Mycorrhizal Fungi as Bioprotectors of Crops Against Verticillium Wilt—A Hypothetical Scenario Under Changing Environmental Conditions
Abstract
1. Mycorrhizal Fungi Can Protect Crops against Soil-Borne Pathogens
2. Climate Change Can Influence the Development, Incidence and Impact of Crop Diseases
3. Climate Change Can Affect Mycorrhizal Fungal Communities and Mycorrhizal Fungal-Crop Interactions
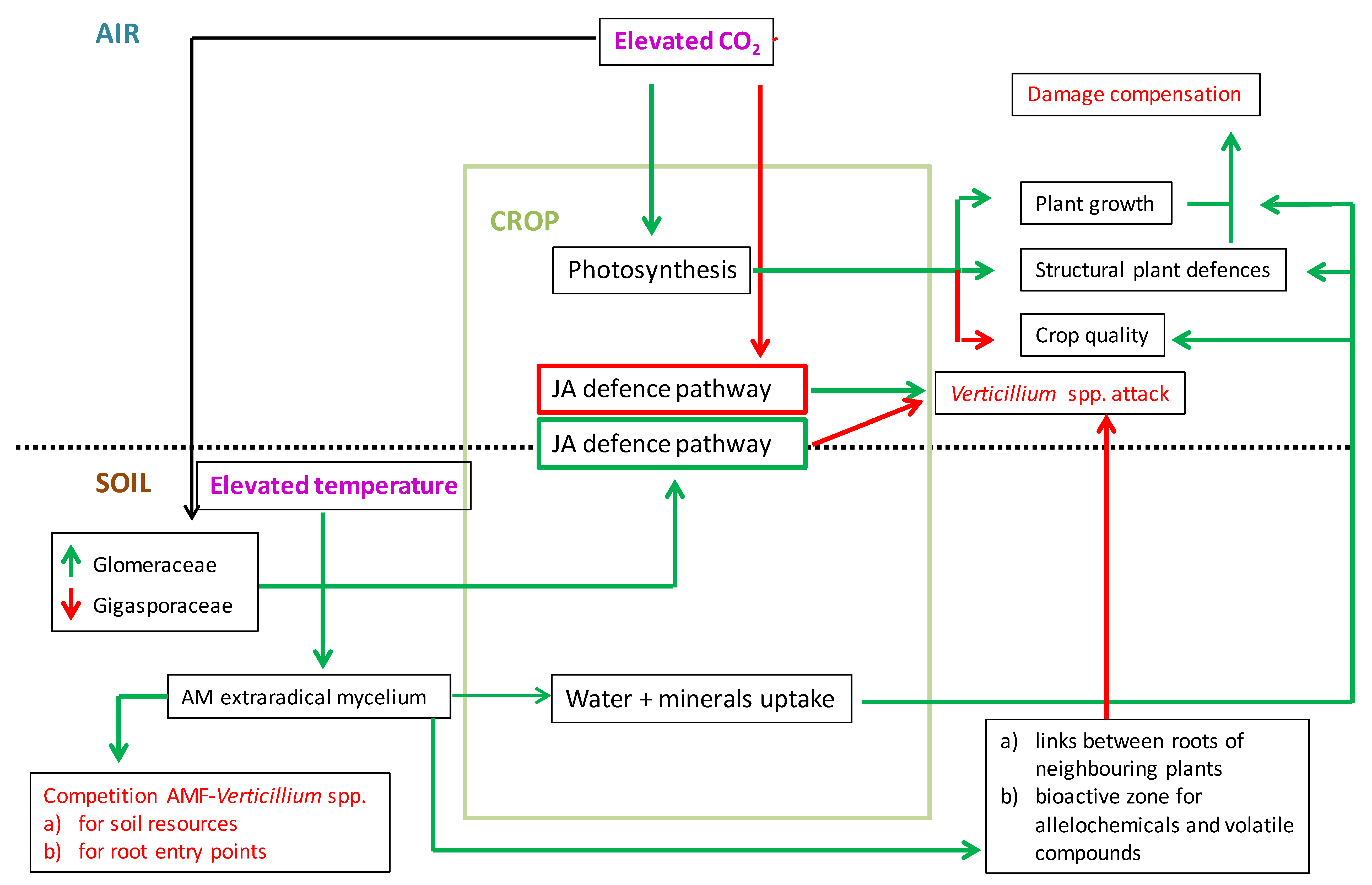
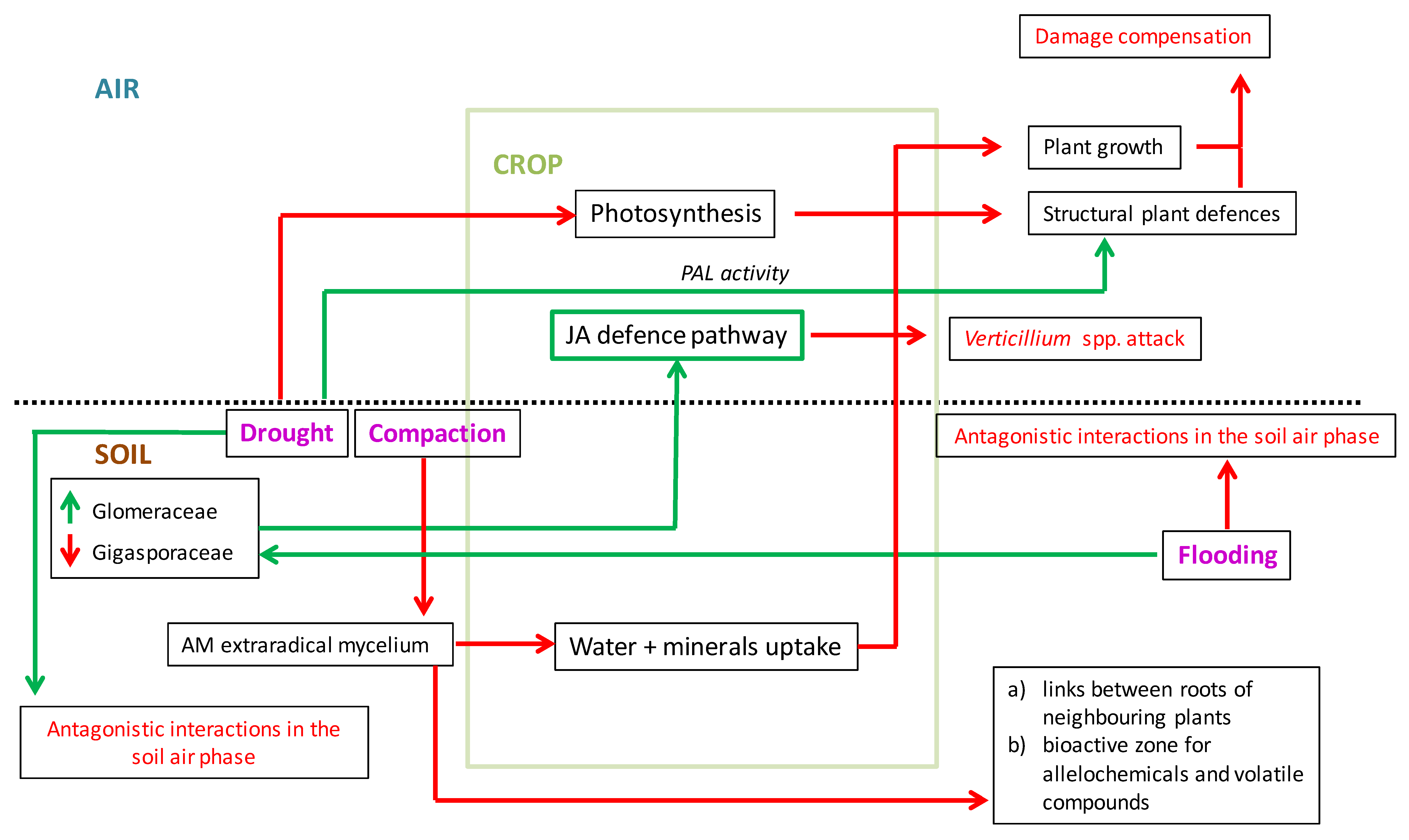
4. Mycorrhizal Protection of Crops Against Verticillium Wilt Under Climate Change Scenarios: A Perspective Based on the Limited Current Scientific Information
5. Conclusions and Perspective
Funding
Conflicts of Interest
References
- Johnson, N.C.; Gehring, C.A. Mycorrhizas: Symbiotic Mediators of Rhizosphere and Ecosystem Processes. In The Rhizosphere. An Ecological Perspective; Cardon, Z.G., Whitbeck, J.L., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2007; pp. 73–100. [Google Scholar]
- Simon, L.; Bousquet, J.; Lévesque, R.C.; LaLonde, M. Origin and diversification of endomycorrhizal fungi and coincidence with vascular land plants. Nat. Cell Biol. 1993, 363, 67–69. [Google Scholar] [CrossRef]
- Yanan, W.; Xusheng, A.; Baozhong, Y.; Wenchao, Z.; Jintang, G. Biochemical defences induced by mycorrhizae fungi Glomus mosseae in controlling strawberry Fusarium wilt. Open Biomed. Eng. J. 2015, 9, 301–304. [Google Scholar] [CrossRef][Green Version]
- Hu, J.-L.; Lin, X.; Wang, J.-H.; Shen, W.; Wu, S.; Peng, S.-P.; Mao, T.-T. Arbuscular Mycorrhizal Fungal Inoculation Enhances Suppression of Cucumber Fusarium Wilt in Greenhouse Soils. Pedosphere 2010, 20, 586–593. [Google Scholar] [CrossRef]
- Shukla, A.; Dehariya, K.; Vyas, D.; Jha, A. Interactions between arbuscular mycorrhizae and Fusarium oxysporum f. sp. ciceris: Effects on fungal development, seedling growth and wilt disease suppression in Cicer arietinum L. Arch. Phytopathol. Plant Prot. 2014, 48, 240–252. [Google Scholar] [CrossRef]
- Olawuyi, O.J.; Odebode, A.C.; Oyewole, I.O.; Akanmu, A.O.; Afolabi, O. Effect of arbuscular mycorrhizal fungi on Pythium aphanidermatum causing foot rot disease on pawpaw (Carica papaya L.) seedlings. Arch. Phytopathol. Plant Prot. 2014, 47, 185–193. [Google Scholar] [CrossRef]
- Nogales, A.; Aguirreolea, J.; Santamaria, E.; Camprubi, A.; Calvet, C. Response of mycorrhizal grapevine to Armillaria mellea inoculation: Disease development and polyamines. Plant Soil 2008, 317, 177–187. [Google Scholar] [CrossRef]
- Pozo, M.J.; Cordier, C.; Dumas-Gaudot, E.; Gianinazzi, S.; Barea, J.M.; Azcón-Aguilar, C. Localized vs systemic effect of arbuscular mycorrhizal fungi on defence responses to Phytophthora infection in tomato plants. J. Exp. Bot. 2002, 53, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Bødker, L.; Kjøller, R.; Rosendahl, S. Effect of phosphate and the arbuscular mycorrhizal fungus Glomus intraradices on disease severity of root rot of peas (Pisum sativum) caused by Aphanomyces euteiches. Mycorrhiza 1998, 8, 169–174. [Google Scholar] [CrossRef]
- Guillon, C.; St-Arnaud, M.; Hamel, C.; Jabaji, S. Differential and systemic alteration of defence-related gene transcript levels in mycorrhizal bean plants infected with Rhizoctonia solani. Can. J. Bot. 2002, 80, 305–315. [Google Scholar] [CrossRef]
- A Harrier, L.; A Watson, C. The potential role of arbuscular mycorrhizal (AM) fungi in the bioprotection of plants against soil-borne pathogens in organic and/or other sustainable farming systems. Pest Manag. Sci. 2004, 60, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.J.; Azcón-Aguilar, C. Unraveling mycorrhiza-induced resistance. Curr. Opin. Plant Biol. 2007, 10, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.S.; Schmidt-Heck, W.; Guthke, R.; Furch, A.C.U.; Reichelt, M.; Gershenzon, J.; Oelmüller, R. Verticillium dahliae-Arabidopsis Interaction Causes Changes in Gene Expression Profiles and Jasmonate Levels on Different Time Scales. Front. Microbiol. 2018, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Bååth, E.; Hayman, D.S. Plant Growth Responses To Vesicular-Arbuscular Mycorrhiza. Xiv. Interactions With Verticillium Wilt On Tomato Plants. New Phytol. 1983, 95, 419–426. [Google Scholar] [CrossRef]
- Porras-Soriano, A.; Marcilla-Goldaracena, I.; Soriano-Martín, M.L.; Porras-Piedra, A. Development and resistance to Verticillium dahliae of olive plantlets inoculated with mycorrhizal fungi during the nursery period. J. Agric. Sci. 2006, 144, 151–157. [Google Scholar] [CrossRef]
- Liu, R.-J. Effect of vesicular-arbuscular mycorrhizal fungi on verticillium wilt of cotton. Mycorrhiza 1995, 5, 293–297. [Google Scholar] [CrossRef]
- Goicoechea, N.; Garmendia, I.; Sanchezdiaz, M.; Aguirreolea, J. Arbuscular mycorrhizal fungi (AMF) as bioprotector agents against wilt induced by Verticillium spp. in pepper: A review. Span. J. Agric. Res. 2010, 8, 25. [Google Scholar] [CrossRef]
- Zhang, G.; Raza, W.; Wang, X.; Ran, W.; Shen, Q. Systemic modification of cotton root exudates induced by arbuscular mycorrhizal fungi and Bacillus vallismortis HJ-5 and their effects on Verticillium wilt disease. Appl. Soil Ecol. 2012, 61, 85–91. [Google Scholar] [CrossRef]
- Demír, S.; Şensoy, S.; Ocak, E.; Tüfenkçi, Ş.; Durak, E.D.; Erdinç, Ç.; Ünsal, H. Effects of arbuscular mycorrhizal fungus, humic acid, and whey on wilt disease caused by Verticillium dahliae Kleb. in three solanaceous crops. Turk. J. Agric. For. 2015, 39, 300–309. [Google Scholar] [CrossRef]
- Sowik, I.; Borkowska, B.; Markiewicz, M. The activity of mycorrhizal symbiosis in suppressing Verticillium wilt in susceptible and tolerant strawberry (Fragaria x ananassa Duch.) genotypes. Appl. Soil Ecol. 2016, 101, 152–164. [Google Scholar] [CrossRef]
- Boutaj, H.; Chakhchar, A.; Meddich, A.; Wahbi, S.; El Alaoui-Talibi, Z.; Douira, A.; Filali-Maltouf, A.; El Modafar, C. Bioprotection of olive tree from Verticillium wilt by autochthonous endomycorrhizal fungi. J. Plant Dis. Prot. 2020, 127, 349–357. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, X.; Ren, Y.; Ding, X.; Qiu, J.; Li, N.; Zeng, F.; Chu, Z. Improvement of Verticillium Wilt Resistance by Applying Arbuscular Mycorrhizal Fungi to a Cotton Variety with High Symbiotic Efficiency under Field Conditions. Int. J. Mol. Sci. 2018, 19, 241. [Google Scholar] [CrossRef] [PubMed]
- Audet, P.; Charest, C. Identification of Constraining Experimental-Design Factors in Mycorrhizal Pot-Growth Studies. J. Bot. 2010, 2010, 1–6. [Google Scholar] [CrossRef]
- Singh, R.; Adholeya, A.; Mukerji, K.G. Mycorrhiza in Control of Soil Borne Pathogens. In Mycorrhizal Biology; Springer: Boston, MA, USA, 2000; pp. 173–196. [Google Scholar]
- Jaizme-Vega, M.; Tenoury, P.; Pinochet, J.; Jaumot, M. Interactions between the root-knot nematode Meloidogyne incognita and Glomus mosseae in banana. Plant Soil 1997, 196, 27–35. [Google Scholar] [CrossRef]
- Pautasso, M.; Döring, T.F.; Garbelotto, M.; Pellis, L.; Jeger, M.J. Impacts of climate change on plant diseases—opinions and trends. Eur. J. Plant Pathol. 2012, 133, 295–313. [Google Scholar] [CrossRef]
- Ghini, R.; Hamada, E.; Bettiol, W. Climate change and plant diseases. Sci. Agricola 2008, 65, 98–107. [Google Scholar] [CrossRef]
- Singh, P.; Hussain, T.; Patel, S.; Akhtar, N. Impact of climage change on root-pathogen interactions. In Root Biology, Soil Biology 52; Giri, B., Prasad, R., Varma, A., Eds.; Springer: New York, NY, USA, 2018; pp. 409–427. [Google Scholar]
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N. Climate change, food security and mycotoxins: Do we know enough? Fungal Biol. Rev. 2017, 31, 143–154. [Google Scholar] [CrossRef]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant–Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef]
- Molinero-Ruiz, L. Recent advances on the characterization and control of sunflower soilborne pathogens under climate change conditions. OCL 2018, 26, 2. [Google Scholar] [CrossRef]
- Gioria, R.; Brunelli, K.R.; Kobori, R.F. Impacto potencial das mudanças climáticas sobre as doenças de hortaliças: Tomate, um estudo de caso. Summa Phytopathol. 2008, 34, S121–S122. [Google Scholar]
- Zhou, Y.; Van Leeuwen, S.K.; Pieterse, C.M.J.; Bakker, P.A.H.M.; Van Wees, S.C. Effect of atmospheric CO2 on plant defense against leaf and root pathogens of Arabidopsis. Eur. J. Plant Pathol. 2019, 154, 31–42. [Google Scholar] [CrossRef]
- Osswald, W.F.; Fleischmann, F.; Heiser, I. Investigations on the effect of ozone, elevated CO2 and nitrogen fertilization on host-parasite interactions. Summa Phytopathol. 2006, 32S, S111–S113. [Google Scholar]
- Frew, A.; Price, J.N. Mycorrhizal-mediated plant–herbivore interactions in a high CO2 world. Funct. Ecol. 2019, 33, 1376–1385. [Google Scholar] [CrossRef]
- Gao, W.; Long, L.; Zhu, L.-F.; Xu, L.; Gao, W.-H.; Sun, L.-Q.; Liu, L.-L.; Zhang, X.-L. Proteomic and Virus-induced Gene Silencing (VIGS) Analyses Reveal That Gossypol, Brassinosteroids, and Jasmonic acid Contribute to the Resistance of Cotton toVerticillium dahliae. Mol. Cell. Proteom. 2013, 12, 3690–3703. [Google Scholar] [CrossRef]
- Li, C.; He, X.; Luo, X.; Xu, L.; Liu, L.; Min, L.; Jin, L.; Zhu, L.; Zhang, X. Cotton WRKY1 Mediates the Plant Defense-to-Development Transition during Infection of Cotton by Verticillium dahliae by Activating JASMONATE ZIM-DOMAIN1 Expression. Plant Physiol. 2014, 166, 2179–2194. [Google Scholar] [CrossRef] [PubMed]
- Meisner, A.; De Boer, W. Strategies to maintain natural biocontrol of soil-borne crop diseases during severe drought and rainfall events. Front. Microbiol. 2018, 9, 2279. [Google Scholar] [CrossRef]
- Wakelin, S.; Gómez-Gallego, M.; Jones, E.E.; Smaill, S.J.; Lear, G.; Lambie, S. Climate change induced drought impacts on plant diseases in New Zealand. Australas. Plant Pathol. 2018, 47, 101–114. [Google Scholar] [CrossRef]
- Calderón, R.; Lucena, C.; Trapero-Casas, J.L.; Zarco-Tejada, P.J.; Navas-Cortés, J.A. Soil Temperature Determines the Reaction of Olive Cultivars to Verticillium dahliae Pathotypes. PLoS ONE 2014, 9, e110664. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Zhang, L.; He, S.Y. Plant-Microbe Interactions Facing Environmental Challenge. Cell Host Microbe 2019, 26, 183–192. [Google Scholar] [CrossRef]
- Cotton, T.E.A. Arbuscular mycorrhizal fungal communities and global change: An uncertain future. FEMS Microbiol. Ecol. 2018, 94, 11. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, X.; Guo, R.; Guo, J. Response of AM fungi spore population to elevated temperature and nitrogen addition and their influence on the plant community composition and productivity. Sci. Rep. 2016, 6, 24749. [Google Scholar] [CrossRef]
- Goss, M.J.; Carvalho, M.; Brito, I. Functional Diversity of Mycorrhiza and Sustainable Agriculture. Management to Overcome Biotic and Abiotic Stresses; Academic Press: London, Oxford, UK; Boston, MA, USA; New York, NY, USA; San Diego, CA, USA, 2017. [Google Scholar]
- Cotton, T.E.A.; Fitter, A.H.; Miller, R.M.; Dumbrell, A.J.; Helgason, T. Fungi in the future: Interannual variation and effects of atmospheric change on arbuscular mycorrhizal fungal communities. New Phytol. 2015, 205, 1598–1607. [Google Scholar] [CrossRef]
- Veresoglou, S.D.; Anderson, I.C.; De Sousa, N.M.F.; Hempel, S.; Rillig, M.C. Resilience of Fungal Communities to Elevated CO2. Microb. Ecol. 2016, 72, 493–495. [Google Scholar] [CrossRef]
- Zhu, X.; Song, F.; Liu, S.; Liu, F. Role of Arbuscular Mycorrhiza in Alleviating Salinity Stress in Wheat (Triticum aestivum L.) Grown Under Ambient and Elevated CO2. J. Agron. Crop. Sci. 2016, 202, 486–496. [Google Scholar] [CrossRef]
- Asha, H.; Nirmalnath, P.J.; Sagarkar, M.A.; Venkatesh, H. Impact of Elevated CO2 and/or Temperature on the AM Fungal Diversity in Groundnut Rhizosphere under Open Top Chamber facility. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 882–895. [Google Scholar] [CrossRef]
- Bennett, A.E.; Classen, A. Climate change influences mycorrhizal fungal–plant interactions, but conclusions are limited by geographical study bias. Ecologies 2020, 101, e02978. [Google Scholar] [CrossRef]
- Wilson, H.; Johnson, B.R.; Bohannan, B.; Pfeifer-Meister, L.; Mueller, R.; Bridgham, S.D. Experimental warming decreases arbuscular mycorrhizal fungal colonization in prairie plants along a Mediterranean climate gradient. PeerJ 2016, 4, e2083. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.V.; Hartley, I.P.; Ineson, P.; Fitter, A.H. Soil temperature affects carbon allocation within arbuscular mycorrhizal networks and carbon transport from plant to fungus. Glob. Chang. Biol. 2008, 14, 1181–1190. [Google Scholar] [CrossRef]
- Heinemeyer, A.; Fitter, A.H. Impact of temperature on the arbuscular mycorrhizal (AM) symbiosis: Growth responses of the host plant and its AM fungal partner. J. Exp. Bot. 2004, 55, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Mohan, J.E.; Cowden, C.C.; Baas, P.; Dawadi, A.; Frankson, P.T.; Helmick, K.; Hughes, E.; Khan, S.; Lang, A.; Machmuller, M.; et al. Mycorrhizal fungi mediation of terrestrial ecosystem responses to global change: Mini-review. Fungal Ecol. 2014, 10, 3–19. [Google Scholar] [CrossRef]
- Singer, M.J.; Munns, D.N. Soils, an Introduction; Macmillan Publishing Co.: New York, NY, USA, 1987. [Google Scholar]
- Goicoechea, N.; Merino, S.; Sanchez-Diaz, M.F. Contribution of arbuscular mycorrhizal fungi (AMF) to the adaptations exhibited by the deciduous shrub Anthyllis cytisoides L. under water deficit. Physiol. Plant. 2004, 122, 453–464. [Google Scholar] [CrossRef]
- Wipf, D.; Krajinski, F.; Van Tuinen, D.; Recorbet, G.; Courty, P. Trading on the arbuscular mycorrhiza market: From arbuscules to common mycorrhizal networks. New Phytol. 2019, 223, 1127–1142. [Google Scholar] [CrossRef]
- Raklami, A.; Bechtaoui, N.; Tahiri, A.-I.; Anli, M.; Meddich, A.; Oufdou, K. Use of Rhizobacteria and Mycorrhizae Consortium in the Open Field as a Strategy for Improving Crop Nutrition, Productivity and Soil Fertility. Front. Microbiol. 2019, 10, 1106. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-Mediated Stress Resistance in Plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Allison, C.E.; Francey, R.J. Verifying Southern Hemisphere trends in atmospheric carbon dioxide stable isotopes. J. Geophys. Res. Space Phys. 2007, 112. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change, IPCC. Carbon Dioxide: Projected Emissions and Concentrations. 2020. Available online: https://www.esrl.noaa.gov/gmd/ccgg/trends/index.html#mlo_growth (accessed on 6 July 2020).
- Intergovernmental Panel on Climate Change, IPCC. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation; A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V., Stocker, T.F., Qin, D., Dokken, D.J., Ebi, K.L., Mastrandrea, M.D., Mach, K.J., Plattner, G.-K., Allen, S.K., et al., Eds.; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Intergovernmental Panel on Climate Change, IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Loladze, I. Rising atmospheric CO2 and human nutrition: Toward globally imbalanced plant stoichiometry? Trends Ecol. Evol. 2002, 17, 457–461. [Google Scholar] [CrossRef]
- Sun, X.-G.; Tang, M. Effect of arbuscular mycorrhizal fungi inoculation on root traits and root volatile organic compound emissions of Sorghum bicolor. South Afr. J. Bot. 2013, 88, 373–379. [Google Scholar] [CrossRef]
- Wonglom, P.; Ito, S.-I.; Sunpapao, A. Volatile organic compounds emitted from endophytic fungus Trichoderma asperellum T1 mediate antifungal activity, defense response and promote plant growth in lettuce (Lactuca sativa). Fungal Ecol. 2020, 43, 100867. [Google Scholar] [CrossRef]
- Sharma, N.K.; Gupta, S.K.; Dwivedi, V.; Chattopadhyay, D. Lignin deposition in chickpea root xylem under drought. Plant Signal. Behav. 2020, 15, 1754621. [Google Scholar] [CrossRef]
- Shi, H.; Liu, Z.; Zhu, L.; Zhang, C.; Chen, Y.; Zhou, Y.; Li, F.; Li, X. Overexpression of cotton (Gossypium hirsutum) dirigent1 gene enhances lignification that blocks the spread of Verticillium dahliae. Acta Biochim. Biophys. Sin. 2012, 44, 555–564. [Google Scholar] [CrossRef]
- Hattori, R.; Matsumura, A.; Yamawaki, K.; Tarui, A.; Daimon, H. Effects of flooding on arbuscular mycorrhizal colonization and root-nodule formation in different roots of soybeans. Agric. Sci. 2013, 4, 673–677. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Qiu, Q.; Xin, G.; Yang, Z.; Shi, S. Flooding Greatly Affects the Diversity of Arbuscular Mycorrhizal Fungi Communities in the Roots of Wetland Plants. PLoS ONE 2011, 6, e24512. [Google Scholar] [CrossRef] [PubMed]
- Fougnies, L.; Renciot, S.; Müller, F.; Plenchette, C.; Prin, Y.; De Faria, S.M.; Bouvet, J.M.; Sylla, S.N.; Dreyfus, B.; Ba, A.M. Arbuscular mycorrhizal colonization and nodulation improve flooding tolerance in Pterocarpus officinalis Jacq. seedlings. Mycorrhiza 2006, 17, 159–166. [Google Scholar] [CrossRef]
| Crop | Growth Conditions | Application of AMF | Effects on Plants | Reference |
|---|---|---|---|---|
| Cotton | Greenhouse (pots) | Pre-inoculation | ● Increased plant dry weight ● Reduced disease index ● Decreased levels of phenolics in root exudates | [18] |
| Pots | Simultaneous | ● Increased plant growth ● Advanced flowering ● Increased number of flowers and bolls ● Decreased disease incidence | [16] | |
| Field | Simultaneous | ● Induced expression of pathogenesis-related genes and lignin synthesis-related genes | [22] | |
| Pepper | Greenhouse (pots) | Pre-inoculation | ● Delayed the appearance and development of the disease symptoms ● Increased chitinase and superoxide dismutase activity in roots ● Induced the lignification of xylem in stems ● Increased the antioxidant metabolism in leaves ● Improved plant water status ● Favoured the maintenance of photosynthesis ● Diminished the reduction of yield caused by V. dahliae | [17] |
| Strawberry | Greenhouse | Pre-inoculation | ● Reduced disease development ● Increased stomatal conductance and transpiration rate ● Improved plant water relations ● Counteracted the decreased photochemical activity in susceptible cultivars | [20] |
| Olive | Greenhouse (pots) | Pre-inoculation | ● Reduced dwarfing and leaf alterations ● Decreased presence of Verticillium in roots and stems | [21] |
| Tomato Pepper Eggplant | Growth chamber | Pre-inoculation | ● Reduced wilt disease severity and microsclerotia number (AMF combined with humic acids and/or whey) | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goicoechea, N. Mycorrhizal Fungi as Bioprotectors of Crops Against Verticillium Wilt—A Hypothetical Scenario Under Changing Environmental Conditions. Plants 2020, 9, 1468. https://doi.org/10.3390/plants9111468
Goicoechea N. Mycorrhizal Fungi as Bioprotectors of Crops Against Verticillium Wilt—A Hypothetical Scenario Under Changing Environmental Conditions. Plants. 2020; 9(11):1468. https://doi.org/10.3390/plants9111468
Chicago/Turabian StyleGoicoechea, Nieves. 2020. "Mycorrhizal Fungi as Bioprotectors of Crops Against Verticillium Wilt—A Hypothetical Scenario Under Changing Environmental Conditions" Plants 9, no. 11: 1468. https://doi.org/10.3390/plants9111468
APA StyleGoicoechea, N. (2020). Mycorrhizal Fungi as Bioprotectors of Crops Against Verticillium Wilt—A Hypothetical Scenario Under Changing Environmental Conditions. Plants, 9(11), 1468. https://doi.org/10.3390/plants9111468





