Spatial Differentiation of Physical and Chemical Soil Parameters under Integrated, Organic, and Biodynamic Viticulture
Abstract
1. Introduction
2. Results
Spatial Differentiation of Management Effects
3. Discussion
4. Materials and Methods
4.1. Experimental Site
4.2. Soil Sampling
- in the under-vine area which has been kept free of vegetation by herbicide or mechanical means
- in the tractor track of the cover cropped inter-row (which was also the draining zone of the canopy)
- in the middle of the cover cropped inter-row between the two main tractor tracks.
4.3. Soil Physical Analysis
4.4. Chemical Parameter Analysis
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; de Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Parr, J.F.; Papendick, R.I.; Hornick, S.B.; Meyer, R.E. Soil quality: Attributes and relationship to alternative and sustainable agriculture. Am. J. Altern. Agric. 1992, 7, 5–11. [Google Scholar] [CrossRef]
- European Commission. Towards a Thematic Strategy for Soil Protection; European Commission: Brussels, Belgium, 2002. [Google Scholar]
- Miguéns, T.; Leirós, M.A.C.; Gil-Sotres, F.; Trasar-Cepeda, C. Biochemical properties of vineyard soils in Galicia, Spain. Sci. Total Environ. 2007, 378, 218–222. [Google Scholar] [CrossRef]
- Ballabio, C.; Panagos, P.; Lugato, E.; Huang, J.-H.; Orgiazzi, A.; Jones, A.; Fernández-Ugalde, O.; Borrelli, P.; Montanarella, L. Copper distribution in European topsoils: An assessment based on LUCAS soil survey. Sci. Total Environ. 2018, 636, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Riches, D.; Porter, I.J.; Oliver, D.P.; Bramley, R.G.V.; Rawnsley, B.; Edwards, J.; White, R.E. Review: Soil biological properties as indicators of soil quality in Australian viticulture. Aust. J. Grape Wine Res. 2013, 19, 311–323. [Google Scholar] [CrossRef]
- Oliver, D.P.; Bramley, R.G.V.; Riches, D.; Porter, I.; Edwards, J. Review: Soil physical and chemical properties as indicators of soil quality in Australian viticulture. Aust. J. Grape Wine Res. 2013, 19, 129–139. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science. Principles and Applications, 3rd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2008; ISBN 978-0-12-373646-8. [Google Scholar]
- Polge de Combret-Champart, L.; Guilpart, N.; Mérot, A.; Capillon, A.; Gary, C. Determinants of the degradation of soil structure in vineyards with a view to conversion to organic farming. Soil Use Manag. 2013, 29, 557–566. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Directive E 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides; 2009/128/EC, 2009; European Parliament and Council of the European Union: Brussels, Belgium, 2009. [Google Scholar]
- The World of Organic Agriculture. Statistics & Emerging Trends 2020; Research Institute of Organic Agriculture (FiBL): Frick, Switzerland; IFOAM—Organics International: Bonn, Germany, 2020; ISBN 978-3-03736-159-7. [Google Scholar]
- Council of the European Union. Council Regulation (EC) No 834/2007 of 28 June 2007 on Organic Production and Labelling of Organic Products and Repealing Regulation (EEC) No 2092/91; 834/2007/EC, 2007; Council of the European Union: Brussels, Belgium, 2007. [Google Scholar]
- European Commission. Commission Regulation (EC) No 889/2008 of 5 September 2008 Laying Down Detailed Rules for the Implementation of Council Regulation (EC) No 834/2007 on Organic Production and Labelling of Organic Products with Regard to Organic Production, Labelling and Control; 889/2008/EC, 2008; European Commission: Brussels, Belgium, 2008. [Google Scholar]
- Masson, P.; Masson, V. Landwirtschaft, Garten- und Weinbau Biodynamisch; AT-Verl.: Aarau, Switzerland; München, Germany, 2013; ISBN 9783038007128. [Google Scholar]
- Hendgen, M.; Hoppe, B.; Döring, J.; Friedel, M.; Kauer, R.; Frisch, M.; Dahl, A.; Kellner, H. Effects of different management regimes on microbial biodiversity in vineyard soils. Sci. Rep. 2018, 8, 9393. [Google Scholar] [CrossRef]
- Di Giacinto, S.; Friedel, M.; Poll, C.; Döring, J.; Kunz, R.; Kauer, R. Vineyard management system affects soil microbiological properties. OENO ONE 2020, 54. [Google Scholar] [CrossRef]
- Longa, C.M.O.; Nicola, L.; Antonielli, L.; Mescalchin, E.; Zanzotti, R.; Turco, E.; Pertot, I. Soil microbiota respond to green manure in organic vineyards. J. Appl. Microbiol. 2017, 123, 1547–1560. [Google Scholar] [CrossRef]
- Probst, B.; Schüler, C.; Joergensen, R.G. Vineyard soils under organic and conventional management—Microbial biomass and activity indices and their relation to soil chemical properties. Biol. Fertil. Soils 2008, 44, 443–450. [Google Scholar] [CrossRef]
- Parat, C.; Chaussod, R.; Lévêque, J.; Dousset, S.; Andreux, F. The relationship between copper accumulated in vineyard calcareous soils and soil organic matter and iron. Eur. J. Soil Sci. 2002, 53, 663–670. [Google Scholar] [CrossRef]
- Komárek, M.; Száková, J.; Rohošková, M.; Javorská, H.; Chrastný, V.; Balík, J. Copper contamination of vineyard soils from small wine producers: A case study from the Czech Republic. Geoderma 2008, 147, 16–22. [Google Scholar] [CrossRef]
- Rooney, C.P.; Zhao, F.-J.; Mcgrath, S.P. Soil factors controlling the expression of copper toxicity to plants in a wide range of European soils. Environ. Toxicol. Chem. 2006, 25, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Lock, K.; Janssen, C.R. Influence of aging on copper bioavailability in soils. Environ. Toxicol. Chem. 2003, 22, 1162–1166. [Google Scholar] [CrossRef]
- Chaignon, V.; Sanchez-Neira, I.; Herrmann, P.; Jaillard, B.; Hinsinger, P. Copper bioavailability and extractability as related to chemical properties of contaminated soils from a vine-growing area. Environ. Pollut. 2003, 123, 229–238. [Google Scholar] [CrossRef]
- Council of the European Union. Council Directive 86/278/EEC of 12 June 1986 on the Protection of the Environment, and in Particular of the Soil, When Sewage Sludge is Used in Agriculture; 86/278/EEC, 1986; Council of the European Union: Brussels, Belgium, 1986. [Google Scholar]
- Giller, K.E.; Witter, E.; Mcgrath, S.P. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biol. Biochem. 1998, 30, 1389–1414. [Google Scholar] [CrossRef]
- Brun, L.A.; Maillet, J.; Hinsinger, P.; Pépin, M. Evaluation of copper availability to plants in copper-contaminated vineyard soils. Environ. Pollut. 2001, 111, 293–302. [Google Scholar] [CrossRef]
- Federal Office of Consumer Protection and Food Safety. Registration Report—Cuprozin Progress. Art. 51 Extension of Authorisation for Minor Uses. 2020. Available online: https://www.bvl.bund.de/SharedDocs/Downloads/04_Pflanzenschutzmittel/01_zulassungsberichte/006895-00-23.pdf?__blob=publicationFile&v=2 (accessed on 2 September 2020).
- Federal Office of Consumer Protection and Food Safety. Registration Report—Copper Hydroxide. SPU-02720-F. 2011. Available online: https://www.bvl.bund.de/SharedDocs/Downloads/04_Pflanzenschutzmittel/01_zulassungsberichte/006896-00-00.pdf?__blob=publicationFile&v=4 (accessed on 2 September 2020).
- Coll, P.; Le Cadre, E.; Blanchart, E.; Hinsinger, P.; Villenave, C. Organic viticulture and soil quality: A long-term study in Southern France. Appl. Soil Ecol. 2011, 50, 37–44. [Google Scholar] [CrossRef]
- Strumpf, T.; Steindl, A.; Strassemeyer, J.; Riepert, F. Erhebung von Kupfergesamtgehalten in ökologisch und konventionell bewirtschafteten Böden.: Teil 1: Gesamtgehalte in Weinbergsböden deutscher Qualitätsanbaugebiete. J. Kult. 2011, 63, 131–143. [Google Scholar] [CrossRef]
- Radić, T.; Likar, M.; Hančević, K.; Bogdanović, I.; Pasković, I. Occurrence of root endophytic fungi in organic versus conventional vineyards on the Croatian coast. Agric. Ecosyst. Environ. 2014, 192, 115–121. [Google Scholar] [CrossRef]
- Strumpf, T.; Stendel, U.; Vetter, C. Gesamtgehalte von Kupfer in Böden des Kernobstanbaus, Weinbergen und Hopfenanlagen. J. Kult. 2009, 61, 117–125. [Google Scholar] [CrossRef]
- Beni, C.; Rossi, G. Conventional and organic farming: Estimation of some effects on copper, soil accumulation and wine in Central Italy vineyard. Agrochimica 2009, 53, 145–159. [Google Scholar]
- Soane, B.D.; van Ouwerkerk, C. Implications of soil compaction in crop production for the quality of the environment. Soil Tillage Res. 1995, 35, 5–22. [Google Scholar] [CrossRef]
- Ferrero, A.; Usowicz, B.; Lipiec, J. Effects of tractor traffic on spatial variability of soil strength and water content in grass covered and cultivated sloping vineyard. Soil Tillage Res. 2005, 84, 127–138. [Google Scholar] [CrossRef]
- Wheeler, S.A. The Adoption and Diffusion of Organic Agriculture: Economics, Drivers and Constraints. Ph.D. Thesis, University of South Australia, Adelaide, Australia, 2006. [Google Scholar]
- Collins, C.; Penfold, C.; Johnston, L.; Bastian, S.; Marschner, P. The relative sustainability of organic, biodynamic and conventional viticulture. In Proceedings of the 19th International Meeting of Viticulture GiESCO, Pech Rouge—Montpellier, France, 31 May–2 June 2015. [Google Scholar]
- Gutiérrez-Gamboa, G.; Verdugo-Vásquez, N.; Díaz-Gálvez, I. Influence of type of management and climatic conditions on productive behavior, oenological potential, and soil characteristics of a ‘Cabernet Sauvignon’ vineyard. Agronomy 2019, 9, 64. [Google Scholar] [CrossRef]
- Gehlen, P.; Neu, J.; Schröder, D. Bodenchemische und bodenbiologische Vergleichsuntersuchungenkonventionell und biologisch bewirtschafteter Weinstandorte an der Mosel. Weinwissenschaft 1988, 43, 161–173. [Google Scholar]
- Okur, N.; Altindişli, A.; Çengel, M.; Göçmez, S.; Kayikçioğlu, H.H. Microbial biomass and enzyme activity in vineyard soils under organic and conventional farming systems. Turk. J. Agric. For. 2009, 33, 413–423. [Google Scholar]
- Freitas, N.d.O.; Yano-Melo, A.M.; Silva, F.S.B.d.; de Melo, N.F.; Maia, L.C. Soil biochemistry and microbial activity in vineyards under conventional and organic management at Northeast Brazil. Sci. Agric. 2011, 68, 223–229. [Google Scholar] [CrossRef]
- Van Dijck, S.J.E.; van Asch, T.W.J. Compaction of loamy soils due to tractor traffic in vineyards and orchards and its effect on infiltration in southern France. Soil Tillage Res. 2002, 63, 141–153. [Google Scholar] [CrossRef]
- Barik, K.; Aksakal, E.L.; Islam, K.R.; Sari, S.; Angin, I. Spatial variability in soil compaction properties associated with field traffic operations. CATENA 2014, 120, 122–133. [Google Scholar] [CrossRef]
- Hartwig, N.L.; Ammon, H.U. Cover crops and living mulches. Weed Sci. 2002, 50, 688–699. [Google Scholar] [CrossRef]
- Danso, S.K.A.; Curbelo, S.; Labandera, C.; Pastorini, D. Herbage yield and nitrogen-fixation in a triple-species mixed sward of white clover, lotus and fescue. Soil Biol. Biochem. 1991, 23, 65–70. [Google Scholar] [CrossRef]
- Kutschera, L. Wurzelatlas Mitteleuropäischer Ackerunkräuter und Kulturpflanzen. 1. Band der Wurzelatlas-Reihe; DLG-Verlag: Frankfurt, Germany, 1960. [Google Scholar]
- Ampleman, M.D.; Crawford, K.M.; Fike, D.A. Differential soil organic carbon storage at forb- and grass-dominated plant communities, 33 years after tallgrass prairie restoration. Plant Soil 2014, 374, 899–913. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Carbon and nitrogen storage by deep-rooted tall fescue (Lolium arundinaceum) in the surface and subsurface soil of a fine sandy loam in eastern Canada. Agric. Ecosyst. Environ. 2010, 136, 125–132. [Google Scholar] [CrossRef]
- Kay, B.D. Soil structure and organic carbon. A review. In Soil Processes and the Carbon Cycle; Lal, R., Kimble, J.M., Follett, R.F., Steward, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 1998; pp. 169–197. [Google Scholar]
- Reeve, J.R.; Carpenter-Boggs, L.; Reganold, J.P.; York, A.L.; Brinton, W.F. Influence of biodynamic preparations on compost development and resultant compost extracts on wheat seedling growth. Bioresour. Technol. 2010, 101, 5658–5666. [Google Scholar] [CrossRef]
- Scheffer, F.; Schachtschabel, P.; Blume, H.-P.; Brümmer, G.W.; Horn, R.; Kandeler, E.; Kögel-Knabner, I.; Kretzschmar, R.; Stahr, K.; Thiele-Bruhn, S.; et al. Lehrbuch der Bodenkunde, 16. Auflage; Spektrum Akademischer Verlag: Heidelberg, Germany, 2010; ISBN 9783827414441. [Google Scholar]
- VandenBygaart, A.J.; Gregorich, E.G.; Angers, D.A. Influence of agricultural management on soil organic carbon: A compendium and assessment of Canadian studies. Can. J. Soil Sci. 2003, 83, 363–380. [Google Scholar] [CrossRef]
- Kern, J.S.; Johnson, M.G. Conservation tillage impacts on national soil and atmospheric carbon levels. Soil Sci. Soc. Am. J. 1993, 57, 200–210. [Google Scholar] [CrossRef]
- Golchin, A.; Oades, J.M.; Skjemstad, J.O.; Clarke, P. Soil structure and carbon cycling. Soil Res. 1994, 32, 1043. [Google Scholar] [CrossRef]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Fliessbach, A.; Oberholzer, H.-R.; Widmer, F. Ranking the magnitude of crop and farming system effects on soil microbial biomass and genetic structure of bacterial communities. FEMS Microbiol. Ecol. 2006, 57, 378–388. [Google Scholar] [CrossRef]
- VandenBygaart, A.J.; Angers, D.A. Towards accurate measurements of soil organic carbon stock change in agroecosystems. Can. J. Soil Sci. 2006, 86, 465–471. [Google Scholar] [CrossRef]
- Döring, J.; Frisch, M.; Tittmann, S.; Stoll, M.; Kauer, R. Growth, yield and fruit quality of grapevines under organic and biodynamic management. PLoS ONE 2015, 10, e0138445. [Google Scholar] [CrossRef]
- Brun, L.A.; Maillet, J.; Richarte, J.; Herrmann, P.; Remy, J.C. Relationships between extractable copper, soil properties and copper uptake by wild plants in vineyard soils. Environ. Pollut. 1998, 102, 151–161. [Google Scholar] [CrossRef]
- Baker, D.E. Copper. In Heavy Metals in Soils; Alloway, B.J., Ed.; Blackie and Son Ltd.: Glasgow, UK, 1990; pp. 151–175. ISBN 9401045860. [Google Scholar]
- Federal Soil Protection and Contaminated Sites Ordinance. BBodSchV, 2017; Federal Ministry of Justice and Consumer Protection: Berlin, Germany, 2017.
- Dell’Amico, E.; Mazzocchi, M.; Cavalca, L.; Allievi, L.; Andreoni, V. Assessment of bacterial community structure in a long-term copper-polluted ex-vineyard soil. Microbiol. Res. 2008, 163, 671–683. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Soler-Rovira, P.; Polo, A.; Díaz-Raviña, M.; Arias-Estévez, M.; Plaza, C. Enzyme activities in vineyard soils long-term treated with copper-based fungicides. Soil Biol. Biochem. 2010, 42, 2119–2127. [Google Scholar] [CrossRef]
- Arias, M.; Lopez, E.; Fernandez, D.; Soto, B. Copper distribution and dynamics in acid vineyard soils treated with copper-based fungicides. Soil Sci. 2004, 169, 796–805. [Google Scholar] [CrossRef]
- McLaren, R.G.; Williams, J.G.; Swift, R.S. Some observations on the desorption and distribution behaviour of copper with soil components. J. Soil Sci. 1983, 34, 325–331. [Google Scholar] [CrossRef]
- Lagomarsino, A.; Marabottini, R.; Grego, S.; Stazi, S.R. Copper distribution among physical and chemical fractions in a former vineyard soil. Agrochimica 2010, 54, 1–12. [Google Scholar]
- Sims, J.T. Soil pH effects on the distribution and plant availability of manganese, copper, and zinc. Soil Sci. Soc. Am. J. 1986, 50, 367–373. [Google Scholar] [CrossRef]
- Ristić, M.; Bokić, T.; Zečević, T. Copper accumulation and availability in vineyard soils of serbia. Work. Living Environ. Prot. 2006, 3, 35–42. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2016. [Google Scholar]
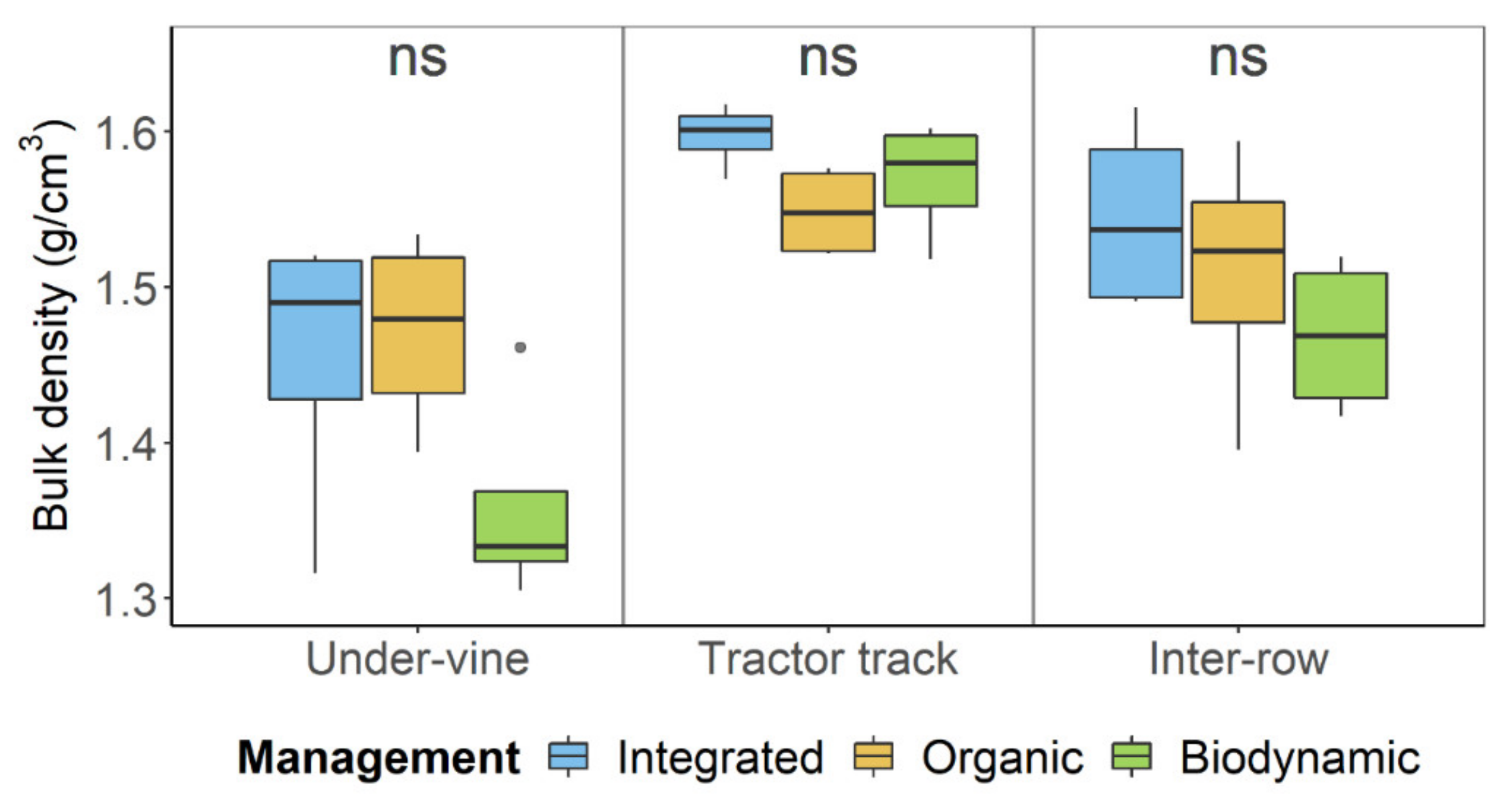
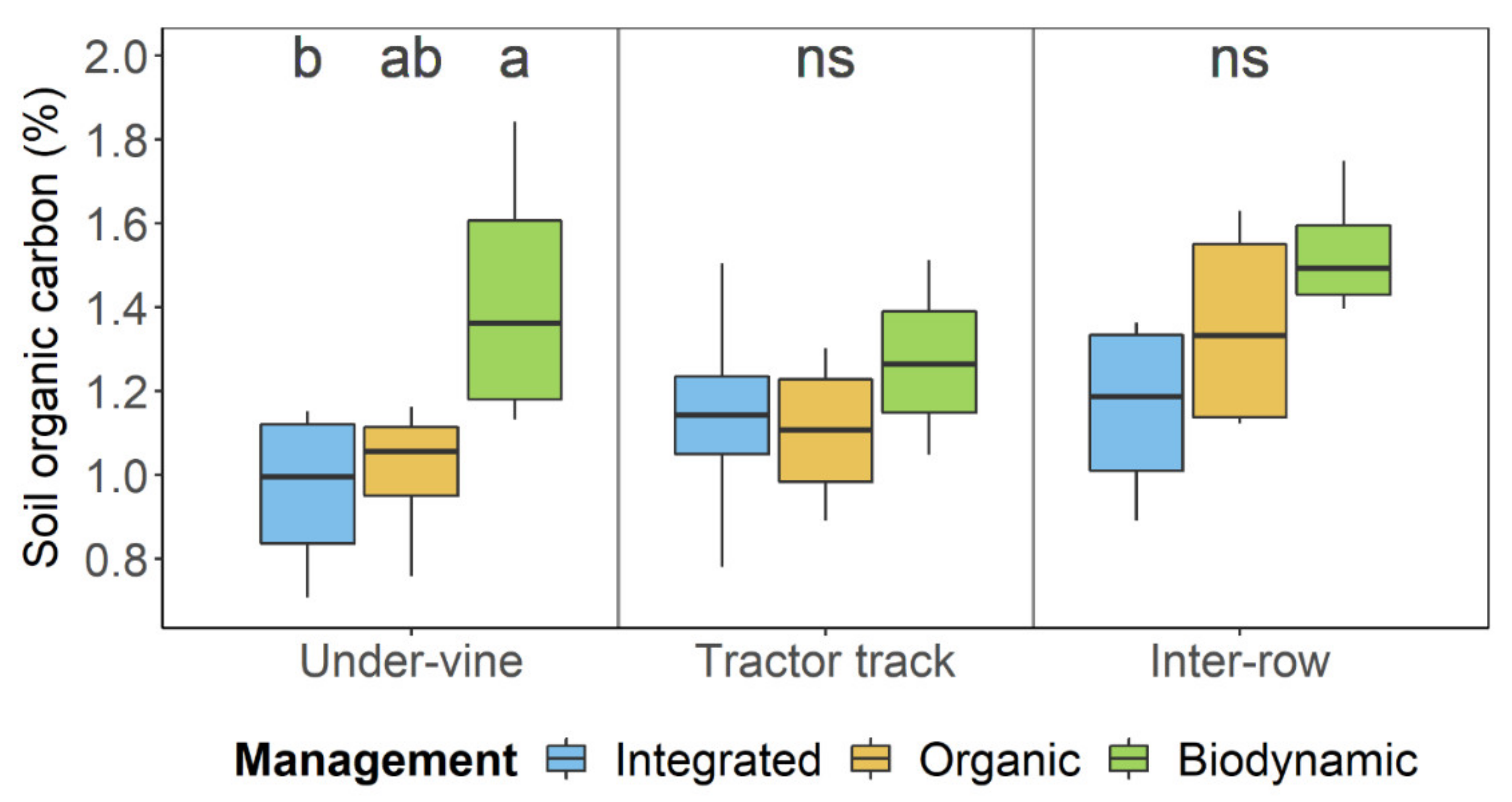
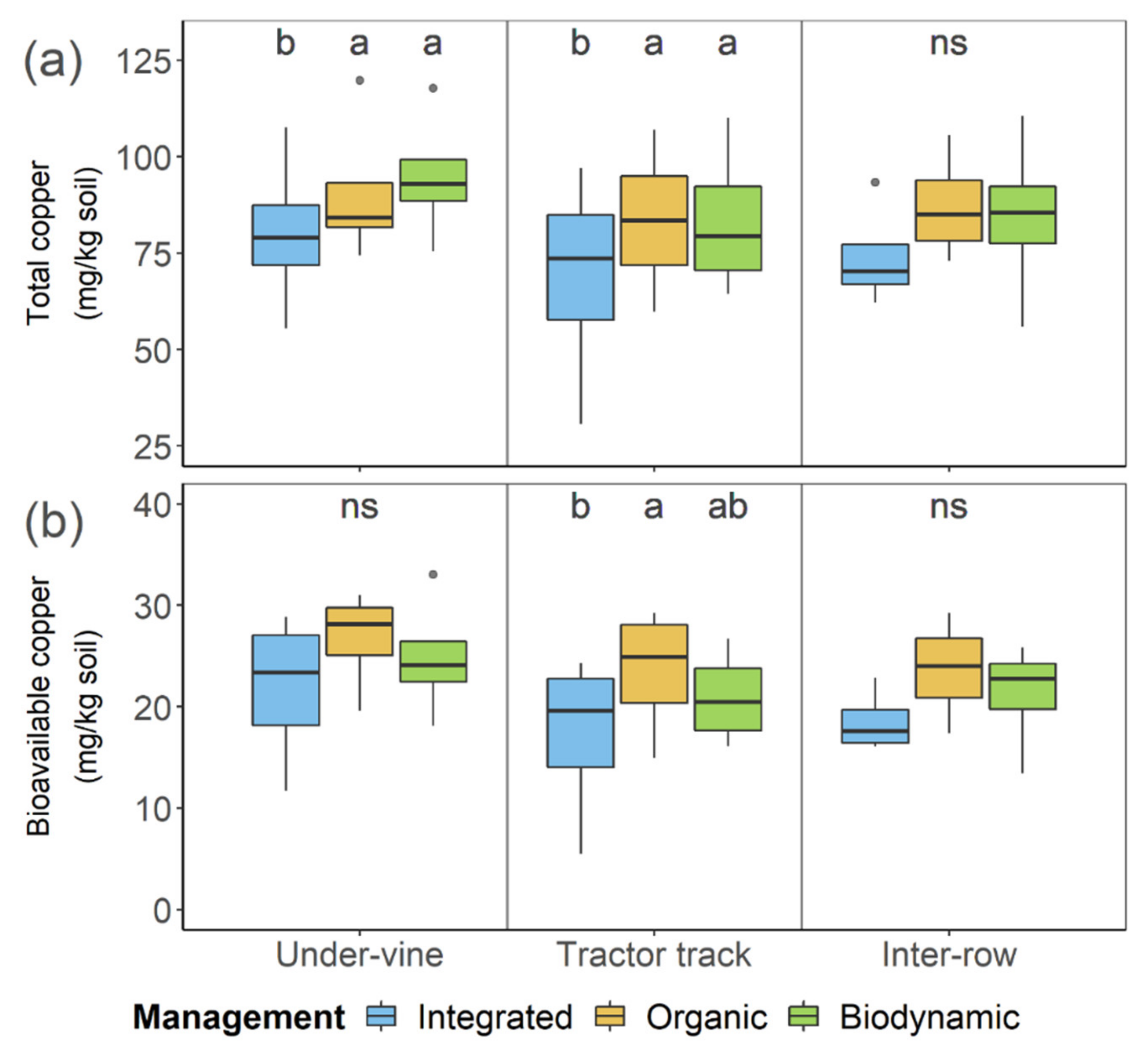

| pmanagement | Integrated | Organic | Biodynamic | pposition | Under-Vine | Tractor Track | Inter-Row | pmanagement:position | |
|---|---|---|---|---|---|---|---|---|---|
| Bulk density (g cm−3) | 0.042 * | 1.53 ± 0.09 a | 1.51 ± 0.07 ab | 1.47 ± 0.1 b | 2.40 × 10−5 * | 1.43 ± 0.09 c | 1.57 ± 0.03 a | 1.51 ± 0.07 b | 0.324 |
| AWC (%) | 0.179 | 15 ± 1.25 | 14.9 ± 1.68 | 15.9 ± 1.99 | 8.88 × 10−3 * | 14.8 ± 1.4 b | 14.6 ± 1.6 b | 16.4 ± 1.6 a | 0.107 |
| pH | 0.257 | 7.13 ± 0.32 | 7.23 ± 0.13 | 7.15 ± 0.08 | 0.32 | 7.19 ± 0.22 | 7.21 ± 0.19 | 7.12 ± 0.21 | 0.992 |
| N (%) | 0.6 | 0.11 ± 0.02 | 0.12 ± 0.05 | 0.12 ± 0.05 | 0.357 | 0.11 ± 0.05 | 0.11 ± 0.02 | 0.13 ± 0.02 | 0.867 |
| SOC (%) | 5.23 × 10−3 * | 1.09 ± 0.24 b | 1.15 ± 0.24 b | 1.41 ± 0.24 a | 0.071 | 1.13 ± 0.31 | 1.17 ± 0.22 | 1.35 ± 0.25 | 0.564 |
| C/N | 0.073 | 10.15 ± 1.36 | 10.2 ± 2.39 | 12.31 ± 2.99 | 0.853 | 10.76 ± 3.57 | 11.22 ± 1.98 | 10.68 ± 1.73 | 0.943 |
| Total Cu (mg kg−1 soil) | 1.52 × 10−4 * | 74.3 ± 20.4 b | 87 ± 16.9 a | 87.4 ± 18.9 a | 7.01 × 10−3 * | 88.5 ± 18.9 a | 78.5 ± 22.1 b | 81.1 ± 16.6 b | 0.879 |
| Bioavailable Cu (mg kg−1 soil) | 1.33 × 10−3 * | 19.2 ± 6.5 b | 24.6 ± 5.3 a | 22.3 ± 5.3 a | 1.28 × 10−2 * | 24.5 ± 6.1 a | 20.6 ± 6.6 b | 21.1 ± 4.7 b | 0.992 |
| Integrated | Organic | Biodynamic | |
|---|---|---|---|
| cover crop | sward (every 2nd row cultivated) | multi-species mixture (every 2nd row cultivated) | |
| under-vine-management | herbicides | mechanically | |
| fertilization | green waste compost + mineral fertilizers (according to Nmin analysis) | farmyard manure + rolling or cultivation of cover crop | farmyard manure with biodynamic preparations (or cow pat pit preparation) + rolling or cultivation of cover crop |
| plant protection | systemic fungicides botryticides | copper (max. 3 kg/ha and year), wettable sulfur plant resistance improvers | |
| mating disruption method against grape berry moth | |||
| biodynamic preparations | - | - | horn manure, horn silica compost preparations |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendgen, M.; Döring, J.; Stöhrer, V.; Schulze, F.; Lehnart, R.; Kauer, R. Spatial Differentiation of Physical and Chemical Soil Parameters under Integrated, Organic, and Biodynamic Viticulture. Plants 2020, 9, 1361. https://doi.org/10.3390/plants9101361
Hendgen M, Döring J, Stöhrer V, Schulze F, Lehnart R, Kauer R. Spatial Differentiation of Physical and Chemical Soil Parameters under Integrated, Organic, and Biodynamic Viticulture. Plants. 2020; 9(10):1361. https://doi.org/10.3390/plants9101361
Chicago/Turabian StyleHendgen, Maximilian, Johanna Döring, Verena Stöhrer, Fabian Schulze, Ruth Lehnart, and Randolf Kauer. 2020. "Spatial Differentiation of Physical and Chemical Soil Parameters under Integrated, Organic, and Biodynamic Viticulture" Plants 9, no. 10: 1361. https://doi.org/10.3390/plants9101361
APA StyleHendgen, M., Döring, J., Stöhrer, V., Schulze, F., Lehnart, R., & Kauer, R. (2020). Spatial Differentiation of Physical and Chemical Soil Parameters under Integrated, Organic, and Biodynamic Viticulture. Plants, 9(10), 1361. https://doi.org/10.3390/plants9101361





