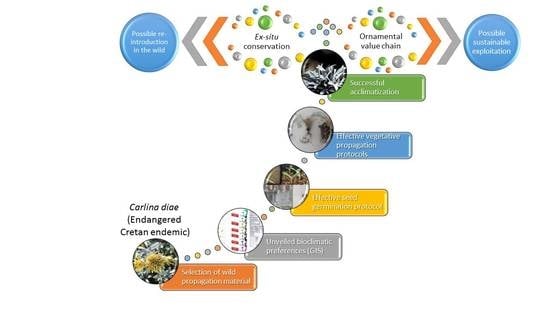
GIS-Facilitated Effective Propagation Protocols of the Endangered Local Endemic of Crete Carlina diae (Rech. f.) Meusel and A. Kástner (Asteraceae): Serving Ex Situ Conservation Needs and Its Future Sustainable Utilization as an Ornamental
Abstract
1. Introduction
2. Results
2.1. Seasonal Bioclimatic Preferences of C. diae
2.2. In Vivo Seed Germination
2.3. In Vitro Seed Germination and Microshoot Development
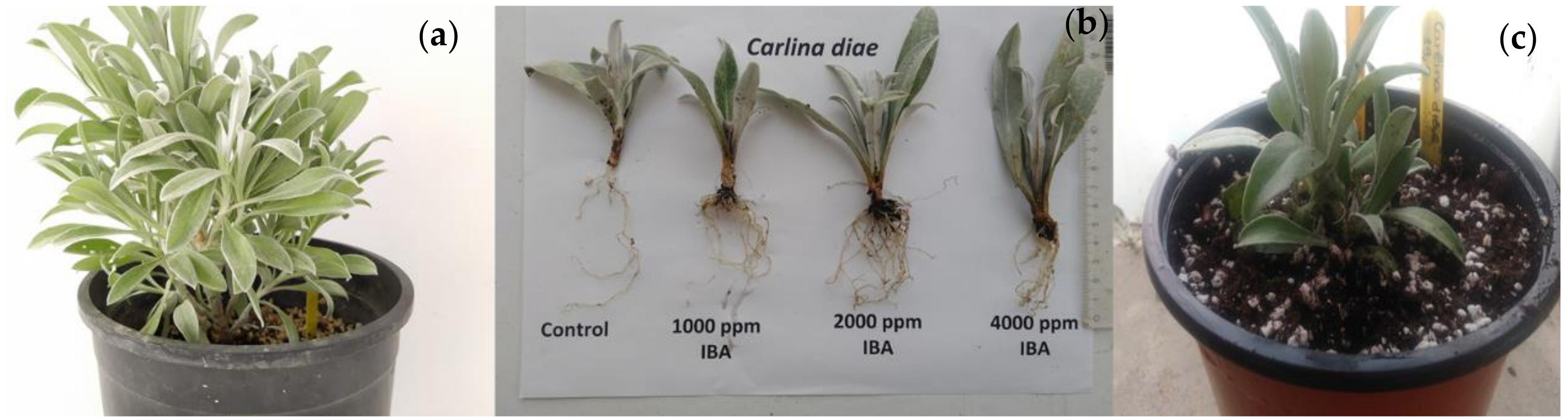
2.4. Vegetative Propagation by Cuttings
2.5. Ex Situ Conservation and GIS-Derived Data
3. Discussion
4. Materials and Methods
4.1. Botanical Collections
4.2. Unveiling the Ecological Preferences of C. diae with GIS
4.3. In Vivo Seed Germination
4.4. In Vitro Seed Germination and Microshoot Production
4.5. Vegetative Propagation by Cuttings
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Bioclimatic Variable | MIN | MAX | MEAN | STD |
|---|---|---|---|---|
| Annual Mean Temperature (°C) | 18.44 | 18.51 | 18.47 | 0.03 |
| Mean Diurnal Range 1 (°C) | 5.77 | 6.14 | 5.92 | 0.16 |
| Isothermality 2 (°C) | 30.51 | 31.18 | 30.73 | 0.31 |
| Temperature Seasonality 3 (°C) | 507.86 | 518.03 | 512.89 | 4.15 |
| Max Temperature of Warmest Month (°C) | 27.80 | 28.30 | 28.03 | 0.21 |
| Min Temperature of Coldest Month (°C) | 8.60 | 8.90 | 8.77 | 0.12 |
| Temperature Annual Range 4 (°C) | 18.90 | 19.70 | 19.27 | 0.33 |
| Mean Temperature of Wettest Quarter (°C) | 12.62 | 12.73 | 12.68 | 0.05 |
| Mean Temperature of Driest Quarter (°C) | 24.67 | 24.88 | 24.77 | 0.09 |
| Mean Temperature of Warmest Quarter (°C) | 25.08 | 25.21 | 25.17 | 0.06 |
| Mean Temperature of Coldest Quarter (°C) | 12.47 | 12.48 | 12.48 | 0.01 |
| Annual Precipitation (mm) | 489.00 | 506.00 | 498.67 | 7.13 |
| Precipitation of Wettest Month (mm) | 94.00 | 98.00 | 96.00 | 1.63 |
| Precipitation of Driest Month (mm) | 1.00 | 1.00 | 1.00 | 0.00 |
| Precipitation Seasonality (Coefficient of Variation) (mm) | 83.89 | 84.28 | 84.12 | 0.17 |
| Precipitation of Wettest Quarter (mm) | 252.00 | 261.00 | 257.00 | 3.74 |
| Precipitation of Driest Quarter (mm) | 5.00 | 6.00 | 5.67 | 0.47 |
| Precipitation of Warmest Quarter (mm) | 1.00 | 3.00 | 2.00 | 0.01 |
| Precipitation of Coldest Quarter (mm) | 229.00 | 237.00 | 233.33 | 3.30 |
| Month | T Min (°C) | T Max (°C) | T Mean (°C) |
|---|---|---|---|
| January | 5.8 | 13.4 | 9.6 |
| February | 5.6 | 13.2 | 9.4 |
| March | 7.1 | 14.7 | 10.9 |
| April | 10.3 | 17.9 | 15.0 |
| May | 14.2 | 21.9 | 18.0 |
| June | 18.4 | 26.0 | 22.2 |
| July | 21.3 | 27.1 | 24.2 |
| August | 21.2 | 28.3 | 24.1 |
| September | 18.8 | 24.6 | 21.7 |
| October | 15.0 | 20.9 | 18.0 |
| November | 11.2 | 17.0 | 14.1 |
| December | 8.2 | 14.1 | 11.1 |
References
- Meusel, H.; Kästner, A. Lebensgeschichte der Gold—Und Silberdisteln, Monographie der Mediterran-Mitteleuropäischen Compositen—Gattung Carlina, Band; Springer: New York, NY, USA, 1990; pp. 23–48. (In Germany) [Google Scholar]
- Kovanda, M. Observations on Carlina biebersteinii. Thaiszia J. Bot. 2002, 12, 75–82. Available online: http://www.upjs.sk/bz/thaiszia/index.html (accessed on 26 October 2020).
- Gunther, R.T. Early British Botanists and Their Gardens; Oxford University Press: Oxford, UK, 1922; pp. 1592–1664. [Google Scholar]
- Loudon, J. The Ladies’ Flower-Garden of Ornamental Perennials; William Smith: London, UK, 1918; pp. 1807–1858. [Google Scholar] [CrossRef]
- Krigas, N.; Menteli, V.; Vokou, D. The electronic trade in Greek endemic plants: Biodiversity, commercial and legal aspects. Econ. Bot. 2014, 68, 85–95. [Google Scholar] [CrossRef]
- Tanaka, T. Tanaka’s Cyclopaedia of Edible Plants of the World, 1st ed.; Keigaku Publishing: Tokyo, Japan, 1976; pp. 791–801. [Google Scholar]
- Kunkel, G. Plants for Human Consumption: An Annotated Checklist of the Edible Phanerogams and Ferns; Koeltz Scientific Books: Koenigstein, Germany, 1984; pp. 1–393. ISBN 3874292169. [Google Scholar]
- Jacke, D.; Toensmeier, E. Edible Forest Gardens; Chelsea Green Publishing, Co.: Hartford, VT, USA, 2005; Volume 2, pp. 1–1068. ISBN 9781890132606. [Google Scholar]
- Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Załuski, D.; Verpoorte, R. Historical and traditional medical applications of Carlina acualis L.—A critical ethnopharmacological review. J. Ethnopharmacol. 2019, 239, 111842. [Google Scholar] [CrossRef] [PubMed]
- Ðjorđjević, S.; Tadić, V.; Petrović, S.; Kukić-Marković, J.; Dobrić, S.; Milenković, M.; Hadžiferjzović, N. Bioactivity assays on Carlina acualis and C. acanthifolia root and herb extracts. Dig. J. Nanomater. Bios. 2012, 7, 1213–1222. Available online: http://www.chalcogen.ro/1213_Djordjevic.pdf (accessed on 26 October 2020).
- Strzemski, M.; Dresler, S.; Sowa, I.; Czubacka, A.; Agacka-Mołdoch, M.; Plachno, B.J.; Granica, S.; Feldo, M.; Wójciak-Kosior, M. The impact of different cultivation systems on the content of selected secondary metabolites and antioxidant activity of Carlina acualis plant material. Molecules 2020, 25, 146. [Google Scholar] [CrossRef]
- Grubišić, D.; Šavikin-Fodulović, K.; Mišić, D.; Zlatko, G.; Konjević, R. In vitro stem elongation of stemless carline thistle. Plant Growth Regul. 2004, 44, 65–69. [Google Scholar] [CrossRef]
- Kravets, N.B.; Tulaidan, N.V.; Mosula, M.Z.; Drobyk, N.M. Microclonal propagation and callus induction of some species of Carlina L. genus. Fakt. Eksperiment. Evol. Org. 2018, 22, 274–281. [Google Scholar] [CrossRef]
- Trejgell, A.; Tretyn, A. Analysis of flowering ability of regenerated Carlina acaulis subsp. simplex plants. Acta Agrobot. 2007, 60, 39–44. [Google Scholar] [CrossRef]
- Trejgell, A.; Bednarek, M.; Tretyn, A. Micropropagation of Carlina acaulis L. Acta Biol. Cracov. Bot. 2009, 51, 97–103. Available online: http://yadda.icm.edu.pl/yadda/element/bwmeta1.element.agro-article-26410976-9891-4b11-b6e6-d20323c759d7 (accessed on 26 October 2020).
- Trejgell, A.; Bednarek, M.; Tretyn, A. In vitro regeneration of Carlina acaulis subsp. simplex from seedling explants. Acta Physiol. Plant. 2009, 31, 445–453. [Google Scholar] [CrossRef]
- Trejgell, A.; Tretyn, A. Shoot multiplication and in vitro rooting of Carlina onopordifolia Basser. Acta Biol. Cracov. Bot. 2011, 53, 68–72. [Google Scholar] [CrossRef]
- Trejgell, A.; Libront, I.; Tretyn, A. The effect of Fe-EDDHA on shoot multiplication and in vitro rooting of Carlina onopordifolia Besser. Acta Physiol. Plant. 2012, 34, 2051–2055. [Google Scholar] [CrossRef]
- Löfgren, P.; Eriksson, O.; Lehtilä, K. Population dynamics and the effect of disturbance in the monocarpic herb Carlina vulgaris (Asteraceae). Ann. Bot. Fenn. 2000, 37, 183–192. Available online: https://www.jstor.org/stable/23726899 (accessed on 26 October 2020).
- Rees, M.; Childs, D.Z.; Metcalf, J.C.; Rose, K.E.; Sheppard, A.W.; Grubb, P.J. Seed dormancy and delayed flowering in monocarpic plants: Selective interactions in a stochastic environment. Am. Nat. 2006, 168, E53–E71. [Google Scholar] [CrossRef]
- Peco, B.; Traba, J.; Levassor, C.; Sanchez, A.M.; Azcarate, F.M. Seed size, shape and persistence in dry Mediterranean grass and scrublands. Seed Sci. Res. 2003, 13, 87–95. [Google Scholar] [CrossRef]
- SID-KEW. Seed Information Database of Royal Botanic Gardens Kew; Kew Royal Botanic Gardens: London, UK, 2008; Available online: https://data.kew.org/sid/ (accessed on 25 March 2020).
- Pogge, F.L.; Bearce, B.C. Germinating common and cat greenbrier. TPN 1989, 40, 34–37. [Google Scholar]
- Eriksson, A.; Eriksson, O. Seedling recruitment in semi-natural pastures: The effect of disturbance, size, phenology and seed bank. Nord. J. Bot. 1997, 17, 469–482. [Google Scholar] [CrossRef]
- Plants of the World Online. Available online: www.plantsoftheworldonline.org (accessed on 3 October 2020).
- The Euro+Med PlantBase—The Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://ww2.bgbm.org/EuroPlusMed/ (accessed on 3 October 2020).
- Bazos, I. Calina diae. IUCN Red List Threat. Species. e.T165174A5986277. 2011. Available online: https://www.iucn.org/resources/conservation-tools/iucn-red-list-threatened-species (accessed on 26 October 2020).
- Nordenstam, B. Studies in the Aegean Flora II. Genus Lyrolepis. Bot. Not. 1960, 3, 451–457. Available online: https://journals.lub.lu.se/bn/article/view/11215 (accessed on 3 October 2020).
- Krigas, N.; Mendeli, V.; Vokou, D. Analysis of the ex situ conservation of the Greek endemic flora at national European and global scales and of its effectiveness in meeting GSPC Target 8. Plant Biosyst. 2016, 150, 573–582. [Google Scholar] [CrossRef]
- Maloupa, E.; Krigas, N.; Grigoriadou, K.; Lazari, D.; Tsoktouridis, G. Conservation strategies for native plant species and their sustainable exploitation: Case of the Balkan Botanic Garden of Kroussia, N. Greece. In Floriculture Ornamental Plant Biotechnology: Advances and Topical Issues, 1st ed.; Teixeira da Silva, J.A., Ed.; Global Science Books: Isleworth, UK, 2008; Volume 4, pp. 37–56. [Google Scholar]
- Krigas, N.; Maloupa, E. The Balkan Botanic Garden of Kroussia, Northern Greece: A garden dedicated to the conservation of native plants of Greece and the Balkans. Sibbaldia 2008, 6, 9–27. [Google Scholar] [CrossRef]
- Krigas, N.; Maloupa, E. The collection policy of the Balkan Botanic Garden of Kroussia: Almost 20 years of documentation and ex situ conservation of the Greek flora. BG J. 2019, 16, 42–44. Available online: https://www.bgci.org/resources/bgci-tools-and-resources/bgjournal/ (accessed on 26 October 2020).
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 437–497. [Google Scholar] [CrossRef]
- Maunder, M.; Higgens, S.; Culham, A. The effectiveness of botanic garden collections in supporting plant conservation: A European case study. Biodivers. Conserv. 2001, 10, 383–401. [Google Scholar] [CrossRef]
- Krigas, Ν.; Mouflis, G.; Grigoriadou, K.; Maloupa, E. Conservation of important plants from the Ionian Islands at the Balkan Botanic Garden of Kroussia, N Greece: Using GIS to link the in situ collection data with plant propagation and ex situ cultivation. Biodiver. Conserv. 2010, 19, 3583–3603. [Google Scholar] [CrossRef]
- Krigas, N.; Papadimitriou, K.; Mazaris, A.D. GIS and ex situ plant conservation. In Application of Geographic Information Systems; Alam, B.M., Ed.; InTechopen.com: Rijeka, Croatia, 2012; pp. 153–174. [Google Scholar] [CrossRef]
- Bunn, E.; Turner, S.R.; Dixon, K.W. Biotechnology for saving rare and threatened flora in a biodiversity hotspot. In Vitro Cell Dev. Biol. Plant 2011, 47, 188–200. [Google Scholar] [CrossRef]
- Benson, E.; Danaher, J.E.; Pimbley, I.M.; Anderson, C.T.; Wake, J.E.; Daley, S.; Adams, L.K. In vitro micropropagation of Primula scotica: A rare Scottish plant. Biodivers. Conserv. 2002, 9, 711–726. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Khan, M.A.; Mahmood, T.; Ahmad, M.; Chaudhary, M.F.; Khan, M.A. Shoot regeneration and free-radical scavenging activity in Silybum marianum L. Plant Cell Tissue Organ Cult. 2010, 101, 371–376. [Google Scholar] [CrossRef]
- Kirmizi, S.; Guleryuz, G.; Arslan, H. Germination responses to GA3 and short-time chilling of three endemic species: Tripleurospermum pichleri, Cirsium leucopsis and Senecio olympicus (Asteraceae). Plant Spec. Biol. 2011, 26, 51–57. [Google Scholar] [CrossRef]
- Smith, D.; Williams, D.; Houseal, G.; Henderson, K. The Tallgrass Prairie Center Guide to Prairie Restoration in the Upper Midwest; University of Iowa Press: Iowa City, IA, USA, 2010; pp. 1–342. [Google Scholar]
- Fournaraki, C. Conservation of Threatened Plants of Crete—Seed Ecology, Operation and Management of a Gene Bank. Ph.D. Thesis, National and Kapodistrian University of Athens, Faculty of Biology, Department of Botany, Athens, Greece, 2010. (In Greek with English Abstract). [Google Scholar]
- Cassells, A. Pathogen and biological contamination management in plant tissue culture: Phytopathogens, vitro pathogens, and vitro pests. In Plant Cell Culture Protocols—Methods in Molecular Biology (Methods and Protocols); Loyola-Vargas, V., Ochoa-Alejo, N., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 877, pp. 57–80. [Google Scholar] [CrossRef]
- Chory, J.; Li, J. Gibberellins, brassinosteroids and light-regulated development. Plant Cell Environ. 1997, 20, 801–806. [Google Scholar] [CrossRef]
- Talon, M.; Zeevaart, J.A.D. Gibberellins and stem growth as related to photoperiod in Silene armeria L. Plant Physiol. 1990, 92, 1094–1100. [Google Scholar] [CrossRef]
- Joshi, M.; Dhar, U. In vitro propagation of Saussurea obvallata (DC.) Edgew.—An endangered ethnoreligious medicinal herb of Himalaya. Plant Cell Rep. 2003, 21, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, P.; Jayabalan, N. An efficient micropropagation system for Eclipa alba—A valuable medicinal herb. In Vitro Cell Dev. Biol. Plant 2005, 41, 532–539. [Google Scholar] [CrossRef]
- Orlikowska, T.; Nowak, E.; Marasek, A.; Kucharska, D. Effect of growth regulators and incubation period on in vitro regeneration of adventitious shoots from gerbera petioles. Plant Cell Tissue Organ Cult. 1999, 59, 95–102. [Google Scholar] [CrossRef]
- Sujata, G.; Ranjitha Kumari, B.D. Effect of phytohormones on micropropagation of Artemisia vulgaris L. Acta Physiol. Plant. 2007, 29, 189–195. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Datta, S.K. Micropropagation of gerbera: Lipid peroxidation and antioxidant enzyme activities during acclimatization process. Acta Physiol. Plant. 2008, 30, 325–331. [Google Scholar] [CrossRef]
- Galek, R.; Kukułczanka, K. Propagation of Carlina acaulis in vitro Culture Methods. In Proceedings of the IX All-Poland Conference In Vitro Cultures and Plant Biotechnology, Gdańsk, Sobieszewo, Poland, 10–13 September 2000; p. 132. [Google Scholar]
- Xu, J.; Wang, Y.; Zhang, Y.; Chai, T. Rapid in vitro multiplication and ex vitro rooting of Malus zumi (Matsumura) Rehd. Acta Physiol. Plant. 2008, 30, 129–132. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Davies, J.R.; Geneve, R.L. Plant Propagation: Principles and Practices, 7th ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 2002; pp. 1–880. [Google Scholar]
- Ofori, D.A.; Newton, A.C.; Leaky, R.R.B.; Grace, J. Vegetative propagation of Milicia excelsa by leafy stem cuttings: Effects of auxin concentration, leaf area and rooting medium. For. Ecol. Manag. 1996, 84, 39–48. [Google Scholar] [CrossRef]
- Ingle, M.R.; Venugopal, C.K. Effect of different growth regulators on rooting of stevia (Stevia rebaudiana Bertoni) cuttings. Karnataka J. Agric. Sci. 2009, 22, 460–461. [Google Scholar]
- Arya, S.T.; Tomar, R.; Tokyt, O.P. Effect of plant age and auxin treatment on rooting response in stem cuttings of Prosopis cineraria. J. Arid Environ. 1994, 27, 99–103. [Google Scholar] [CrossRef]
- Grewal, H.S.; Ramesh, K.; Rupinder, C. Effect of IBA and NAA on rooting of chrysanthemum (Dendranthema grandiflora Tzevlev) terminal cuttings. J. Ornam. Hortic. 2005, 8, 230–232. Available online: https://eurekamag.com/research/013/037/013037549.php (accessed on 26 October 2020).
- Wiesman, Z.J.; Riov Epstein, E. Comparison of movement and metabolism of indole-3-acetic acid in mung bean cuttings. Physiol. Plant. 1988, 74, 556–560. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Davies, J.R.; Geneve, R.L. Plant Propagation: Principles and Practices, 6th ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 1997; pp. 1–770. [Google Scholar]
- Lebrun, A.; Toussaint, A.N.; Roggemans, J. Description of Syzygium paniculatum Gaertn. ‘Verlaine’ and its propagation by stem cuttings. Sci. Hortic. 1998, 75, 103–111. [Google Scholar] [CrossRef]
- Haissig, B.E. Influences of auxin synergists on adventitious root primordium initiation and development. N. Z. J. For. Sci. 1974, 4, 311–323. [Google Scholar]
- Al-Saqri, F.; Alderson, P.G. Effect of IBA, cutting type and rooting media on rooting of Rosa centifolia. J. Hortic. Sci. 1996, 71, 729–737. [Google Scholar] [CrossRef]
- Jawanda, J.S.; Singh, A.; Singh, S.; Bal, J.S. Effect of indolebutyric acid and shoot portion on the rooting of cuttings in Japanese plum. Acta Hortic. 1991, 283, 189–197. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Davies, J.R.; Geneve, R.L. Plant Propagation: Principles and Practices, 5th ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1990; pp. 1–647. [Google Scholar]
- Strid, A. Atlas of the Aegean Flora, Part 1: Text & Plates; Part 2: Maps; Englera; Botanic Garden and Botanical Museum Berlin, Freie Universität Berlin: Berlin, Germany, 2016; Volume 33, pp. 1–2. [Google Scholar]
- Lazarina, M.; Charalampopoulos, A.; Psaralexi, M.; Krigas, N.; Michailidou, D.E.; Kallimanis, A.S.; Sgardelis, S.P. Diversity patterns of different life forms of plants along an elevational gradient in Crete, Greece. Diversity 2019, 11, 200. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Maloupa, E. Micropropagation and salt tolerance of in vitro grown Crithmum maritimum L. Plant Cell Tissue Organ Cult. 2008, 94, 209–217. [Google Scholar] [CrossRef]
- Makunga, N.P.; Jager, A.K.; Van Staden, J. Micropropagation of Thapsia garganica—A medicinal plant. Plant Cell Rep. 2003, 21, 967–973. [Google Scholar] [CrossRef]
- Carr, G.D.; Medeiros, A.C. A remnant greensword population from Pu‘u ‘Alea, Maui, with characteristics of Argyroxiphium virescens (Asteraceae). Pac. Sci. 1998, 52, 61–68. Available online: https://www.semanticscholar.org/paper/A-Remnant-Greensword-Population-from-Pu%27u-%27Alaea%2C-Carr-Medeiros/c075149a1ad64b531f56efb86224f8653b077140 (accessed on 26 October 2020).
- Kurt, S.; Erdağ, B. In vitro germination and axillary shoot propagation of Centaurea zeybekii. Biologia 2009, 64, 97–101. [Google Scholar] [CrossRef]


| Days after Sowing | No. of Germinated Seeds after | Germination (%) after | ||
|---|---|---|---|---|
| Drying | Drying and Cold Storage | Drying | Drying and Cold Storage | |
| 15th | 70 c | 95 b | 63.33 c | 70 bc |
| 30th | 90 b | 116 a | 77.33 b | 90 a |
| 45th | 90 b | 116 a | 77.33 b | 90 a |
| 60th | 90 b | 116 a | 77.33 b | 90 a |
| p-values (general linear model/two-way ANOVA) | ||||
| Number of sowing days (Α) | 0.000 2 | |||
| Seed pre-treatment (Β) | 0.000 2 | |||
| (Α)*(Β) | 0.997 1 | |||
| In Vitro Germination (%) | ||
|---|---|---|
| Days of Culture | MS + GA3 | Plain MS |
| 4 | 41.38 c | 10.00 d |
| 10 | 96.55 a | 44.00 c |
| 20 | 96.55 a | 70.00 b |
| 30 | 96.55 a | 100.00 a |
| 40 | 96.55 a | 100.00 a |
| p-values (general linear model/two-way ANOVA) | ||
| Culture days in MS medium (Α) | 0.000 1 | |
| GA3 in the medium (yes/no) (Β) | 0.000 1 | |
| (Α)*(Β) | 0.000 1 | |
| Treatments | Rooting (%) | Root Number/Rooted Cutting | Root Length (cm) | Necrosis (%) |
|---|---|---|---|---|
| Control | 71.43 b | 5.00 ± 0.58 b | 4.26 ± 0.43 a | 0 b |
| 1000 ppm IBA | 100.00 a | 11.43 ± 1.84 ab | 2.97 ± 0.24 b | 0 b |
| 2000 ppm IBA | 100.00 a | 16.00 ± 3.45 a | 2.08 ± 0.42 b | 0 b |
| 4000 ppm IBA | 57.14 c | 17.00 ± 2.04 a | 2.88 ± 0.51 b | 42.86 a |
| p-values | 0.000 2 | 0.003 1 | 0.010 1 | 0.000 2 |
| Season | Rooting (%) | Root Number/Rooted Cutting | Root Length (cm) | Rooting Period (Days) |
|---|---|---|---|---|
| Summer (June) | 100.00 a | 13.17 ± 1.28 a | 1.82 ± 0.19 b | 30 b |
| Autumn (November) | 100.00 a | 9.86 ± 1.45 b | 3.01 ± 0.20 a | 30 b |
| Winter (January) | 52.38 b | 9.73 ± 1.33 b | 1.37 ± 0.18 c | 50 a |
| p-values | 0.000 2 | 0.009 1 | 0.000 2 | 0.004 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoriadou, K.; Sarropoulou, V.; Krigas, N.; Maloupa, E.; Tsoktouridis, G. GIS-Facilitated Effective Propagation Protocols of the Endangered Local Endemic of Crete Carlina diae (Rech. f.) Meusel and A. Kástner (Asteraceae): Serving Ex Situ Conservation Needs and Its Future Sustainable Utilization as an Ornamental. Plants 2020, 9, 1465. https://doi.org/10.3390/plants9111465
Grigoriadou K, Sarropoulou V, Krigas N, Maloupa E, Tsoktouridis G. GIS-Facilitated Effective Propagation Protocols of the Endangered Local Endemic of Crete Carlina diae (Rech. f.) Meusel and A. Kástner (Asteraceae): Serving Ex Situ Conservation Needs and Its Future Sustainable Utilization as an Ornamental. Plants. 2020; 9(11):1465. https://doi.org/10.3390/plants9111465
Chicago/Turabian StyleGrigoriadou, Katerina, Virginia Sarropoulou, Nikos Krigas, Eleni Maloupa, and Georgios Tsoktouridis. 2020. "GIS-Facilitated Effective Propagation Protocols of the Endangered Local Endemic of Crete Carlina diae (Rech. f.) Meusel and A. Kástner (Asteraceae): Serving Ex Situ Conservation Needs and Its Future Sustainable Utilization as an Ornamental" Plants 9, no. 11: 1465. https://doi.org/10.3390/plants9111465
APA StyleGrigoriadou, K., Sarropoulou, V., Krigas, N., Maloupa, E., & Tsoktouridis, G. (2020). GIS-Facilitated Effective Propagation Protocols of the Endangered Local Endemic of Crete Carlina diae (Rech. f.) Meusel and A. Kástner (Asteraceae): Serving Ex Situ Conservation Needs and Its Future Sustainable Utilization as an Ornamental. Plants, 9(11), 1465. https://doi.org/10.3390/plants9111465