Profiling Volatile Terpenoids from Calabrian Pine Stands Infested by the Pine Processionary Moth
Abstract
1. Introduction
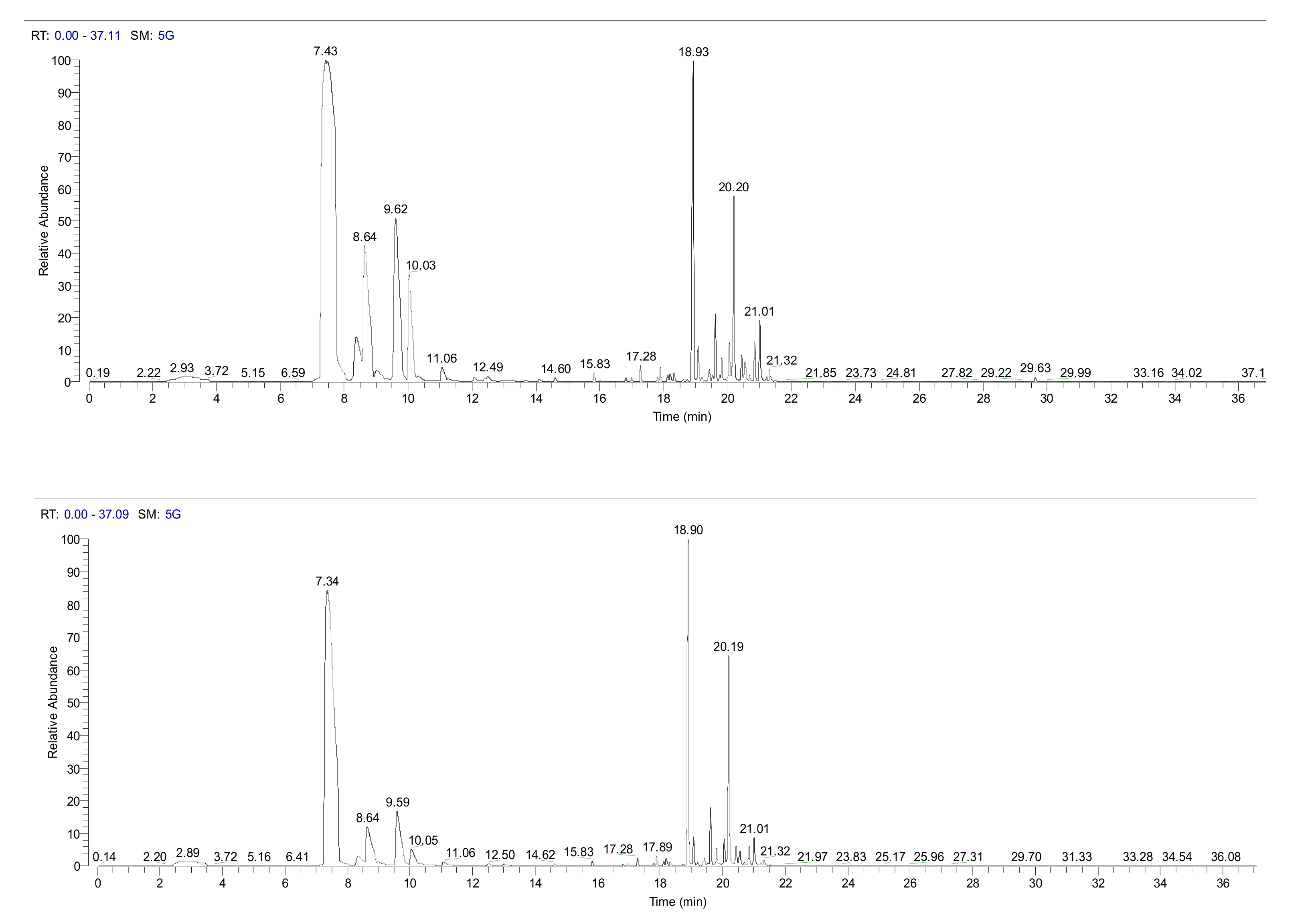
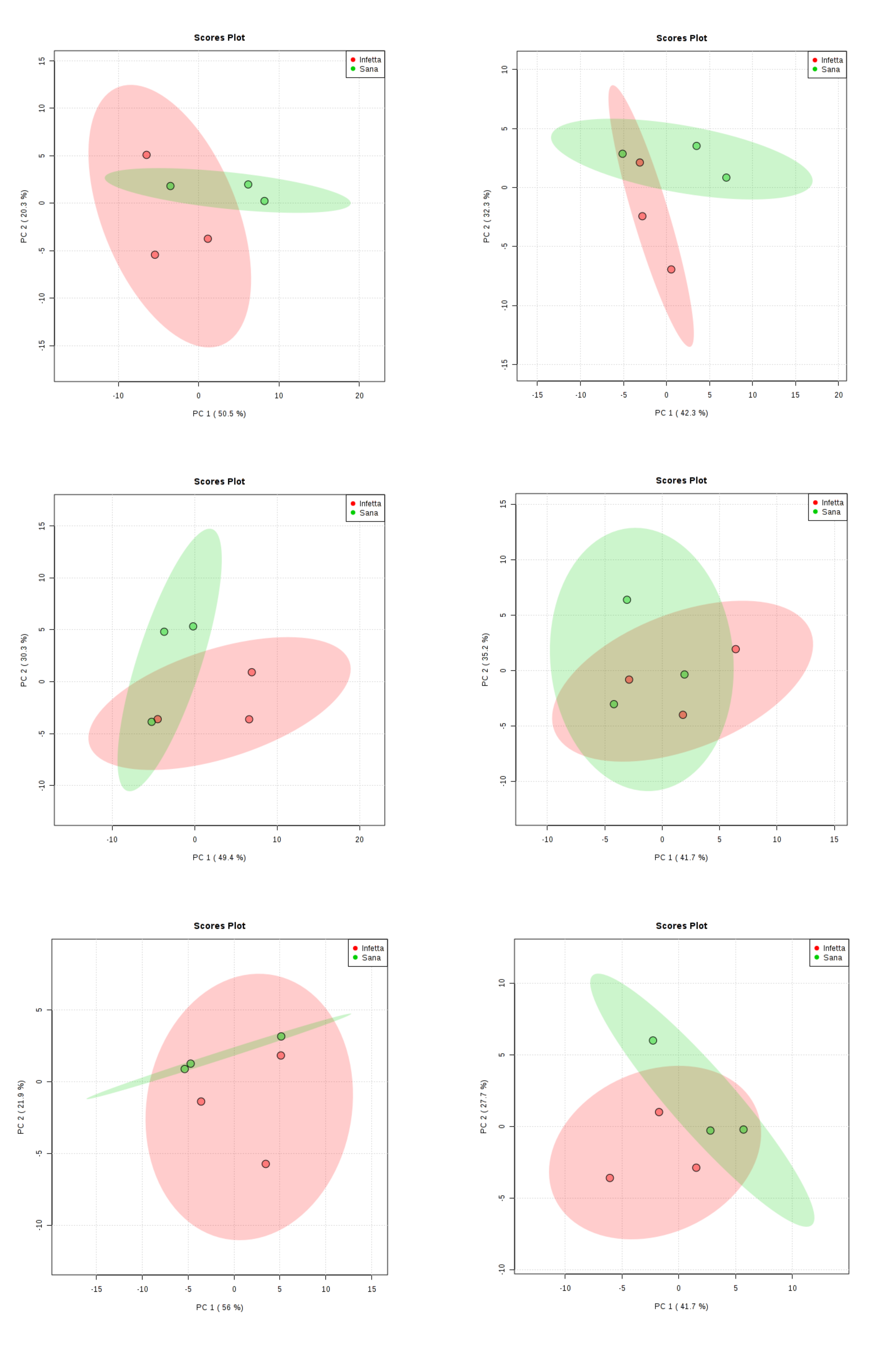
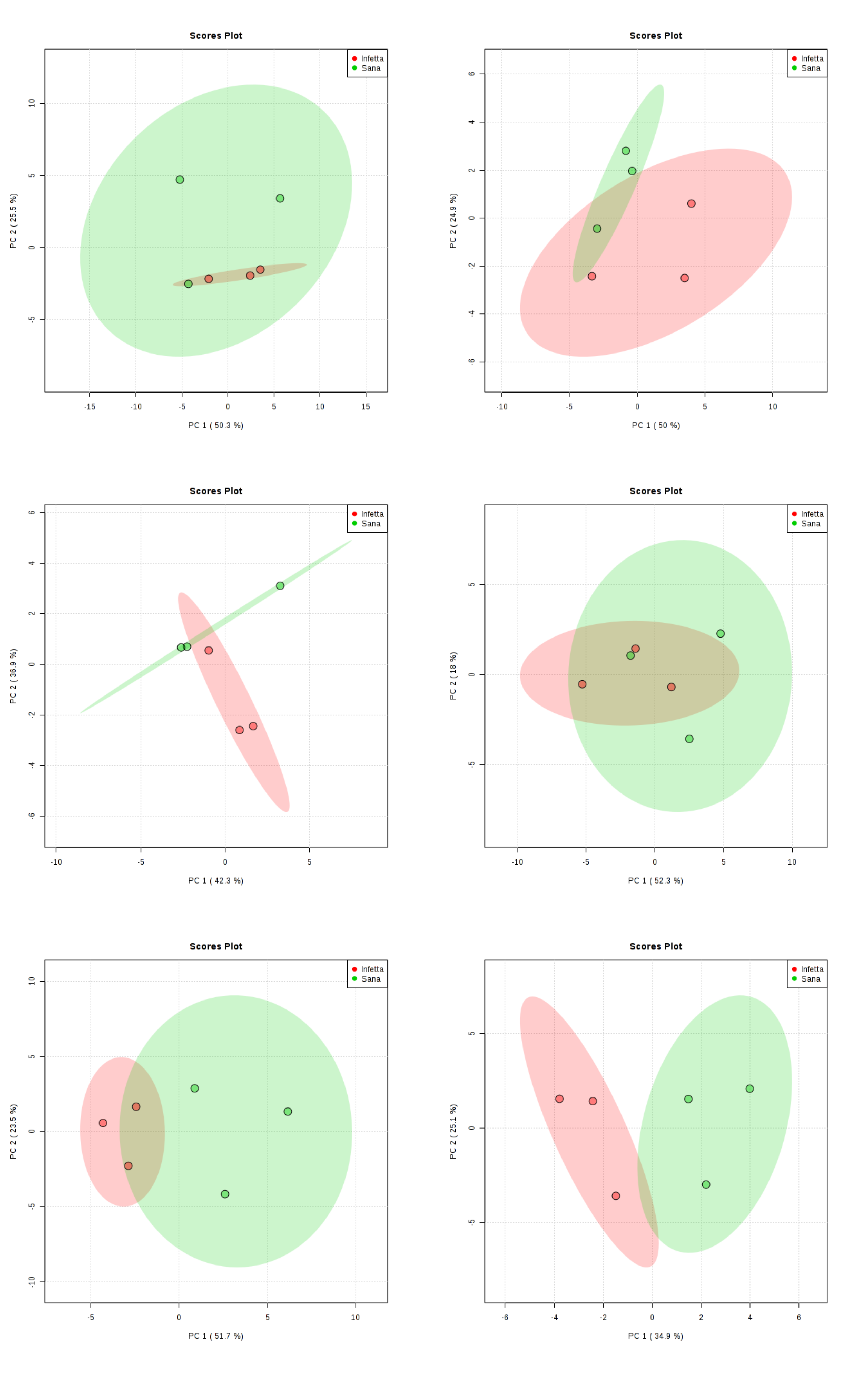
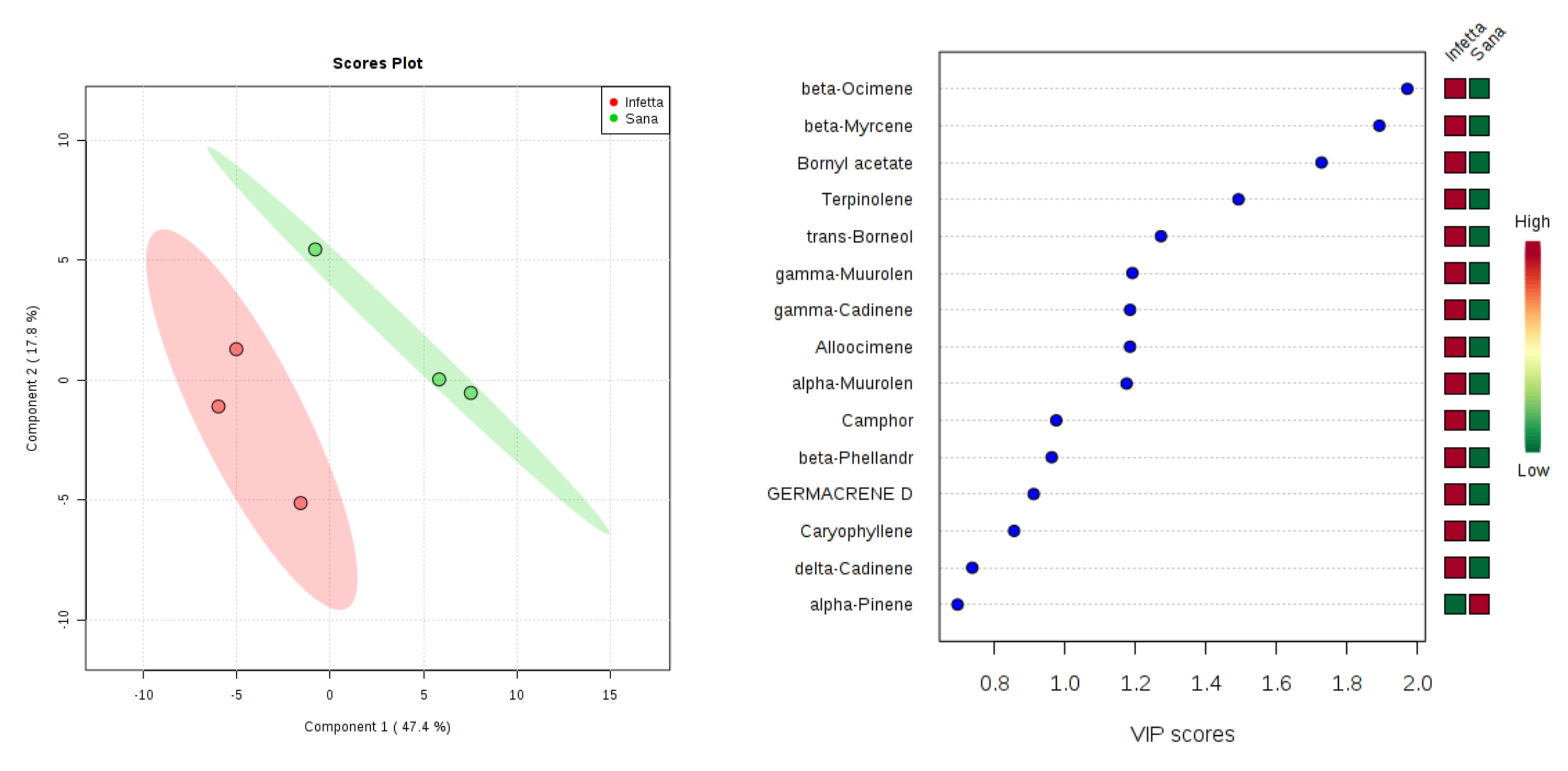
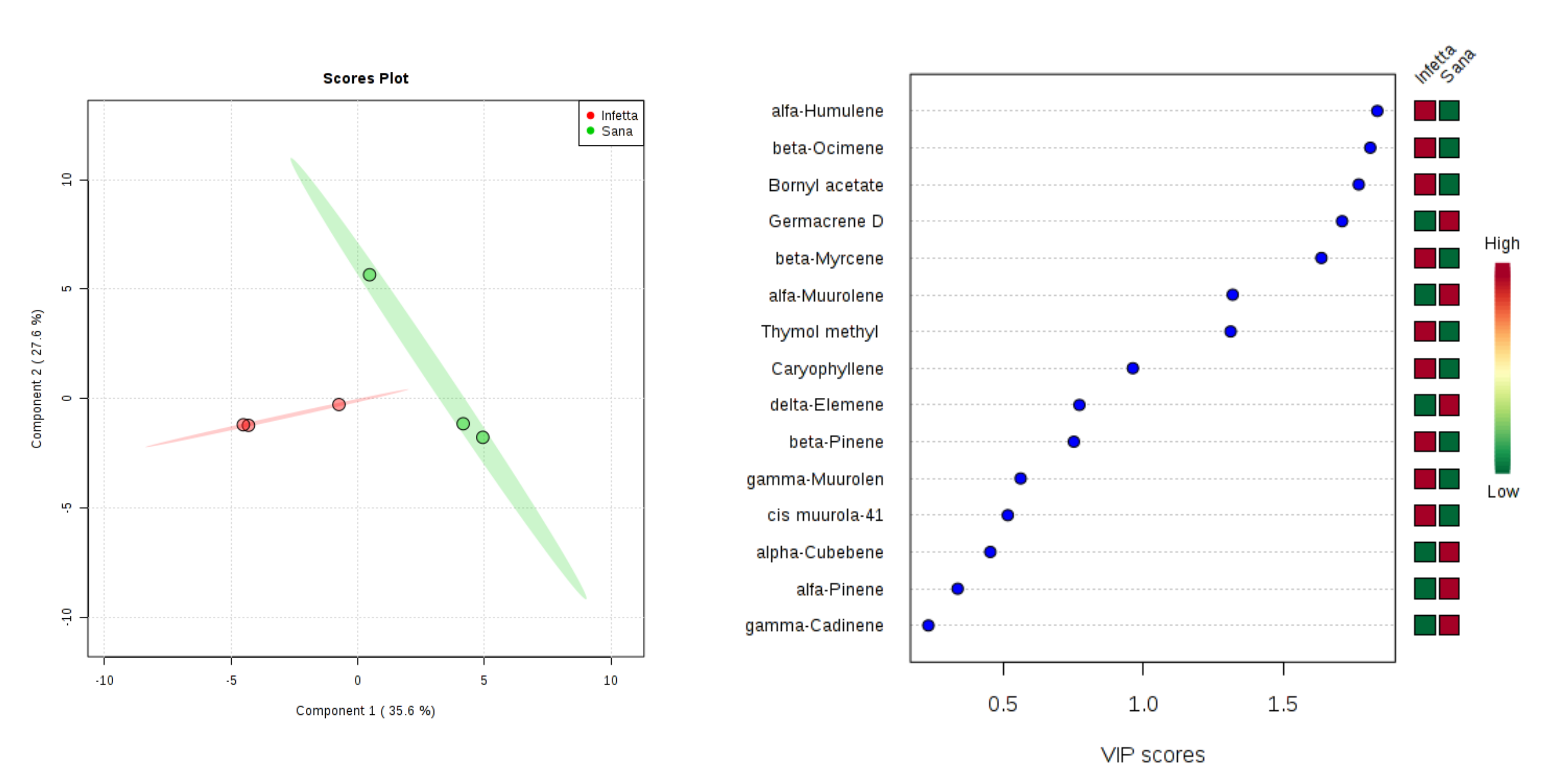
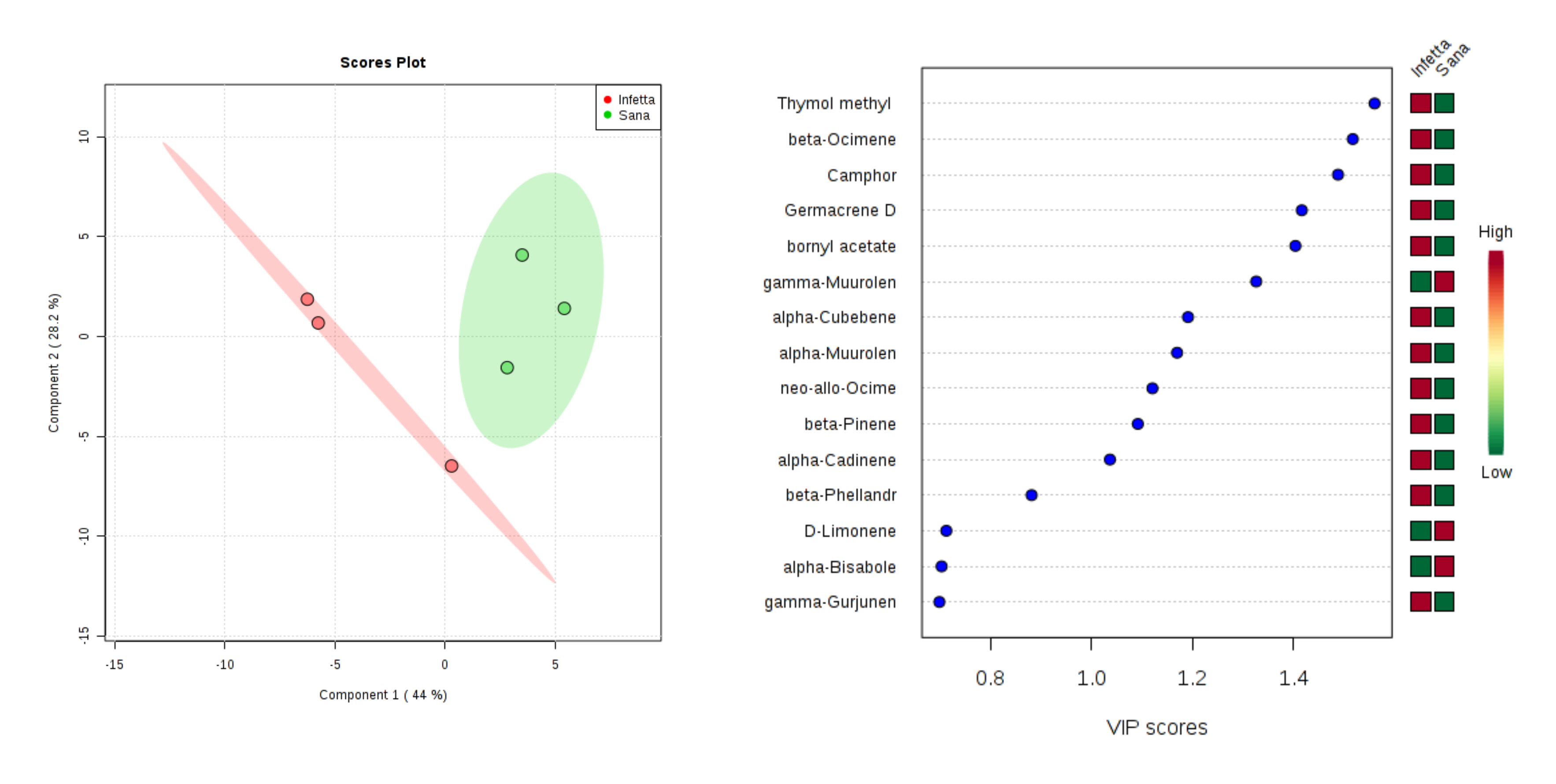
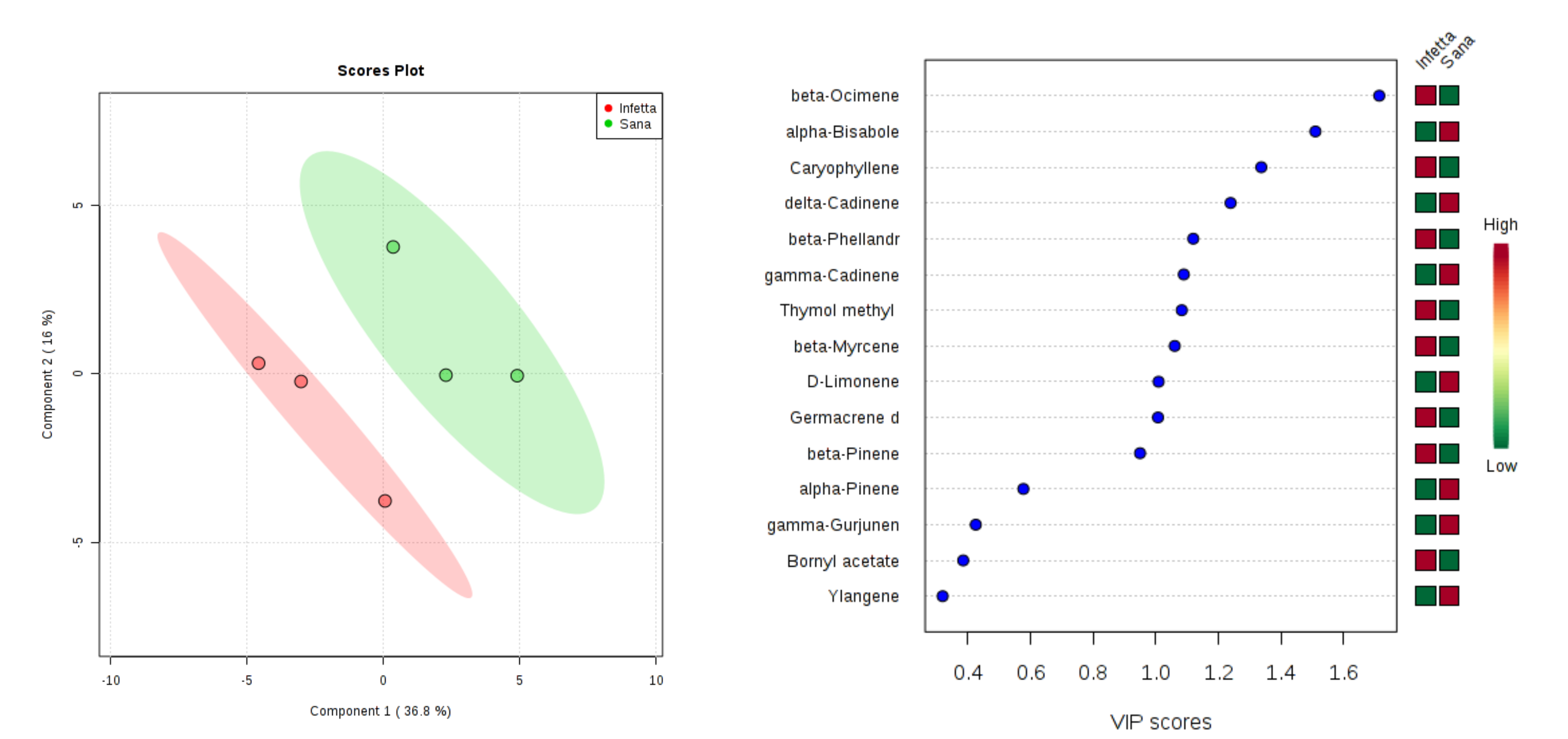
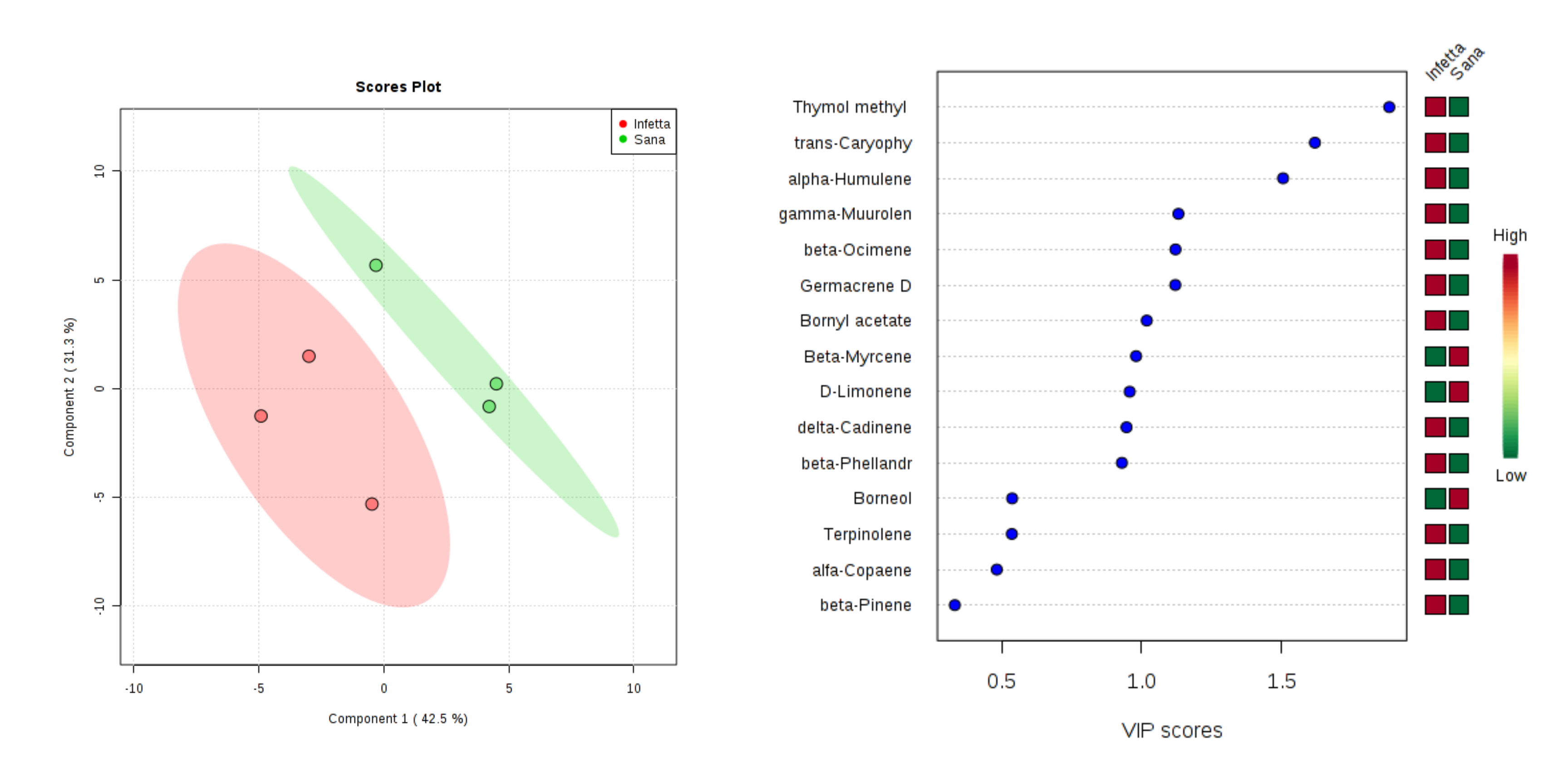
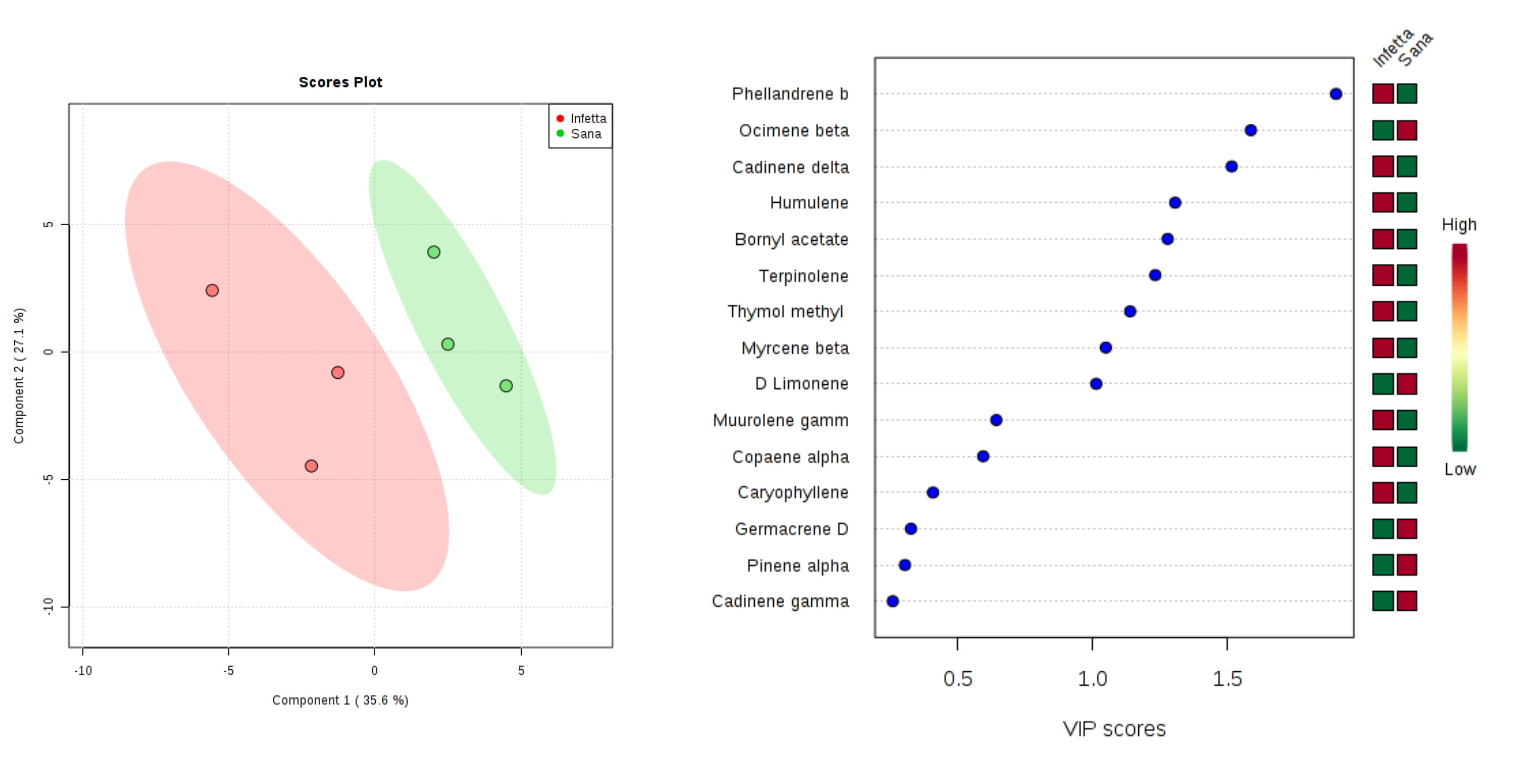
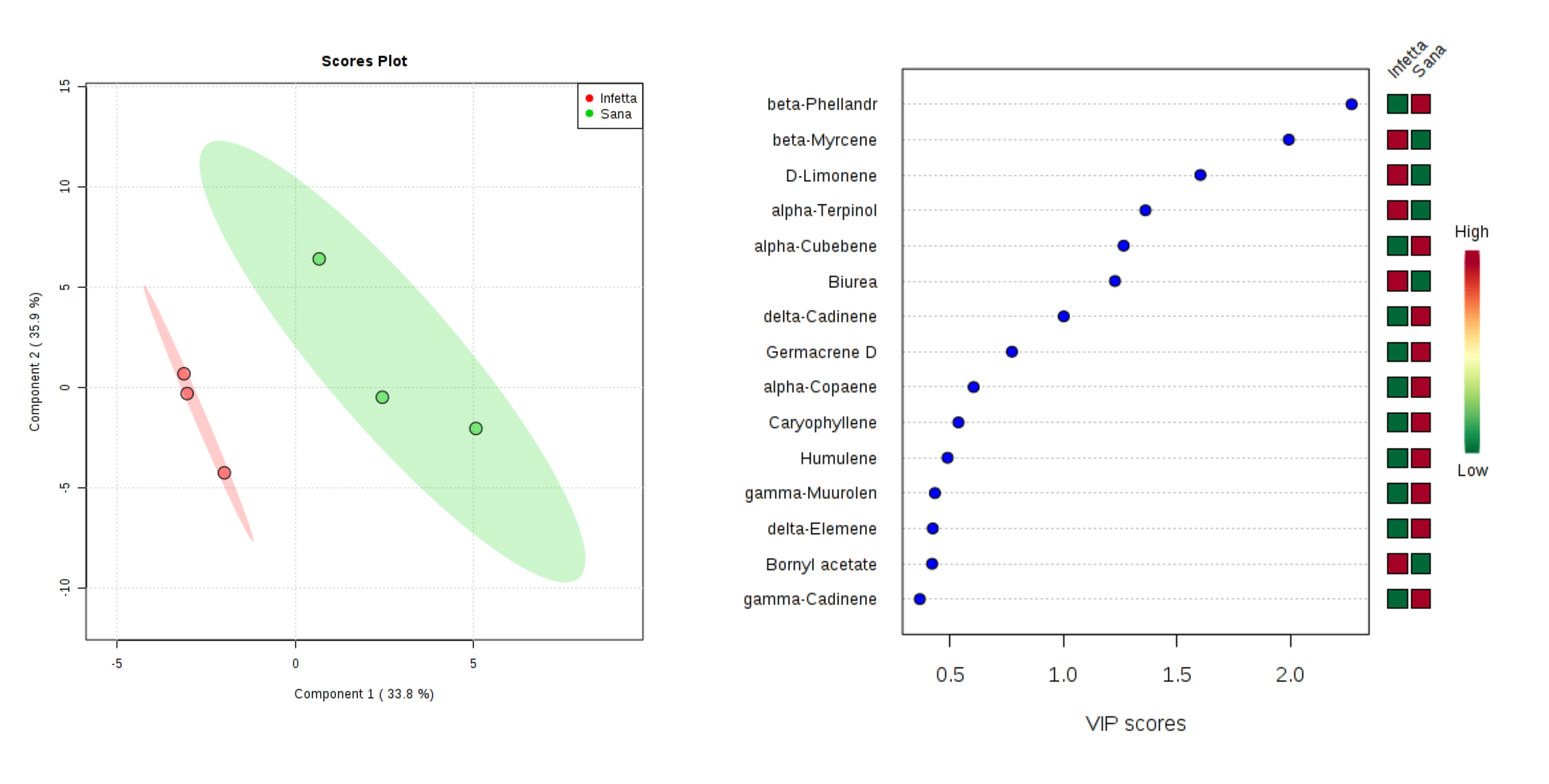
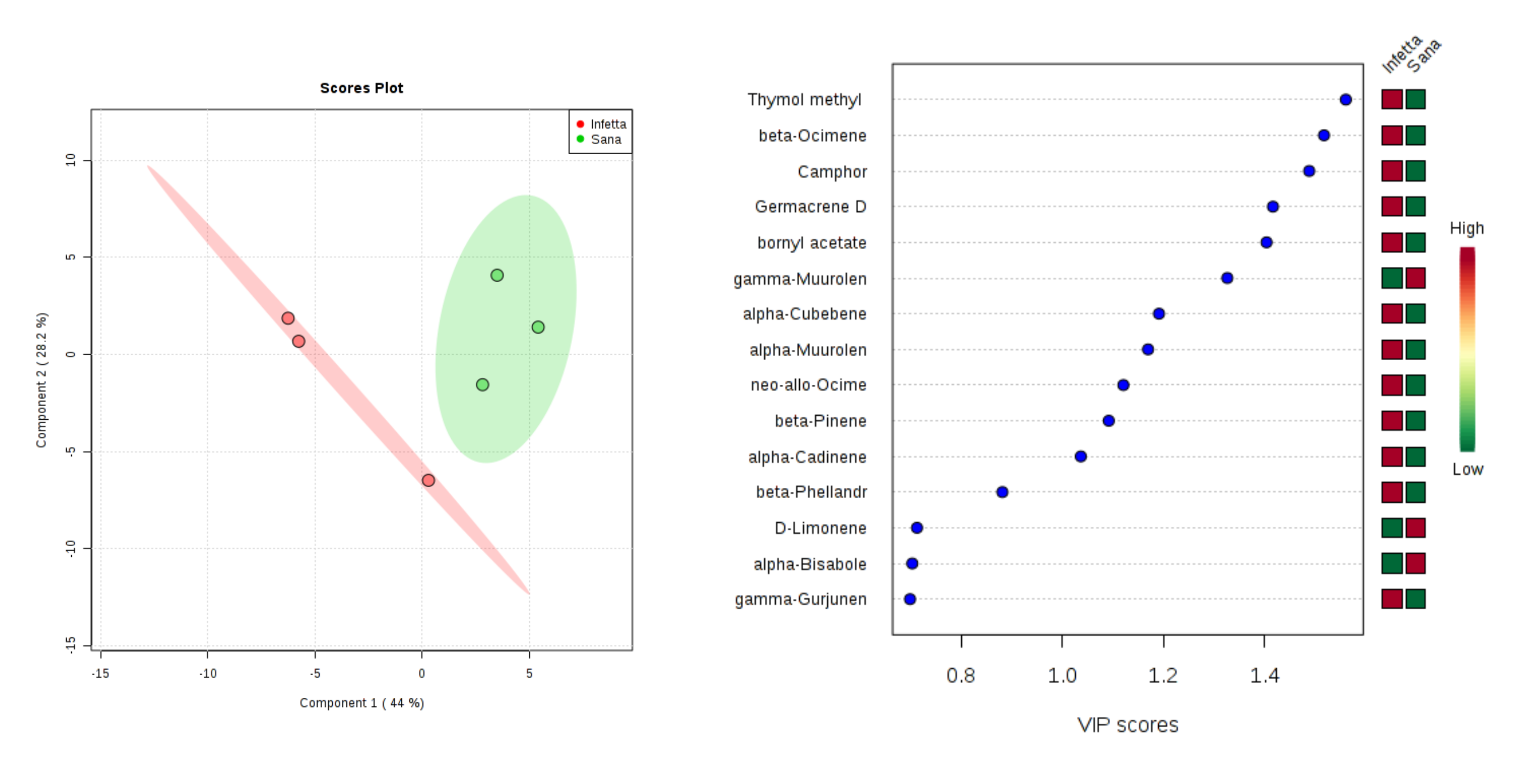
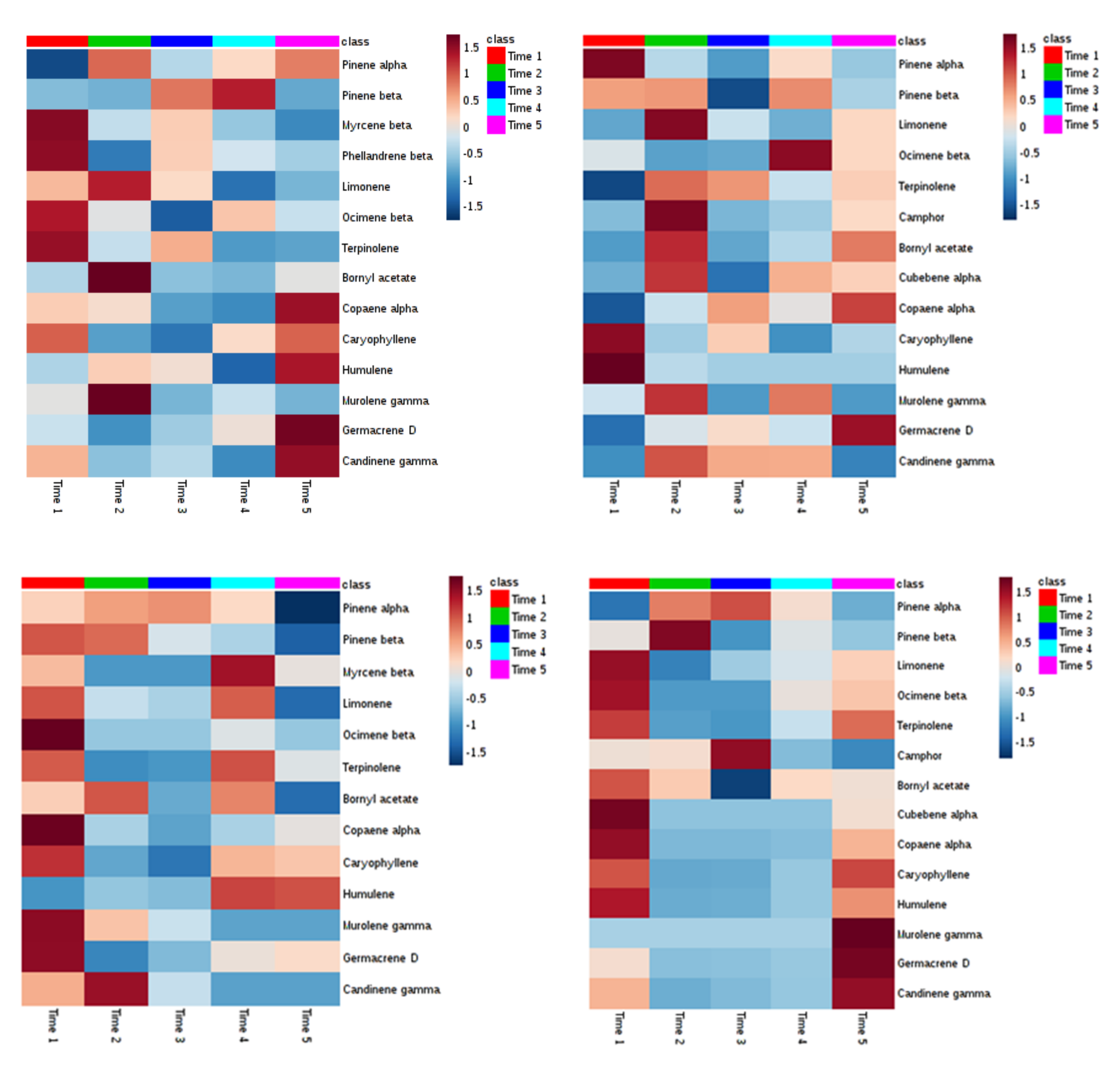
2. Results
3. Discussion
4. Materials and Methods
4.1. Plants Sampling Sites
4.2. Sampling of Pine Needles
4.3. Head Space GC/MS Analysis of Volatiles from Pine Needles
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tholl, D. Biosynthesis and Biological Functions of Terpenoids in Plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 2014, 5, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.M.; Fan, Y. Volatile terpenoids: Multiple functions, biosynthesis, modulation and manipulation by genetic engineering. Planta 2017, 246, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Alicandri, E.; Paolacci, A.R.; Osadolor, S.; Sorgonà, A.; Badiani, M.; Ciaffi, M. On the Evolution and Functional Diversity of Terpene Synthases in the Pinus Species: A Review. J. Mol. Evol. 2020, 88, 253–283. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D.; Lee, S. Terpene Specialized Metabolism inArabidopsis thaliana. Arab. Book 2011, 9, e0143. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Gao, Y.; Hoy, J.A.; Mann, F.M.; Honzatko, R.B.; Peters, R.J. Insights into Diterpene Cyclization from Structure of Bifunctional Abietadiene Synthase from Abies grandis. J. Biol. Chem. 2012, 287, 6840–6850. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Dicke, M.; Schnitzler, J.-P.; Turlings, T.C.J. Plant volatiles and the environment. Plant Cell Environ. 2014, 37, 1905–1908. [Google Scholar] [CrossRef]
- Gols, R. Direct and indirect chemical defences against insects in a multitrophic framework. Plant Cell Environ. 2014, 37, 1741–1752. [Google Scholar] [CrossRef]
- Pierik, R.; Ballaré, C.L.; Dicke, M. Ecology of plant volatiles: Taking a plant community perspective. Plant Cell Environ. 2014, 37, 1845–1853. [Google Scholar] [CrossRef]
- Büchel, K.; Malskies, S.; Mayer, M.; Fenning, T.M.; Gershenzon, J.; Hilker, M.; Meiners, T. How plants give early herbivore alert: Volatile terpenoids attract parasitoids to egg-infested elms. Basic Appl. Ecol. 2011, 12, 403–412. [Google Scholar] [CrossRef]
- Heil, M. Herbivore-induced plant volatiles: Targets, perception and unanswered questions. New Phytol. 2014, 204, 297–306. [Google Scholar] [CrossRef]
- Keeling, C.I.; Bohlmann, J. Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytol. 2006, 170, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Zulak, K.G.; Bohlmann, J. Terpenoid Biosynthesis and Specialized Vascular Cells of Conifer Defense. J. Integr. Plant Biol. 2010, 52, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.E.; Robert, J.A.; Keeling, C.I.; Domanski, D.; Quesada, A.L.; Jancsik, S.; Kuzyk, M.A.; Hamberger, B.; Borchers, C.H.; Bohlmann, J. An integrated genomic, proteomic and biochemical analysis of (+)-3-carene biosynthesis in Sitka spruce (Picea sitchensis) genotypes that are resistant or susceptible to white pine weevil. Plant J. 2011, 65, 936–948. [Google Scholar] [CrossRef]
- Isajev, V.; Fady, B.; Semerci, H.; Andonovski, V. EUFORGEN Technical Guidelines for Genetic Conservation and Use for European Black Pine (Pinus nigra); International Plant Genetic Resources Institute: Rome, Italy, 2004. [Google Scholar]
- Enescu, C.M. Pinus nigra in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Office of the European Union: Luxembourg, 2016; p. e015138. [Google Scholar]
- Nicolaci, A.; Travaglini, D.; Menguzzato, G.; Nocentini, S.; Veltri, A.; Iovino, F. Ecological and anthropogenic drivers of Calabrian pine (Pinus nigra J.F. Arn. ssp. laricio (Poiret) Maire) distribution in the Sila mountain range. iForest Biogeosci. For. 2015, 8, 497–508. [Google Scholar] [CrossRef]
- Werno, J.; Lamy, M. Animal atmospheric pollution: The urticating hairs of the pine processionary caterpillar (Thaumetopoea pityocampa Schiff.) (Insecta, Lepidoptera). Comptes Rendus l’Acadl. Sci. Serl. 3 Scil. Vie 1990, 310, 325–331. [Google Scholar]
- Battisti, A.; Stastny, M.; Netherer, S.; Robinet, C.; Schopf, A.; Roques, A.; Larsson, S. Expansion of Geographic Range In The Pine Processionary Moth Caused By Increased Winter Temperatures. Ecol. Appl. 2005, 15, 2084–2096. [Google Scholar] [CrossRef]
- Paine, T.D.; Raffa, K.F.; Harrington, T.C. Interactions Among Scolytid Bark Beetles, Their Associated Fungi, And Live Host Conifers. Annu. Rev. Entomol. 1997, 42, 179–206. [Google Scholar] [CrossRef]
- Koricheva, J.; Nykänen, H.; Gianoli, E. Meta-analysis of Trade-offs among Plant Antiherbivore Defenses: Are Plants Jacks-of-All-Trades, Masters of All? Am. Nat. 2004, 163, E64–E75. [Google Scholar] [CrossRef]
- Mumm, R.; Hilker, M. Direct and indirect chemical defence of pine against folivorous insects. Trends Plant Sci. 2006, 11, 351–358. [Google Scholar] [CrossRef]
- Trapp, S.C.; Croteau, R.B. Genomic organization of plant terpene synthases and molecular evolutionary implications. Genetics 2001, 158, 811–832. [Google Scholar] [PubMed]
- Jacquet, J.-S.; Orazio, C.; Jactel, H. Defoliation by processionary moth significantly reduces tree growth: A quantitative review. Ann. For. Sci. 2012, 69, 857–866. [Google Scholar] [CrossRef]
- Sampedro, L.; Moreira, X.; Zas, R. Costs of constitutive and herbivore-induced chemical defences in pine trees emerge only under low nutrient availability. J. Ecol. 2011, 99, 818–827. [Google Scholar] [CrossRef]
- Litvak, M.E.; Monson, R. Patterns of induced and constitutive monoterpene production in conifer needles in relation to insect herbivory. Oecologia 1998, 114, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Staudt, M.; Lhoutellier, L. Volatile organic compound emission from holm oak infested by gypsy moth larvae: Evidence for distinct responses in damaged and undamaged leaves. Tree Physiol. 2007, 27, 1433–1440. [Google Scholar] [CrossRef]
- Hilker, M.; Meiners, T. Induction of plant responses to oviposition and feeding by herbivorous arthropods: A comparison. Entomol. Exp. Appl. 2002, 104, 181–192. [Google Scholar] [CrossRef]
- Peñuelas, J. Plant VOC emissions: Making use of the unavoidable. Trends Ecol. Evol. 2004, 19, 402–404. [Google Scholar] [CrossRef]
- Arbez, M.; Bernard-Dagan, C.; Fillon, C. Variabilité intraspécifique des monoterpènes de Pinus nigra Arn.: Bilan des premiers résultats. Ann. Sci. For. 1974, 31, 57–70. [Google Scholar] [CrossRef][Green Version]
- Achotegui Castells, A. The Role of Terpenes in the Defensive Responses of Conifers against Herbivores and Pathogens. Ph.D. Thesis, Universitat Autònoma de Barcelona, Barcelona, Spain, September 2015. [Google Scholar]
- Achotegui-Castells, A.; Llusià, J.; Hódar, J.A.; Epenuelas, J. Needle terpene concentrations and emissions of two coexisting subspecies of Scots pine attacked by the pine processionary moth (Thaumetopoea pityocampa). Acta Physiol. Plant. 2013, 35, 3047–3058. [Google Scholar] [CrossRef]
- Cates, R.G.; Henderson, C.B.; Redak, R.A. Responses of the western spruce budworm to varying levels of nitrogen and terpenes. Oecologia 1987, 73, 312–316. [Google Scholar] [CrossRef]
- Ryan, M.F.; Guerin, P.M. Behavioural responses of the carrot fly larva, Psila rosae, to carrot root volatiles. Physiol. Entomol. 1982, 7, 315–324. [Google Scholar] [CrossRef]
- Nishino, C.; Manabe, S. Olfactory receptor systems for sex pheromone mimics in the American cockroach, Periplaneta americana L. Cell. Mol. Life Sci. 1983, 39, 1340–1342. [Google Scholar] [CrossRef]
- Rozman, V.; Kalinovic, I.; Korunic, Z. Toxicity of naturally occurring compounds of Lamiaceae and Lauraceae to three stored-product insects. J. Stored Prod. Res. 2007, 43, 349–355. [Google Scholar] [CrossRef]
- Feng, Y.-X.; Wang, Y.; Geng, Z.-F.; Zhang, D.; Borjigidai, A.; Du, S.S. Contact toxicity and repellent efficacy of Valerianaceae spp. to three stored-product insects and synergistic interactions between two major compounds camphene and bornyl acetate. Ecotoxicol. Environ. Saf. 2020, 190, 110106. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-X.; Wang, Y.; Chen, Z.-Y.; Guo, S.-S.; You, C.-X.; Du, S.S. Efficacy of bornyl acetate and camphene from Valeriana officinalis essential oil against two storage insects. Environ. Sci. Pollut. Res. 2019, 26, 16157–16165. [Google Scholar] [CrossRef]
- Araniti, F.; Lupini, A.; Sunseri, F.; Abenavoli, M.R. Allelopatic Potential of Dittrichia viscosa (L.) W. Greuter Mediated by VOCs: A Physiological and Metabolomic Approach. PLoS ONE 2017, 12, e0170161. [Google Scholar] [CrossRef]
- Hodgson, E.; Smart, R.C. Molecular and Biochemical Toxicology: Definition and Scope; Wiley: Hoboken, NJ, USA, 2008; pp. 1–4. [Google Scholar]





| N. | Common Name | IUPAC Name | Type of Terpene | RT (min) | KI | Structural Formula |
|---|---|---|---|---|---|---|
| 1 | α-pinene | (1S.5S)-2.6.6-trimethylbicyclo[3.1.1]hept-2-ene ((−)-α-pinene) | mono | 7.38 | 939 | 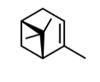 |
| 2 | β-pinene | 6.6-dimethyl-2-methylidenebicyclo[3.1.1]heptane | mono | 8.35 | 982 | 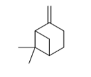 |
| 3 | β-myrcene | 7-methyl-3-methylene-1.6-octadiene | mono | 8.75 | 1000 | 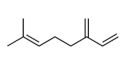 |
| 4 | β-phellandrene | 2-methyl-5-(1-methylethyl)-1.3-cyclohexadiene | mono | 9.61 | 1035 | 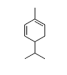 |
| 5 | limonene | 1-methyl-4-(prop-1-en-2-yl)cyclohex-1-ene | mono | 9.83 | 1043 |  |
| 6 | β-ocimene | (Z)-3.7-dimethyl-1.3.6-octatriene | mono | 10.06 | 1053 |  |
| 7 | terpinolene | 4-methyl-1-(1-methylethyl)-1.3-cyclohexadiene | mono | 11.09 | 1095 |  |
| 8 | thymol methyl ether | 2-methoxy-4-methyl-1-propan-2-ylbenzene | mono | 11.98 | 1131 |  |
| 9 | camphor | 1.7.7-trimethylbicyclo[2.2.1]heptan-2-one | mono | 12.48 | 1152 | 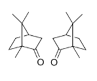 |
| 10 | bornyl acetate | 4.7.7-trimethyl-3-bicyclo[2.2.1]heptanyl) acetate | mono | 15.82 | 1292 |  |
| 11 | γ-gurjunene | (1R.3aR.4R.7R)-1.4-dimethyl-7-prop-1-en-2-yl-1.2.3.3a.4.5.6.7-octahydroazulene | sesqui | 16.41 | 1318 | 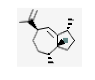 |
| 12 | δ-elemene | (3 R. 4 R ) -1-isopropil-4-metil-3- (prop-1-en- 2-il) -4-vinylcyclohex-1-ene | sesqui | 16.99 | 1344 | 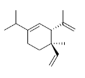 |
| 13 | α-cubebene | (1R.5S.6R.7S.10R)-10-methyl-4-methylidene-7-(propan-2-yl)tricyclo[4.4.0.0¹.⁵]decane | sesqui | 17.27 | 1357 | 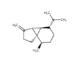 |
| 14 | α-copaene | (1S.6S.7S.8S)-1.3-dimethyl-8-(propan-2-yl)tricyclo[4.4.0.02.⁷]dec-3-ene | sesqui | 17.89 | 1384 |  |
| 15 | β-bourbonene | 1-methyl-5-methylidene-8-(propan-2-yl)tricyclo[5.3.0.02.⁶]decane | sesqui | 18.82 | 1427 | 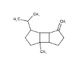 |
| 16 | caryophyllene | (1R.4E.9S)-4.11.11-trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene | sesqui | 18.88 | 1430 | 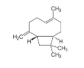 |
| 17 | α-bisabolene | (E)-1-methyl-4-(6-methylhepta-2.5-dien-2-yl)cyclohex-1-ene | sesqui | 19.63 | 1466 |  |
| 18 | humulene | 2.6.6.9-tetramethyl-1.4-8-cycloundecatriene | sesqui | 19.93 | 1480 | 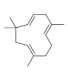 |
| 19 | γ-muurolene | (1S.4aS.8aR)-7-methyl-4-methylidene-1-propan-2-yl-2.3.4a.5.6.8a-hexahydro-1H-naphthalene | sesqui | 20.44 | 1492 | 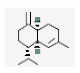 |
| 20 | germacrene D | (1E.5E.8S)-1.5-dimethyl-8-(prop-1-en-2-yl)cyclodeca-1.5-diene | sesqui | 20.18 | 1502 | 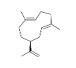 |
| 21 | γ-cadinene | (1S.4aR.8aR)-7-methyl-4-methylidene-1-propan-2-yl-2.3.4a.5.6.8a-hexahydro-1H-naphthalene | sesqui | 20.89 | 1526 | 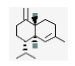 |
| Common Name | Type of Terpene | Sampling Time | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1°-NEST | 2°-DEFO_1 | 3°-DEFO_2 | 4°-PROCE_1 | 5°-PROCE_2 | 6°-OVI | ||||||||
| Area % | Area % | Area % | Area % | Area % | Area % | ||||||||
| Canolo | Bova | Canolo | Bova | Canolo | Bova | Canolo | Bova | Canolo | Bova | Canolo | Bova | ||
| α-pinene | mono | 73.043 | 82.013 | 89.573 | 29.990 | 91.603 | 75.727 | 83.950 | 78.380 | 76.787 | 76.630 | 73.770 | 77.380 |
| β-pinene | mono | 3.106 | 2.320 | 4.117 | - | 2.527 | - | 3.063 | 2.403 | 2.760 | 2.260 | 3.980 | 2.840 |
| limonene | Mono | 5.285 | 4.070 | 2.410 | 10.790 | 2.057 | 5.680 | 3.475 | 4.245 | 3.935 | 6.945 | 4.910 | 4.990 |
| β-ocimene | mono | 4.787 | - | - | - | - | - | - | - | 3.855 | - | 6.990 | 2.620 |
| terpinolene | mono | 0.205 | - | 0.145 | 0.280 | 0.140 | 0.263 | 0.220 | 0.193 | 0.360 | 0.350 | 0.810 | 0.160 |
| camphor | mono | - | - | 0.050 | - | 0.100 | - | - | - | - | 0.200 | - | - |
| bornyl acetate | mono | - | - | - | 0.295 | - | - | 0.035 | 0.080 | - | 0.235 | 0.130 | 0.040 |
| α-cubebene | sesqui | 0.120 | 0.195 | - | 0.265 | - | - | - | 0.190 | - | - | - | 0.360 |
| α-copaene | sesqui | 0.145 | 0.135 | - | 0.445 | - | 0.190 | - | 0.180 | 0.080 | - | 0.040 | 0.280 |
| caryophyllene | sesqui | 2.780 | 2.590 | - | 1.483 | 0.147 | 1.903 | 0.500 | 1.217 | 2.867 | 1.530 | 2.180 | 2.700 |
| humulene | sesqui | 0.485 | - | - | - | - | - | 0.055 | - | 0.330 | - | 0.160 | 0.070 |
| γ-muurolene | sesqui | - | - | 0.715 | - | 0.540 | - | 0.585 | - | - | - | 0.310 | |
| germacrene D | sesqui | 0.510 | 0.830 | - | 1.690 | - | 2.120 | - | 1.550 | 1.043 | 2.693 | 0.100 | 2.480 |
| γ-cadinene | sesqui | 0.500 | 0.565 | - | 1.230 | - | 0.697 | - | 0.800 | 0.203 | - | 0.030 | 0.670 |
| Common Name | Type of Terpene | Sampling Time | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1°-NEST | 2°-DEFO_1 | 3°-DEFO_2 | 4°-PROCE_1 | 5°-PROCE_2 | 6°-OVI | ||||||||
| Area % | Area % | Area % | Area % | Area % | Area % | ||||||||
| Canolo | Bova | Canolo | Bova | Canolo | Bova | Canolo | Bova | Canolo | Bova | Canolo | Bova | ||
| α-pinene | mono | 1.910 | 44.673 | 86.830 | 66.403 | 89.867 | 55.060 | 72.280 | 60.040 | 2.040 | 65.31 | 81.517 | 63.003 |
| β-pinene | mono | 74.383 | 7.505 | 3.443 | 4.640 | 2.607 | 16.485 | 3.590 | 12.930 | 2.435 | 6.565 | 6.643 | 2.940 |
| β-myrcene | mono | 5.060 | 11.330 | - | 4.383 | - | 9.800 | 8.920 | 5.040 | 5.225 | - | 0.513 | 3.230 |
| β-phellandrene | mono | - | 17.105 | - | - | 11.890 | - | 9.960 | - | 8.785 | - | 3.460 | |
| limonene | mono | 10.110 | - | - | 8.160 | 3.803 | - | 9.900 | - | - | - | 7.530 | 3.270 |
| β-ocimene | mono | 2.080 | 6.240 | - | 4.545 | - | 7.485 | - | 3.940 | - | 3.880 | 0.850 | 0.190 |
| terpinolene | mono | 0.447 | 0.607 | 0.205 | 0.360 | 0.153 | 0.470 | 0.485 | 0.270 | 0.283 | 0.280 | 0.240 | 0.310 |
| bornyl acetate | mono | - | 0.287 | 0.270 | 1.803 | 0.065 | 0.133 | 0.240 | 0.077 | 0.100 | 0.530 | 0.200 | 0.380 |
| α-copaene | sesqui | 0.060 | 0.220 | - | 0.210 | - | 0.097 | - | 0.130 | - | 0.203 | 0.100 | 0.220 |
| caryophyllene | sesqui | 1.487 | 4.987 | 0.467 | 3.610 | 0.270 | 3.350 | 1.100 | 4.390 | 1.050 | 6.603 | 1.210 | 5.610 |
| humulene | sesqui | 0.225 | - | - | 0.387 | 0.033 | - | 0.135 | - | 0.130 | 0.965 | 0.100 | 0.770 |
| γ-muurolene | sesqui | - | 0.387 | - | 0.930 | - | 0.275 | - | 0.480 | - | 0.265 | - | 0.330 |
| germacrene D | sesqui | 0.145 | 1.137 | - | - | - | 1.110 | - | 1.570 | - | 4.057 | 0.250 | 0.410 |
| γ-cadinene | sesqui | - | 0.380 | - | 0.375 | - | 0.420 | - | 0.300 | - | 0.503 | - | 0.240 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foti, V.; Araniti, F.; Manti, F.; Alicandri, E.; Giuffrè, A.M.; Bonsignore, C.P.; Castiglione, E.; Sorgonà, A.; Covino, S.; Paolacci, A.R.; et al. Profiling Volatile Terpenoids from Calabrian Pine Stands Infested by the Pine Processionary Moth. Plants 2020, 9, 1362. https://doi.org/10.3390/plants9101362
Foti V, Araniti F, Manti F, Alicandri E, Giuffrè AM, Bonsignore CP, Castiglione E, Sorgonà A, Covino S, Paolacci AR, et al. Profiling Volatile Terpenoids from Calabrian Pine Stands Infested by the Pine Processionary Moth. Plants. 2020; 9(10):1362. https://doi.org/10.3390/plants9101362
Chicago/Turabian StyleFoti, Vincenza, Fabrizio Araniti, Francesco Manti, Enrica Alicandri, Angelo Maria Giuffrè, Carmelo Peter Bonsignore, Elvira Castiglione, Agostino Sorgonà, Stefano Covino, Anna Rita Paolacci, and et al. 2020. "Profiling Volatile Terpenoids from Calabrian Pine Stands Infested by the Pine Processionary Moth" Plants 9, no. 10: 1362. https://doi.org/10.3390/plants9101362
APA StyleFoti, V., Araniti, F., Manti, F., Alicandri, E., Giuffrè, A. M., Bonsignore, C. P., Castiglione, E., Sorgonà, A., Covino, S., Paolacci, A. R., Ciaffi, M., & Badiani, M. (2020). Profiling Volatile Terpenoids from Calabrian Pine Stands Infested by the Pine Processionary Moth. Plants, 9(10), 1362. https://doi.org/10.3390/plants9101362












