27, 1609–1619. [Google Scholar] [CrossRef]
- Basso, B.; Ritchie, J.T. Evapotranspiration in high-yielding maize and under increased vapor pressure deficit in the US Midwest. Agric. Environ. Lett. 2018, 3, 170039. [Google Scholar] [CrossRef]
- Héroult, A.; Lin, Y.S.; Bourne, A.; Medlyn, B.E.; Ellsworth, D.S. Optimal stomatal conductance in relation to photosynthesis in climatically contrasting Eucalyptus species under drought. Plant Cell Environ. 2013, 36, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Holbrook, N.M.; Zwieniecki, M.A.; Palma, B. Leaf hydraulic capacity in ferns, conifers and angiosperms: Impacts on photosynthetic maxima. New Phytol. 2005, 165, 839–846. [Google Scholar] [CrossRef]
- Franks, P.J. Higher rates of leaf gas exchange are associated with higher leaf hydrodynamic pressure gradients. Plant Cell Environ. 2006, 29, 584–592. [Google Scholar] [CrossRef]
- Dias, M.C.; Brüggemann, W. Water-use efficiency in Flaveria species under drought-stress conditions. Photosynthetica 2010, 48, 469–473. [Google Scholar] [CrossRef]


| Taxon | Location | Exposure | Altitude (m) | TI. (°C) | TVII. (°C) | Precipitation (mm) | Type |
|---|---|---|---|---|---|---|---|
| P. pyraster | Kremnica hills (Tŕnie) | S | 540 | −3 | 18 | 750 | MW |
| S. torminalis | Central Moravian Carpathians (Vršava) | S | 500 | −3 | 18 | 500–550 | W |
| p-Value | Control | Drought | |||||
|---|---|---|---|---|---|---|---|
| Parameter | T | R | T*R | P. pyraster | S. torminalis | P. pyraster | S. torminalis |
| Stem length (mm) | <0.01 | <0.01 | 0.63 | 188.36 (±47.37) a | 148.50 (±54.25) ac | 117.62 (±15.11) c | 88.45 (±20.40) b |
| Stem increment (mm) | <0.01 | <0.01 | 0.57 | 161.54 (±46.09) a | 122.00 (±53.21) a | 86.92 (±19.00) c | 59.64 (±21.48) b |
| Root length (mm) | 0.06 | <0.01 | 0.06 | 3494.27 (±1013.42) b | 4765.23 (±1669.71) a | 2861.40 (±803.77) b | 2840.07(±983.51) b |
| Specific root length (mm·mg−1) | <0.01 | <0.01 | 0.76 | 8.12 (±3.65) a | 11.04 (±2.92) b | 10.73 (±3.06) ab | 14.23 (±3.76) c |
| Leaf area (mm2) | <0.01 | <0.01 | 0.43 | 11,186.40 (±3571.79) a | 16.515.60 (±8089.85) b | 4806.37 (±1547.99) c | 8083.09 (±2364.56) d |
| Specific leaf area (mm2·mg−1) | <0.05 | <0.05 | <0.01 | 19.78 (±1.11) a | 19.35 (±2.22) a | 16.45 (±2.49) b | 19.67 (±1.85) a |
| Dry weight of stem (mg) | 0.71 | <0.01 | 0.32 | 500.29 (±185.16) a | 536.92 (±325.40) a | 287.77 (±97.57) b | 209.55 (±118.07) b |
| Dry weight of leaves (mg) | <0.05 | <0.01 | 0.18 | 567.07 (±184.30) a | 886.08 (±484.80) b | 299.39 (±104.10) c | 412.88 (±120.87) d |
| Dry weight of shoot (mg) | 0.14 | <0.01 | 0.22 | 1067.36 (±356.08) a | 1423.00 (±793.26) a | 587.16 (±197.83) b | 622.42 (±214.24) b |
| Dry weight of root (mg) | 0.08 | <0.01 | 0.72 | 478.64 (±171.11) a | 385.75 (±215.24) ac | 278.69 (±80.95) bc | 217.64 (±106.81) b |
| Total biomass (mg) | 0.48 | <0.01 | 0.39 | 1546.00 (±503.63) a | 1808.75 (±991.87) a | 865.86 (±245.19) b | 840.06 (±307.14) b |
| R:S (root to shoot ratio) | <0.01 | 0.07 | 0.79 | 0.45 (±0.10) a | 0.27 (±0.07) b | 0.50 (±0.18) a | 0.35 (±0.09) c |
| Duration of Experiment | 30th Day of Treatment | 40th Day of Treatment | 50th Day of Treatment | ||||
|---|---|---|---|---|---|---|---|
| Parameter | Taxon | Drought | Control | Drought | Control | Drought | Control |
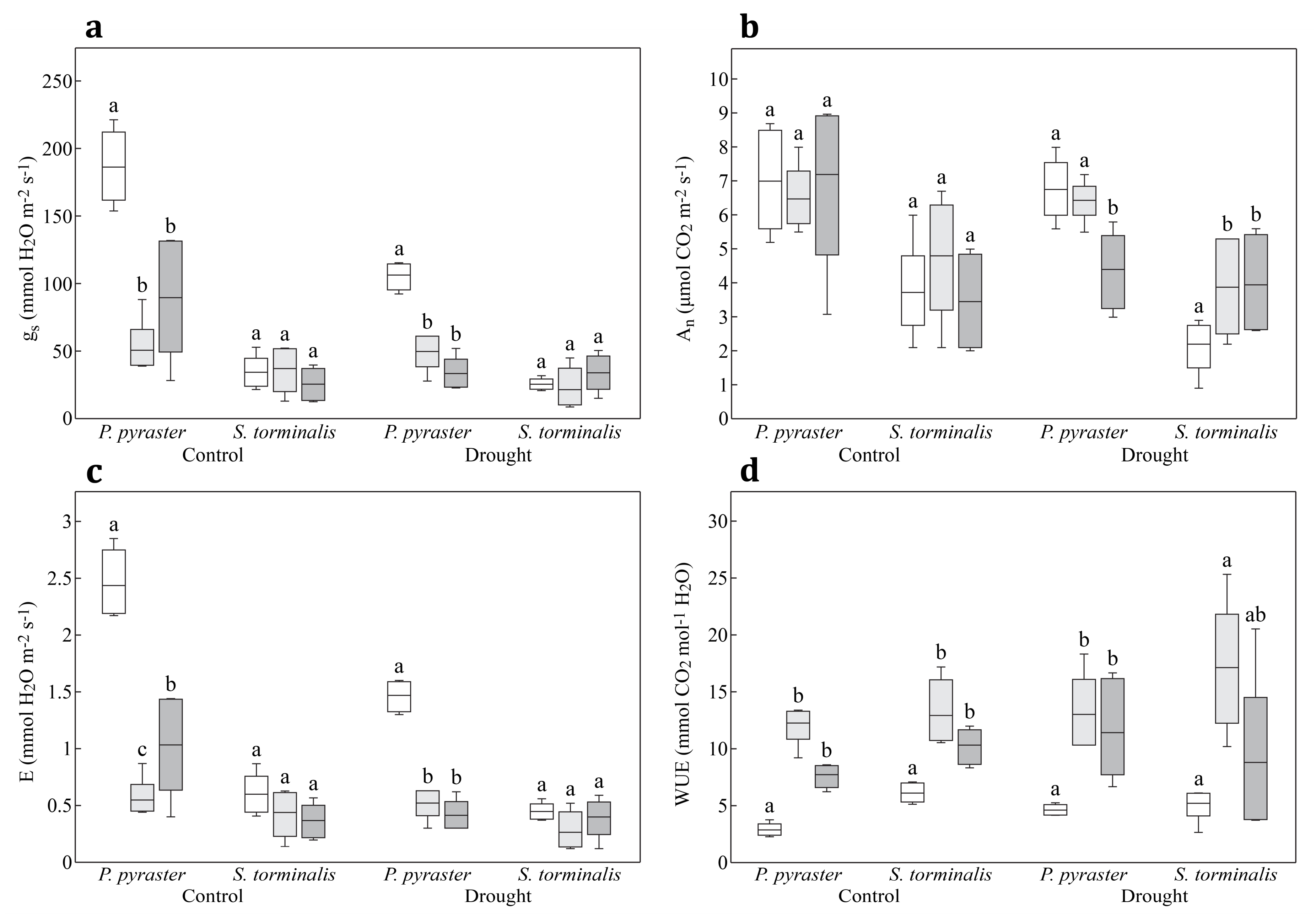
| gs mmol H2O m−2·s−1 | P. pyraster | 106.00 ± 10.32 a | 185.20 ± 26.47 b | 49.80 ± 13.48 a | 50.60 ± 20.19 a | 33.60 ± 11.72 a | 91.00 ± 31.21 b |
| S. torminalis | 25.8 ± 4.21 c | 34.60 ± 11.85 c | 21.80 ± 15.35 b | 36.00 ± 11.57 ab | 31.25 ± 9.74 a | 26.00 ± 11.92 a | |
| An μmol CO2 m−2·s−1 | P. pyraster | 6.76 ± 0.88 a | 7.00 ± 1.49 a | 6.44 ± 0.61 a | 6.48 ± 0.94 a | 4.40 ± 1.14 a | 7.15 ± 1.12 b |
| S. torminalis | 2.20 ± 0.78 b | 3.72 ± 1.41 b | 3.88 ± 1.42 b | 4.80 ± 1.76 ab | 3.95 ± 1.53 a | 3.45 ± 1.47 a | |
| E mmol H2O m−2·s−1 | P. pyraster | 1.47 ± 0.14 b | 2.44 ± 0.30 a | 0.52 ± 0.13 a | 0.55 ± 0.18 a | 0.41 ± 0.13 a | 1.03 ± 0.29 b |
| S. torminalis | 0.45 ± 0.07 c | 0.60 ± 0.18 c | 0.26 ± 0.17 b | 0.45 ± 0.14 ab | 0.40 ± 0.18 a | 0.37 ± 0.15 a | |
| WUE mmol CO2 mol−1 H2O | P. pyraster | 4.60 ± 0.48 b | 2.88 ± 0.57 a | 13.02 ± 3.32 a | 12.27 ± 1.75 a | 11.42 ± 4.46 ac | 7.73 ± 1.06 a |
| S. torminalis | 5.49 ± 0.29 c | 6.11 ± 0.85 c | 17.13 ± 5.59 a | 12.93 ± 2.97 a | 7.85 ± 2.47 ab | 10.33 ± 1.60 bc | |
| DOT | Parameter | p-Value | Control | Drought | ||||
|---|---|---|---|---|---|---|---|---|
| T | R | T*R | P. pyraster | S. torminalis | P. pyraster | S. torminalis | ||
| 40th | Ψwl (MPa) | <0.01 | 0.04 | <0.01 | −0.44 (±0.10) a | −0.81 (±0.18) b | −1.27 (±0.17) c | −1.15 (±0.13) c |
| RWC (%) | 0.59 | <0.01 | 0.05 | 93.62 (±3.24) a | 90.59 (±1.72) ad | 76.50 (±3.80) b | 81.52 (±3.02) bc | |
| 50th | Ψwl (MPa) | <0.01 | <0.01 | 0.31 | −0.49 (±0.13) a | −0.84 (±0.34) b | −1.33 (±0.18) c | −1.53 (±0.08) d |
| RWC (%) | 0.14 | <0.01 | 0.30 | 94.56 (±0.55) a | 89.30 (±2.45) bd | 79.92 (±5.83) bc | 78.89 (±1.82) c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paganová, V.; Hus, M.; Jureková, Z. Physiological Performance of Pyrus pyraster L. (Burgsd.) and Sorbus torminalis (L.) Crantz Seedlings under Drought Treatment. Plants 2020, 9, 1496. https://doi.org/10.3390/plants9111496
Paganová V, Hus M, Jureková Z. Physiological Performance of Pyrus pyraster L. (Burgsd.) and Sorbus torminalis (L.) Crantz Seedlings under Drought Treatment. Plants. 2020; 9(11):1496. https://doi.org/10.3390/plants9111496
Chicago/Turabian StylePaganová, Viera, Marek Hus, and Zuzana Jureková. 2020. "Physiological Performance of Pyrus pyraster L. (Burgsd.) and Sorbus torminalis (L.) Crantz Seedlings under Drought Treatment" Plants 9, no. 11: 1496. https://doi.org/10.3390/plants9111496
APA StylePaganová, V., Hus, M., & Jureková, Z. (2020). Physiological Performance of Pyrus pyraster L. (Burgsd.) and Sorbus torminalis (L.) Crantz Seedlings under Drought Treatment. Plants, 9(11), 1496. https://doi.org/10.3390/plants9111496





