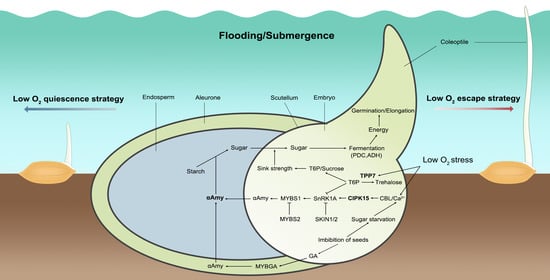
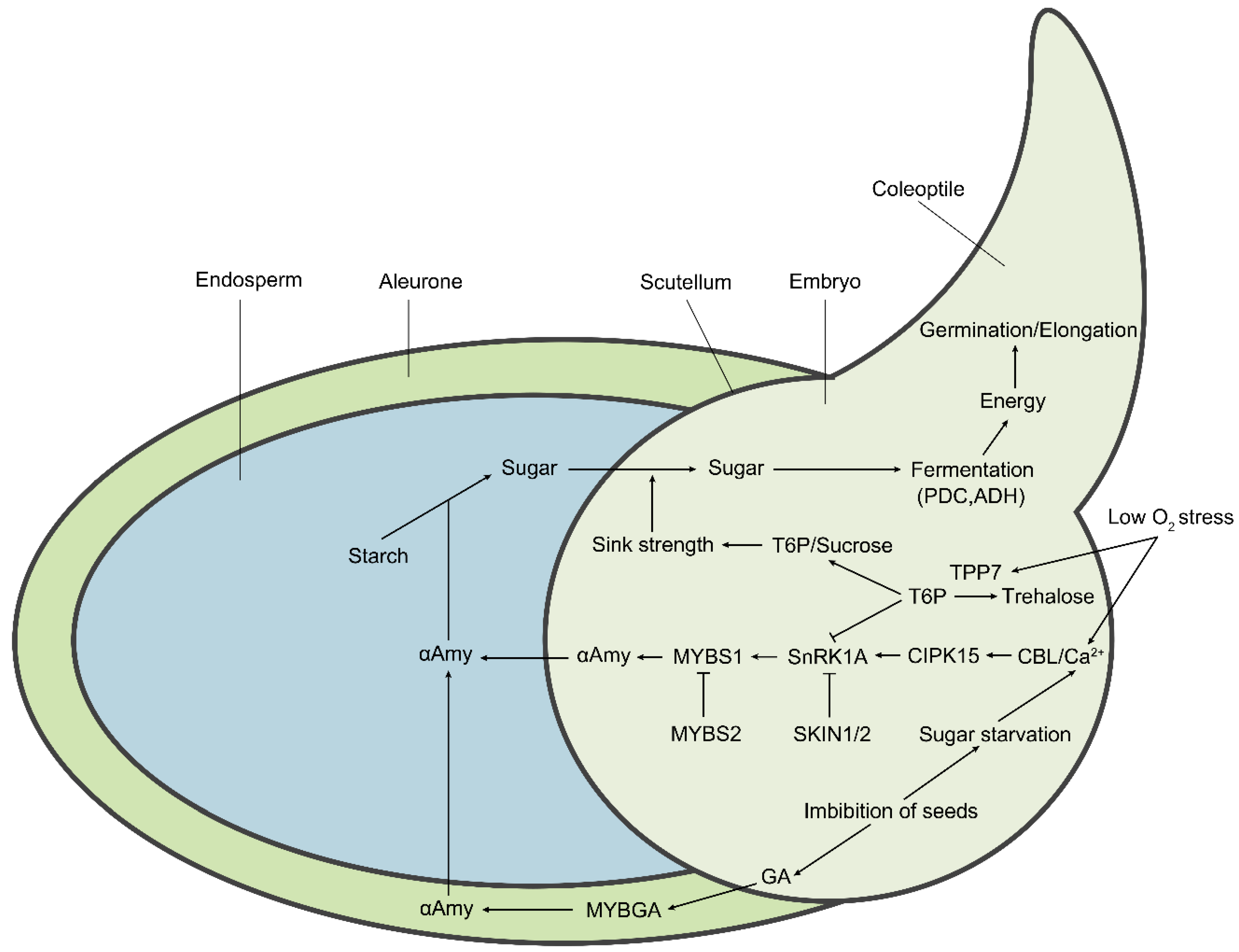
The Molecular Regulatory Pathways and Metabolic Adaptation in the Seed Germination and Early Seedling Growth of Rice in Response to Low O2 Stress
Abstract
1. Introduction
2. Strategies are Adopted in Rice under Submerged Germination
3. Metabolic Adaption to Anaerobic Germination in Rice
4. The Molecular Regulatory Pathway of Anaerobic Germination in Rice
5. Identification of QTLs/Genes for AG Tolerance
6. Breeding Applications Using QTLs/Genes Underlying AG Tolerance
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Khush, G. Productivity Improvements in Rice. Nutr. Rev. 2003, 61 (Suppl. 6), S114–S116. [Google Scholar] [CrossRef]
- Kumar, V.; Ladha, J.K. Direct Seeding of Rice. Recent Developments and Future Research Needs. Adv. Agron. 2011, 111, 297–413. [Google Scholar]
- Pandey, S.; Velasco, M.L. Economics of direct seeding in Asia: Patterns of adoption and research priorities. IRRI 1999, 24, 6–11. [Google Scholar]
- Miro, B.; Ismail, A.M. Tolerance of anaerobic conditions caused by flooding during germination and early growth in rice (Oryza sativa L.). Front. Plant Sci. 2013, 4, 269. [Google Scholar] [CrossRef]
- Farooq, M.; Siddique, K.H.M.; Rehman, H.; Aziz, T.; Lee, D.-J.; Wahid, A. Rice direct seeding: Experiences, challenges and opportunities. Soil Till. Res. 2011, 111, 87–98. [Google Scholar] [CrossRef]
- Gibbs, J.; Greenway, H. Review: Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct. Plant Biol. 2003, 30, 353. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, R.; Bailey-Serres, J.; Ashikari, M.; Atwell, B.J.; Colmer, T.D.; Fagerstedt, K.; Fukao, T.; Geigenberger, P.; Hebelstrup, K.H.; Hill, R.D.; et al. Community recommendations on terminology and procedures used in flooding and low oxygen stress research. New Phytol. 2017, 214, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, R.; Hartman, S.; Liu, Z.; Martopawiro, S.; Sajeev, N.; van Veen, H.; Yeung, E.; Voesenek, L. Signal Dynamics and Interactions during Flooding Stress. Plant Physiol. 2018, 176, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Voesenek, L.A.; Sasidharan, R.; Visser, E.J.; Bailey-Serres, J. Flooding stress signaling through perturbations in oxygen, ethylene, nitric oxide and light. New Phytol. 2016, 209, 39–43. [Google Scholar] [CrossRef]
- Wegner, L.H. Oxygen Transport in Waterlogged Plants. In Waterlogging Signalling and Tolerance in Plants; Springer: Berlin, Heidelberg, 2010; pp. 3–22. [Google Scholar]
- Armstrong, W.; Webb, T.; Darwent, M.; Beckett, P. Measuring and interpreting respiratory critical oxygen pressures in roots. Ann. Bot. 2008, 103, 281–293. [Google Scholar] [CrossRef]
- Drew, M.; Lynch, J. Soil Anaerobiosis, Microorganisms, and Root Function. Annu. Rev. Phytopathol. 1980, 18, 37–66. [Google Scholar] [CrossRef]
- Hafeez-ur-Rehman; Nawaz, A.; Awan, M.I.; Ijaz, M.; Hussain, M.; Ahmad, S.; Farooq, M. Direct Seeding in Rice: Problems and Prospects. In Agronomic Crops: Volume 1: Production Technologies; Hasanuzzaman, M., Ed.; Springer: Singapore, 2019; pp. 199–222. [Google Scholar]
- Pathak, D.S.; Tewari, A.; Sankhyan, S.; Dubey, D.; Mina, U.; Kumar, V.; Jain, N.; Bhatia, A. Direct-seeded rice: Potential, performance and problems-A review. Curr. Adv. Agric. Sci. 2011, 3, 77–88. [Google Scholar]
- Lee, K.-W.; Chen, P.-W.; Lu, C.-A.; Chen, S.; Ho, T.-H.; Yu, S.-M. Coordinated Responses to Oxygen and Sugar Deficiency Allow Rice Seedlings to Tolerate Flooding. Sci. Signal. 2009, 2, ra61. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.H.; Wang, F.Z.; Shi, L.; Chen, M.X.; Cao, Y.Y.; Zhu, F.Y.; Wu, Y.Z.; Xie, L.J.; Liu, T.Y.; Su, Z.Z.; et al. Natural variation in the promoter of rice calcineurin B-like protein10 (OsCBL10) affects flooding tolerance during seed germination among rice subspecies. Plant J. 2018, 94, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.A.; Lin, C.C.; Lee, K.W.; Chen, J.L.; Huang, L.F.; Ho, S.L.; Liu, H.J.; Hsing, Y.I.; Yu, S.M. The SnRK1A protein kinase plays a key role in sugar signaling during germination and seedling growth of rice. Plant Cell 2007, 19, 2484–2499. [Google Scholar] [CrossRef]
- Kretzschmar, T.; Pelayo, M.A.; Trijatmiko, K.R.; Gabunada, L.F.; Alam, R.; Jimenez, R.; Mendioro, M.S.; Slamet-Loedin, I.H.; Sreenivasulu, N.; Bailey-Serres, J.; et al. A trehalose-6-phosphate phosphatase enhances anaerobic germination tolerance in rice. Nat. Plants 2015, 1, 15124. [Google Scholar] [CrossRef]
- Guglielminetti, L.; Yamaguchi, J.; Perata, P.; Alpi, A. Amylolytic Activities in Cereal Seeds under Aerobic and Anaerobic Conditions. Plant Physiol. 1995, 109, 1069–1076. [Google Scholar] [CrossRef]
- Matsumura, H.; Takano, T.; Yoshida, K.; Takeda, G. A Rice Mutant Lacking Alcohol Dehydrogenase. Jpn. J. Breed. 1995, 45, 365–367. [Google Scholar] [CrossRef]
- Ho, V.T.; Tran, A.; Cardarelli, F.; Perata, P.; Pucciariello, C. A calcineurin B-like protein participates in low oxygen signalling in rice. Funct. Plant Biol. 2017, 44, 917–928. [Google Scholar] [CrossRef]
- Lin, C.R.; Lee, K.W.; Chen, C.Y.; Hong, Y.F.; Chen, J.L.; Lu, C.A.; Chen, K.T.; Ho, T.H.; Yu, S.M. SnRK1A-interacting negative regulators modulate the nutrient starvation signaling sensor SnRK1 in source-sink communication in cereal seedlings under abiotic stress. Plant Cell 2014, 26, 808–827. [Google Scholar] [CrossRef]
- Chen, Y.-S.; David Ho, T.-H.; Liu, L.; Lee, D.H.; Lee, C.-H.; Chen, Y.-R.; Lin, S.-Y.; Lu, C.-A.; Yu, S.-M. Sugar starvation-regulated MYBS2 and 14-3-3 protein interactions enhance plant growth, stress tolerance, and grain weight in rice. Proc. Natl. Acad. Sci. USA 2019, 116, 21925. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.M.; Ella, E.S.; Vergara, G.V.; Mackill, D.J. Mechanisms associated with tolerance to flooding during germination and early seedling growth in rice (Oryza sativa). Ann. Bot. 2009, 103, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Magneschi, L.; Perata, P. Rice germination and seedling growth in the absence of oxygen. Ann. Bot. 2009, 103, 181–196. [Google Scholar] [CrossRef]
- Voesenek, L.A.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Voesenek, L.A. Flooding Stress: Acclimations and Genetic Diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Life in the balance: A signaling network controlling survival of flooding. Curr. Opin. Plant Biol. 2010, 13, 489–494. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.J.; Bailey-Serres, J. Flooding tolerance: O2 sensing and survival strategies. Curr. Opin. Plant Biol. 2013, 16, 647–653. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Lee, S.C.; Brinton, E. Waterproofing crops: Effective flooding survival strategies. Plant Physiol. 2012, 160, 1698–1709. [Google Scholar] [CrossRef]
- Xu, K.; Xu, X.; Fukao, T.; Canlas, P.; Maghirang-Rodriguez, R.; Heuer, S.; Ismail, A.M.; Bailey-Serres, J.; Ronald, P.C.; Mackill, D.J. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 2006, 442, 705–708. [Google Scholar] [CrossRef]
- Fukao, T.; Xu, K.; Ronald, P.C.; Bailey-Serres, J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 2006, 18, 2021–2034. [Google Scholar] [CrossRef]
- Fukao, T.; Bailey-Serres, J. Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellin responses in rice. Proc. Natl. Acad. Sci. USA 2008, 105, 16814–16819. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Nagai, K.; Furukawa, S.; Song, X.-J.; Kawano, R.; Sakakibara, H.; Wu, J.; Matsumoto, T.; Yoshimura, A.; Kitano, H.; et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 2009, 460, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Atwell, B.J.; Waters, I.; Greenway, H. The Effect of Oxygen and Turbulence on Elongation of Coleoptiles of Submergence-Tolerant and -Intolerant Rice Cultivars. J. Exp. Bot. 1982, 33, 1030–1044. [Google Scholar] [CrossRef]
- Takahashi, H.; Saika, H.; Matsumura, H.; Nagamura, Y.; Tsutsumi, N.; Nishizawa, N.K.; Nakazono, M. Cell division and cell elongation in the coleoptile of rice alcohol dehydrogenase 1-deficient mutant are reduced under complete submergence. Ann. Bot. 2011, 108, 253–261. [Google Scholar] [CrossRef]
- Huang, J.; Takano, T.; Akita, S. Expression of α-expansin genes in young seedlings of rice (Oryza sativa L.). Planta 2000, 211, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Lee, Y.; Cho, H.T.; Kende, H. Regulation of expansin gene expression affects growth and development in transgenic rice plants. Plant Cell 2003, 15, 1386–1398. [Google Scholar] [CrossRef]
- Lasanthi-Kudahettige, R.; Magneschi, L.; Loreti, E.; Gonzali, S.; Licausi, F.; Novi, G.; Beretta, O.; Vitulli, F.; Alpi, A.; Perata, P. Transcript Profiling of the Anoxic Rice Coleoptile. Plant Physiol. 2007, 144, 218–231. [Google Scholar] [CrossRef]
- Lee, K.W.; Chen, P.W.; Yu, S.M. Metabolic adaptation to sugar/O2 deficiency for anaerobic germination and seedling growth in rice. Plant Cell Environ. 2014, 37, 2234–2244. [Google Scholar]
- Kuroha, T.; Ashikari, M. Molecular mechanisms and future improvement of submergence tolerance in rice. Mol. Breed. 2020, 40, 41. [Google Scholar] [CrossRef]
- Hsu, S.K.; Tung, C.W. RNA-Seq Analysis of Diverse Rice Genotypes to Identify the Genes Controlling Coleoptile Growth during Submerged Germination. Front. Plant Sci. 2017, 8, 762. [Google Scholar] [CrossRef]
- Greenway, H.; Gibbs, J. Mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Funct. Plant Biol. 2003, 30, 999–1036. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.A.F.D.; Sodek, L. The metabolic response of plants to oxygen deficiency. Braz. J. Plant Physiol. 2002, 14, 83–94. [Google Scholar] [CrossRef]
- Tadege, M.; Dupuis, I.; Kuhlemeier, C. Ethanolic fermentation: New functions for an old pathway. Trends Plant Sci. 1999, 4, 320–325. [Google Scholar] [CrossRef]
- Edwards, J.M.; Roberts, T.H.; Atwell, B.J. Quantifying ATP turnover in anoxic coleoptiles of rice (Oryza sativa) demonstrates preferential allocation of energy to protein synthesis. J. Exp. Bot. 2012, 63, 4389–4402. [Google Scholar] [CrossRef]
- Miro, B.; Longkumer, T.; Entila, F.D.; Kohli, A.; Ismail, A.M. Rice Seed Germination Underwater: Morpho-Physiological Responses and the Bases of Differential Expression of Alcoholic Fermentation Enzymes. Front. Plant Sci. 2017, 8, 1857. [Google Scholar] [CrossRef]
- Damaris, R.N.; Lin, Z.; Yang, P.; He, D. The Rice Alpha-Amylase, Conserved Regulator of Seed Maturation and Germination. Int. J. Mol. Sci 2019, 20, 450. [Google Scholar] [CrossRef]
- Senapati, S.; Kuanar, S.; Sarkar, R. Anaerobic Germination Potential in Rice (Oryza sativa L.): Role of Amylases, Alcohol deydrogenase and Ethylene. J. Stress Physiol. Biochem. 2019, 15, 39–52. [Google Scholar]
- Pujadas, G.; Palau, J. Evolution of alpha-amylases: Architectural features and key residues in the stabilization of the (beta/alpha)(8) scaffold. Mol. Biol. Evol. 2001, 18, 38–54. [Google Scholar] [CrossRef]
- Huang, N.; Sutliff, T.D.; Litts, J.C.; Rodriguez, R.L. Classification and characterization of the rice alpha-amylase multigene family. Plant Mol. Biol. 1990, 14, 655–668. [Google Scholar] [CrossRef]
- Huang, N.; Stebbins, G.; Rodriguez, R. Classification and evolution of α-amylase genes in plants. Proc. Natl. Acad. Sci. USA 1992, 89, 7526–7530. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Thomas, B.R.; Rodriguez, R.L. Differential expression of rice α-amylase genes during seedling development under anoxia. Plant Mol. Biol. 1999, 40, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Greenway, H.; Matsumura, H.; Tsutsumi, N.; Nakazono, M. Rice alcohol dehydrogenase 1 promotes survival and has a major impact on carbohydrate metabolism in the embryo and endosperm when seeds are germinated in partially oxygenated water. Ann. Bot. 2014, 113, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, J.; Senapati, S.; Ray, S.; Chakraborty, K.; Molla, K.; Basak, N.; Pradhan, B.; Yeasmin, L.; Chattopadhyay, K.; Sarkar, R. Transcriptomic and physiological studies identify cues for germination stage oxygen deficiency tolerance in rice. Environ. Exp. Bot. 2017, 147, 234–248. [Google Scholar] [CrossRef]
- Saika, H.; Matsumura, H.; Takano, T.; Tsutsumi, N.; Nakazono, M. A Point Mutation of Adh1 Gene is Involved in the Repression of Coleoptile Elongation under Submergence in Rice. Breed. Sci. 2006, 56, 69–74. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Morokuma, M. Ethanolic fermentation and anoxia tolerance in four rice cultivars. J. Plant Physiol. 2007, 164, 168–173. [Google Scholar] [CrossRef]
- Hakata, M.; Kuroda, M.; Miyashita, T.; Yamaguchi, T.; Kojima, M.; Sakakibara, H.; Mitsui, T.; Yamakawa, H. Suppression of α-amylase genes improves quality of rice grain ripened under high temperature. Plant Biotechnol. J. 2012, 10, 1110–1117. [Google Scholar] [CrossRef]
- Hu, Q.; Fu, Y.; Guan, Y.; Lin, C.; Cao, D.; Hu, W.; Sheteiwy, M.; Hu, J. Inhibitory effect of chemical combinations on seed germination and pre-harvest sprouting in hybrid rice. Plant Growth Regul. 2016, 80, 281–289. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Karrer, E.E.; Thomas, B.R.; Chen, L.; Rodriguez, R.L. Three cis-elements required for rice α-amylase Amy3D expression during sugar starvation. Plant Mol. Biol. 1998, 36, 331–341. [Google Scholar] [CrossRef]
- Yu, S.M.; Lee, Y.C.; Fang, S.C.; Chan, M.T.; Hwa, S.F.; Liu, L.F. Sugars act as signal molecules and osmotica to regulate the expression of alpha-amylase genes and metabolic activities in germinating cereal grains. Plant Mol. Biol. 1996, 30, 1277–1289. [Google Scholar] [CrossRef]
- Knight, H.; Knight, M. Abiotic stress signaling pathways: Specificity and cross-talk. Trends Plant Sci. 2001, 6, 1360–1385. [Google Scholar] [CrossRef]
- Batistic, O.; Kudla, J. Integration and channeling of calcium signaling through the CBL calcium sensor/CIPK protein kinase network. Planta 2004, 219, 915–924. [Google Scholar] [CrossRef]
- Das, R.; Pandey, G.K. Expressional analysis and role of calcium regulated kinases in abiotic stress signaling. Curr. Genom. 2010, 11, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Luan, S. The CBL-CIPK network in plant calcium signaling. Trends Plant Sci. 2009, 14, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, V.; Ritz, O.; Linder, S.; Harter, K.; Kudla, J. The NAF domain defines a novel protein-protein interaction module conserved in Ca2+-regulated kinases. EMBO J. 2001, 20, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Kurusu, T.; Hamada, J.; Nokajima, H.; Kitagawa, Y.; Kiyoduka, M.; Takahashi, A.; Hanamata, S.; Ohno, R.; Hayashi, T.; Okada, K.; et al. Regulation of Microbe-Associated Molecular Pattern-Induced Hypersensitive Cell Death, Phytoalexin Production, and Defense Gene Expression by Calcineurin B-Like Protein-Interacting Protein Kinases, OsCIPK14/15, in Rice Cultured Cells. Plant Physiol. 2010, 153, 678–692. [Google Scholar] [CrossRef]
- Halford, N.G.; Hey, S.; Jhurreea, D.; Laurie, S.; McKibbin, R.S.; Paul, M.; Zhang, Y. Metabolic signalling and carbon partitioning: Role of Snf1-related (SnRK1) protein kinase. J. Exp. Bot. 2003, 54, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed]
- Ghillebert, R.; Swinnen, E.; Wen, J.; Vandesteene, L.; Ramon, M.; Norga, K.; Rolland, F.; Winderickx, J. The AMPK/SNF1/SnRK1 fuel gauge and energy regulator: Structure, function and regulation. FEBS J. 2011, 278, 3978–3990. [Google Scholar] [CrossRef]
- Hong, Y.F.; Ho, T.H.; Wu, C.F.; Ho, S.L.; Yeh, R.H.; Lu, C.A.; Chen, P.W.; Yu, L.C.; Chao, A.; Yu, S.M. Convergent starvation signals and hormone crosstalk in regulating nutrient mobilization upon germination in cereals. Plant Cell 2012, 24, 2857–2873. [Google Scholar] [CrossRef]
- Lu, C.A.; Ho, T.H.; Ho, S.L.; Yu, S.M. Three novel MYB proteins with one DNA binding repeat mediate sugar and hormone regulation of alpha-amylase gene expression. Plant Cell 2002, 14, 1963–1980. [Google Scholar] [CrossRef]
- Lu, C.A.; Lim, E.K.; Yu, S.M. Sugar response sequence in the promoter of a rice α-amylase gene serves as a transcriptional enhancer. J. Biol. Chem. 1998, 273, 10120–10131. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.W.; Chiang, C.M.; Tseng, T.H.; Yu, S.M. Interaction between rice MYBGA and the gibberellin response element controls tissue-specific sugar sensitivity of alpha-amylase genes. Plant Cell 2006, 18, 2326–2340. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.W.; Lu, C.A.; Yu, T.S.; Tseng, T.H.; Wang, C.S.; Yu, S.M. Rice alpha-amylase transcriptional enhancers direct multiple mode regulation of promoters in transgenic rice. J. Biol. Chem. 2002, 277, 13641–13649. [Google Scholar] [CrossRef]
- Kaneko, M.; Itoh, H.; Ueguchi-Tanaka, M.; Ashikari, M.; Matsuoka, M. The alpha-amylase induction in endosperm during rice seed germination is caused by gibberellin synthesized in epithelium. Plant Physiol. 2002, 128, 1264–1270. [Google Scholar] [CrossRef]
- Tsuji, H.; Aya, K.; Ueguchi-Tanaka, M.; Shimada, Y.; Nakazono, M.; Watanabe, R.; Nishizawa, N.K.; Gomi, K.; Shimada, A.; Kitano, H.; et al. GAMYB controls different sets of genes and is differentially regulated by microRNA in aleurone cells and anthers. Plant J. 2006, 47, 427–444. [Google Scholar] [CrossRef]
- Gubler, F.; Kalla, R.; Roberts, J.K.; Jacobsen, J.V. Gibberellin-Regulated Expression of a myb Gene in Barley Aleurone Cells: Evidence for Myb Transactivation of a High-pl a-Amylase Gene Promoter. Plant Cell 1995, 7, 1879. [Google Scholar] [CrossRef]
- Gubler, F.; Raventos, D.; Keys, M.; Watts, R.; Mundy, J.; Jacobsen, J. Target genes and regulatory domains of the GAMYB transcription activator in cereal aleurone. Plant J. 1999, 17, 1–9. [Google Scholar] [CrossRef]
- Lunn, J.; Delorge, I.; Figueroa, C.; Van Dijck, P.; Stitt, M. Trehalose metabolism in plants. Plant J. 2014, 79, 544–567. [Google Scholar] [CrossRef]
- Zhang, Y.; Primavesi, L.F.; Jhurreea, D.; Andralojc, P.J.; Mitchell, R.A.; Powers, S.J.; Schluepmann, H.; Delatte, T.; Wingler, A.; Paul, M.J. Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol. 2009, 149, 1860–1871. [Google Scholar] [CrossRef]
- Figueroa, C.M.; Lunn, J.E. A Tale of Two Sugars: Trehalose 6-Phosphate and Sucrose. Plant Physiol. 2016, 172, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.P.; Ivakov, A.; Feil, R.; Duan, G.Y.; Walther, D.; Giavalisco, P.; Piques, M.; Carillo, P.; Hubberten, H.M.; Stitt, M.; et al. The sucrose-trehalose 6-phosphate (Tre6P) nexus: Specificity and mechanisms of sucrose signalling by Tre6P. J. Exp. Bot. 2014, 65, 1051–1068. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Xiong, L. Genetic mechanisms conferring adaptation to submergence and drought in rice: Simple or complex? Curr. Opin. Plant Biol. 2013, 16, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Hattori, Y.; Ashikari, M. Stunt or elongate? Two opposite strategies by which rice adapts to floods. J. Plant Res. 2010, 123, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Angaji, S.A.; Septiningsih, E.M.; Mackill, D.J.; Ismail, A.M. QTLs associated with tolerance of flooding during germination in rice (Oryza sativa L.). Euphytica 2010, 172, 159–168. [Google Scholar] [CrossRef]
- Baltazar, M.; Ignacio, J.C.; Thomson, M.; Ismail, A.; Mendioro, M.; Septiningsih, E. QTL mapping for tolerance to anaerobic germination in rice from IR64 and the aus landrace Kharsu 80A. Breed. Sci. 2019, 69, 227–233. [Google Scholar] [CrossRef]
- Septiningsih, E.M.; Ignacio, J.C.; Sendon, P.M.; Sanchez, D.L.; Ismail, A.M.; Mackill, D.J. QTL mapping and confirmation for tolerance of anaerobic conditions during germination derived from the rice landrace Ma-Zhan Red. Theor. Appl. Genet. 2013, 126, 1357–1366. [Google Scholar] [CrossRef]
- Baltazar, M.D.; Ignacio, J.C.I.; Thomson, M.J.; Ismail, A.M.; Mendioro, M.S.; Septiningsih, E.M. QTL mapping for tolerance of anaerobic germination from IR64 and the aus landrace Nanhi using SNP genotyping. Euphytica 2014, 197, 251–260. [Google Scholar] [CrossRef]
- Kim, S.-M.; Reinke, R.F. Identification of QTLs for tolerance to hypoxia during germination in rice. Euphytica 2018, 214, 160. [Google Scholar] [CrossRef]
- Ghosal, S.; Casal, C.; Quilloy, F.; Septiningsih, E.; Mendioro, M.; Dixit, S. Deciphering Genetics Underlying Stable Anaerobic Germination in Rice: Phenotyping, QTL Identification, and Interaction Analysis. Rice 2019, 12, 50. [Google Scholar] [CrossRef]
- Hsu, S.K.; Tung, C.W. Genetic Mapping of Anaerobic Germination-Associated QTLs Controlling Coleoptile Elongation in Rice. Rice 2015, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lu, Q.; Wu, W.; Niu, X.; Wang, C.; Feng, Y.; Xu, Q.; Wang, S.; Yuan, X.; Yu, H.; et al. Association Mapping Reveals Novel Genetic Loci Contributing to Flooding Tolerance during Germination in Indica Rice. Front. Plant Sci. 2017, 8, 678. [Google Scholar] [CrossRef] [PubMed]
- Nghi, K.N.; Tondelli, A.; Valè, G.; Tagliani, A.; Marè, C.; Perata, P.; Pucciariello, C. Dissection of coleoptile elongation in japonica rice under submergence through integrated genome-wide association mapping and transcriptional analyses. Plant Cell Environ. 2019, 42, 1832–1846. [Google Scholar] [CrossRef] [PubMed]
- Rohilla, M.; Singh, N.; Mazumder, A.; Sen, P.; Roy, P.; Chowdhury, D.; Singh, N.K.; Mondal, T.K. Genome-wide association studies using 50 K rice genic SNP chip unveil genetic architecture for anaerobic germination of deep-water rice population of Assam, India. Mol. Genet. Genom. 2020, 295, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Nordborg, M.; Tavaré, S. Linkage disequilibrium: What history has to tell us Trends Genet. Trends Genet. TIG 2002, 18, 83–90. [Google Scholar] [CrossRef]
- Singh, S.; Mackill, D.; Ismail, A. Responses of SUB1 rice introgression lines to submergence in the field: Yield and grain quality. Field Crops Res. 2009, 113, 12–23. [Google Scholar] [CrossRef]
- Mackill, D.; Ismail, A.; Singh, U.; Labios, R.; Paris, T. Development and Rapid Adoption of Submergence-Tolerant (Sub1) Rice Varieties. Adv. Agron. 2012, 115, 299–352. [Google Scholar]
- Mondal, S.; Khan, M.; Dixit, S.; Cruz, P.C.S.; Septiningsih, E.; Ismail, A. Growth, productivity and grain quality of AG1 and AG2 QTLs introgression lines under flooding in direct-seeded rice system. Field Crops Res. 2020, 248, 107713. [Google Scholar] [CrossRef]
- Toledo, A.M.U.; Ignacio, J.C.I.; Casal, C.; Gonzaga, Z.J.; Mendioro, M.S.; Septiningsih, E.M. Development of Improved Ciherang-Sub1 Having Tolerance to Anaerobic Germination Conditions. Plant Breed. Biotechnol. 2015, 3, 77–87. [Google Scholar] [CrossRef][Green Version]
- Ismail, A.; Singh, U.; Singh, S.; Dar, M.; Mackill, D. The contribution of submergence-tolerant (Sub1) rice varieties to food security in flood-prone rainfed lowland areas in Asia. Field Crops Res. 2013, 152, 83–93. [Google Scholar] [CrossRef]
- Yu, S.M.; Lee, H.T.; Lo, S.F.; Ho, T.D. How does rice cope with too little oxygen during its early life? New Phytol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Vijayan, J.; Sarkar, R.K. Germination Stage Oxygen Deficiency (GSOD): An Emerging Stress in the Era of Changing Trends in Climate and Rice Cultivation Practice. Front. Plant Sci. 2016, 7, 671. [Google Scholar] [CrossRef] [PubMed]
- Mahender, A.; Anandan, A.; Pradhan, S.K. Early seedling vigour, an imperative trait for direct-seeded rice: An overview on physio-morphological parameters and molecular markers. Planta 2015, 241, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef]
- Mickelbart, M.V.; Hasegawa, P.M.; Bailey-Serres, J. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat. Rev. Genet. 2015, 16, 237–251. [Google Scholar] [CrossRef]

| Gene | Subfamily | Chromosome | Locus Name | CDS Coordinates (5′-3′) | Regulatory Function |
|---|---|---|---|---|---|
| RAmy1 A | RAmy1 | 2 | LOC_Os02g52700 | 32243146-32245056 | High temperature in developing seeds [58] |
| RAmy1 B | 2 | LOC_Os02g52710 | 32250180-32248279 | Chemical inhibition [59] | |
| RAmy1 C | 1 | LOC_Os01g25510 | 14459951-14461849 | High temperature in developing seeds [58] | |
| RAmy2 A | RAmy2 | 6 | LOC_Os06g49970 | 30262778-30266915 | Unknown |
| RAmy3 A | RAmy3 | 9 | LOC_Os09g28400 | 17288993-17291295 | High temperature in developing seeds [58] |
| RAmy3 B | 9 | LOC_Os09g28420 | 17296166-17305076 | Chemical inhibition [59] | |
| RAmy3 C | 8 | LOC_Os08g36900 | 23335165-23337151 | Unknown | |
| RAmy3 D | 8 | LOC_Os08g36910 | 23340676-23343533 | High temperature in developing seeds [58] Sugar starvation [53,60] Calcium signaling [21] | |
| RAmy3 E | 4 | LOC_Os04g33040 | 20006128-2000927 | High temperature in developing seeds [58] Chemical inhibition [59] | |
| RAmy3 F | 1 | LOC_Os01g51754 | 29760719-29770037 | Unknown |
| QTLs/Candidate Genes | Chromosome | Traits | Description & Reference |
|---|---|---|---|
| qAG-1-2 | 1 | survival rate | [87] |
| qAG-3-1 | 3 | ||
| qAG-7-2 | 7 | ||
| qAG-9-1, qAG-9-2 | 9 | ||
| qAG7.1 | 7 | survival rate | [89] |
| qAG7 | 7 | survival rate | [90] |
| qAG11 | 11 | ||
| qAG2.1 | 2 | ||
| qAG1a, qAG1b | 1 | survival rate | [91] |
| qAG8 | 8 | ||
| qAG7.1, qAG7.2, qAG7.3 | 7 | survival rate | [88] |
| qAG3 | 3 | ||
| qSUR6–1 | 6 | survival rateseedling height | [92] |
| qSH1–1 | 1 | ||
| OsTPP7 | 9 | coleoptile length | Enhancing germination and coleoptile elongation [18] |
| HXK6 | 1 | coleoptile length | Encoded a hexokinase [93] |
| LOC_Os06g03520 | 6 | coleoptile length | DUF domain containing protein [94] |
| TIR1 | 5 | coleoptile length | F-Box auxin receptor protein [95] Control lateral root initiation in rice [95] Codes for an E3 ubiquitin ligase complex component [95] The ATP binding cassette transporter [95] |
| AUX1 | 1 | ||
| COI1a | 1 | ||
| ABC1-2 | 2 | ||
| LOC_Os03g31550 | 3 | survival rate and coleoptile | encodes for enzyme xanthine dehydrogenase 1(OsXDH1) [96] encodes for SSXT family protein [96] |
| LOC_Os12g31350 | 12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, M.; Cen, W.; Li, R.; Wang, S.; Luo, J. The Molecular Regulatory Pathways and Metabolic Adaptation in the Seed Germination and Early Seedling Growth of Rice in Response to Low O2 Stress. Plants 2020, 9, 1363. https://doi.org/10.3390/plants9101363
Ma M, Cen W, Li R, Wang S, Luo J. The Molecular Regulatory Pathways and Metabolic Adaptation in the Seed Germination and Early Seedling Growth of Rice in Response to Low O2 Stress. Plants. 2020; 9(10):1363. https://doi.org/10.3390/plants9101363
Chicago/Turabian StyleMa, Mingqing, Weijian Cen, Rongbai Li, Shaokui Wang, and Jijing Luo. 2020. "The Molecular Regulatory Pathways and Metabolic Adaptation in the Seed Germination and Early Seedling Growth of Rice in Response to Low O2 Stress" Plants 9, no. 10: 1363. https://doi.org/10.3390/plants9101363
APA StyleMa, M., Cen, W., Li, R., Wang, S., & Luo, J. (2020). The Molecular Regulatory Pathways and Metabolic Adaptation in the Seed Germination and Early Seedling Growth of Rice in Response to Low O2 Stress. Plants, 9(10), 1363. https://doi.org/10.3390/plants9101363