Improving Horticultural Crops via CRISPR/Cas9: Current Successes and Prospects
Abstract
1. Introduction
1.1. Reaching the CRISPR Age
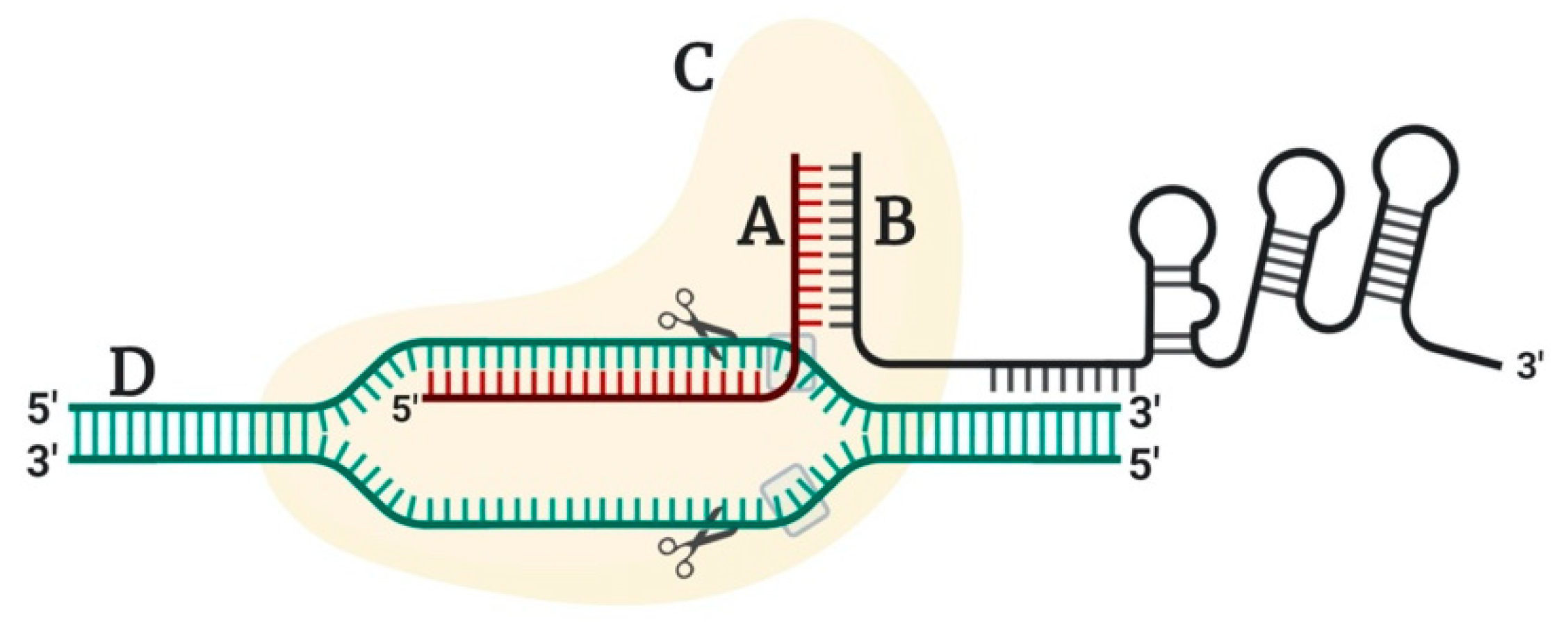
1.2. CRISPR/Cas9, Novel Variants and Challenges
1.3. Applications of CRISPR/Cas9
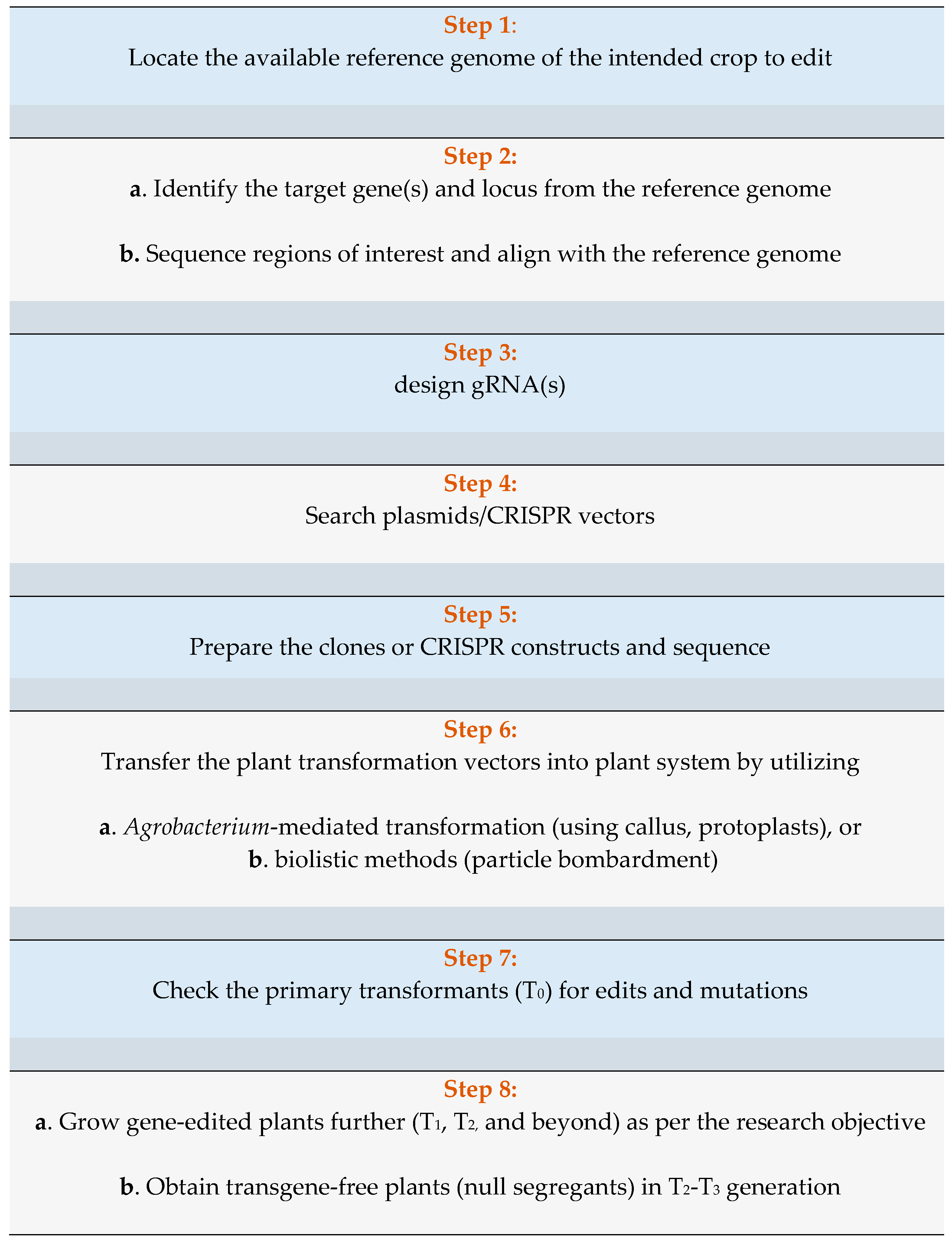
1.4. Existing Resources for CRISPR/Cas9
1.5. The Regulatory Status of Gene-Edited Crops
1.6. Way Forward
Author Contributions
Funding
Conflicts of Interest
References
- United Nations (UN). Probabilistic Population Projections based on the World Population Prospects 2019; United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2019. [Google Scholar]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant–Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef] [PubMed]
- Schaart, J.G.; Van De Wiel, C.C.; Lotz, L.A.; Smulders, M.J. Opportunities for Products of New Plant Breeding Techniques. Trends Plant. Sci. 2016, 21, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.; Al-Sadi, A.M.; Pour-Aboughadareh, A.; Burritt, D.J.; Tran, L.-S.P. Genome editing using CRISPR/Cas9–targeted mutagenesis: An opportunity for yield improvements of crop plants grown under environmental stresses. Plant. Physiol. Biochem. 2018, 131, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Voytas, D.F.; Gao, C. Precision Genome Engineering and Agriculture: Opportunities and Regulatory Challenges. PLoS Biol. 2014, 12, e1001877. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Gurumurthy, C.B.; Grati, M.H.; Ohtsuka, M.; Schilit, S.L.; Quadros, R.M.; Liu, X.Z. CRISPR: A versatile tool for both forward and reverse genetics research. Hum. Genet. 2016, 135, 971–976. [Google Scholar] [CrossRef]
- Xu, X.; Tay, Y.; Sim, B.; Yoon, S.-I.; Huang, Y.; Ooi, J.; Utami, K.H.; Ziaei, A.; Ng, B.; Radulescu, C.; et al. Reversal of Phenotypic Abnormalities by CRISPR/Cas9-Mediated Gene Correction in Huntington Disease Patient-Derived Induced Pluripotent Stem Cells. Stem Cell Rep. 2017, 8, 619–633. [Google Scholar] [CrossRef]
- Li, W.; Cho, M.Y.; Lee, S.; Jang, M.; Park, J.; Park, R. CRISPR-Cas9 mediated CD133 knockout inhibits colon cancer invasion through reduced epithelial-mesenchymal transition. PLoS ONE 2019, 14, e0220860. [Google Scholar] [CrossRef]
- Ye, L.; Wang, J.; Tan, Y.; Beyer, A.I.; Xie, F.; Muench, M.O.; Kan, Y.W. Genome editing using CRISPR-Cas9 to create the HPFH genotype in HSPCs: An approach for treating sickle cell disease and β-thalassemia. Proc. Natl. Acad. Sci. USA 2016, 113, 10661–10665. [Google Scholar] [CrossRef]
- Wang, X.; Niu, Y.; Zhou, J.; Zhu, H.; Ma, B.; Yu, H.; Yan, H.; Hua, J.; Huang, X.; Qu, L.; et al. CRISPR/Cas9-mediatedMSTNdisruption and heritable mutagenesis in goats causes increased body mass. Anim. Genet. 2018, 49, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Koslová, A.; Kučerová, D.; Reinišová, M.; Geryk, J.; Trefil, P.; Hejnar, J. Genetic Resistance to Avian Leukosis Viruses Induced by CRISPR/Cas9 Editing of Specific Receptor Genes in Chicken Cells. Viruses 2018, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.-L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, J.; Brumin, M.; Wolf, D.; Leibman, D.; Klap, C.; Pearlsman, M.; Sherman, A.; Arazi, T.; Gal-On, A. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant. Pathol. 2016, 17, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Malnoy, M.; Viola, R.; Jung, M.-H.; Koo, O.-J.; Kim, S.; Kim, J.-S.; Velasco, R.; Kanchiswamy, C.N. DNA-Free Genetically Edited Grapevine and Apple Protoplast Using CRISPR/Cas9 Ribonucleoproteins. Front. Plant. Sci. 2016, 7, 1904. [Google Scholar] [CrossRef]
- Wang, F.; Wang, C.; Liu, P.; Lei, C.; Hao, W.; Gao, Y.; Liu, Y.-G.; Zhao, K. Enhanced Rice Blast Resistance by CRISPR/Cas9-Targeted Mutagenesis of the ERF Transcription Factor Gene OsERF922. PLoS ONE 2016, 11, e0154027. [Google Scholar] [CrossRef]
- Peng, A.H.; Chen, S.C.; Lei, T.G.; Xu, L.Z.; He, Y.R.; Wu, L.; Yao, L.X.; Zou, X.P. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility geneCsLOB1promoter in citrus. Plant. Biotechnol. J. 2017, 15, 1509–1519. [Google Scholar] [CrossRef]
- Cyranoski, D.; Ledford, H. Genome-edited baby claim provokes international outcry. Nature 2018, 563, 607–608. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Committee on Human Gene Editing: Scientific, Medical, and Ethical Considerations, the Basic Science of Genome Editing. In Human Genome Editing: Science, Ethics, and Governance; National Academies Press (US): Washington, DC, USA, 2017. [Google Scholar]
- Čermák, T.; Curtin, S.J.; Gil-Humanes, J.; Čegan, R.; Kono, T.J.; Konečná, E.; Belanto, J.J.; Starker, C.G.; Mathre, J.W.; Greenstein, R.L.; et al. A Multipurpose Toolkit to Enable Advanced Genome Engineering in Plants. Plant. Cell 2017, 29, 1196–1217. [Google Scholar] [CrossRef]
- Baltes, N.J.; Gil-Humanes, J.; Voytas, D.F. Genome engineering and agriculture: Opportunities and challenges. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2017; Volume 149, pp. 1–26. [Google Scholar]
- Scherer, S.; Davis, R.W. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc. Natl. Acad. Sci. USA 1979, 76, 4951–4955. [Google Scholar] [CrossRef]
- Thomas, K.R.; Folger, K.R.; Capecchi, M.R. High frequency targeting of genes to specific sites in the mammalian genome. Cell 1986, 44, 419–428. [Google Scholar] [CrossRef]
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef] [PubMed]
- Mojica, F.J.; Juez, G.; Rodriguez-Valera, F. Transcription at different salinities of Haloferax mediterranei sequences adjacent to partially modified PstI sites. Mol. Microbiol. 1993, 9, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Mojica, F.J.; Ferrer, C.; Juez, G.; Rodriguez-Valera, F. Long stretches of short tandem repeats are present in the largest replicons of the Archaea Haloferax mediterranei and Haloferax volcanii and could be involved in replicon partitioning. Mol. Microbiol. 1995, 17, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.; Van Embden, J.D.A.; Gaastra, W.; Schouls, L.M. Identification of genes that are associated with DNA repeats in prokaryotes. Mol. Microbiol. 2002, 43, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Pourcel, C.; Salvignol, G.; Vergnaud, G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology 2005, 151, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR Provides Acquired Resistance Against Viruses in Prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Brouns, S.J.J.; Jore, M.M.; Lundgren, M.; Westra, E.R.; Slijkhuis, R.J.H.; Snijders, A.P.L.; Dickman, M.J.; Makarova, K.S.; Koonin, E.V.; Van Der Oost, J. Small CRISPR RNAs Guide Antiviral Defense in Prokaryotes. Science 2008, 321, 960–964. [Google Scholar] [CrossRef]
- Garneau, J.E.; Dupuis, M.-È.; Villion, M.; Romero, D.A.; Barrangou, R.; Boyaval, P.; Fremaux, C.; Horvath, P.; Magadán, A.H.; Moineau, S. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 2010, 468, 67–71. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Jinek, M.; East, A.; Cheng, A.; Lin, S.; Ma, E.; Doudna, J. RNA-programmed genome editing in human cells. eLife 2013, 2, e00471. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, K.; Chaparro-Garcia, A.; Kamoun, S.; Nekrasov, V. Plant genome editing made easy: Targeted mutagenesis in model and crop plants using the CRISPR/Cas system. Plant. Methods 2013, 9, 39. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, Y.; Li, J.; Gao, C. Genome editing in rice and wheat using the CRISPR/Cas system. Nat. Protoc. 2014, 9, 2395–2410. [Google Scholar] [CrossRef] [PubMed]
- Brooks, C.; Nekrasov, V.; Lippman, Z.B.; Van Eck, J. Efficient Gene Editing in Tomato in the First Generation Using the Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-Associated9 System. Plant. Physiol. 2014, 166, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Doudna, J.A. Applications of CRISPR technologies in research and beyond. Nat. Biotechnol. 2016, 34, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, D.; Ramasamy, K.; Sellamuthu, G.; Jayabalan, S.; Venkataraman, G. CRISPR for Crop Improvement: An Update Review. Front. Plant. Sci. 2018, 9, 985. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hua, K.; Lang, Z. Genome editing for horticultural crop improvement. Hortic. Res. 2019, 6, 113–116. [Google Scholar] [CrossRef]
- Ghogare, R.; Williamson-Benavides, B.; Ramírez-Torres, F.; Dhingra, A. CRISPR-associated nucleases: The Dawn of a new age of efficient crop improvement. Transgenic Res. 2019, 29, 1–35. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Kim, Y.G.; Cha, J.; Chandrasegaran, S. Hybrid restriction enzymes: Zinc finger fusions to Fok I cleavage domain. Proc. Natl. Acad. Sci. USA 1996, 93, 1156. [Google Scholar] [CrossRef]
- Christian, M.; Cermak, T.; Doyle, E.L.; Schmidt, C.; Zhang, F.; Hummel, A.; Bogdanove, A.J.; Voytas, D.F. Targeting DNA Double-Strand Breaks with TAL Effector Nucleases. Genetics 2010, 186, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Smith, J.; Yang, M.; Jones, S.; Djukanovic, V.; Nicholson, M.G.; West, A.; Bidney, D.; Falco, S.C.; Jantz, D.; et al. Heritable targeted mutagenesis in maize using a designed endonuclease. Plant. J. 2010, 61, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.K.; Doyon, Y.; Miller, J.C.; DeKelver, R.C.; Moehle, E.A.; Worden, S.E.; Mitchell, J.C.; Arnold, N.L.; Gopalan, S.; Meng, X.; et al. Precise genome modification in the crop species Zea mays using zinc-finger nucleases. Nature 2009, 459, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Maeder, M.L.; Unger-Wallace, E.; Hoshaw, J.P.; Reyon, D.; Christian, M.; Li, X.; Pierick, C.J.; Dobbs, D.; Peterson, T.; et al. High frequency targeted mutagenesis in Arabidopsis thaliana using zinc finger nucleases. Proc. Natl. Acad. Sci. USA 2010, 107, 12028–12033. [Google Scholar] [CrossRef] [PubMed]
- Curtin, S.J.; Zhang, F.; Sander, J.D.; Haun, W.J.; Starker, C.; Baltes, N.J.; Reyon, D.; Dahlborg, E.J.; Goodwin, M.J.; Coffman, A.P.; et al. Targeted Mutagenesis of Duplicated Genes in Soybean with Zinc-Finger Nucleases. Plant. Physiol. 2011, 156, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, B.; Spalding, M.H.; Weeks, D.P.; Yang, B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat. Biotechnol. 2012, 30, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Zhang, Y.; Chen, K.; Zhang, K.; Gao, C. Creation of fragrant rice by targeted knockout of theOsBADH2gene using TALEN technology. Plant. Biotechnol. J. 2015, 13, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhang, K.; Chen, K.; Gao, C. Targeted Mutagenesis in Zea mays Using TALENs and the CRISPR/Cas System. J. Genet. Genom. 2014, 41, 63–68. [Google Scholar] [CrossRef]
- Lor, V.S.; Starker, C.G.; Voytas, D.F.; Weiss, D.; Olszewski, N.E. Targeted Mutagenesis of the Tomato PROCERA Gene Using Transcription Activator-Like Effector Nucleases. Plant. Physiol. 2014, 166, 1288–1291. [Google Scholar] [CrossRef]
- Joung, J.K.; Sander, J.D. TALENs: A widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 2012, 14, 49–55. [Google Scholar] [CrossRef]
- Mishra, R.; Joshi, R.K.; Zhao, K. Genome Editing in Rice: Recent Advances, Challenges, and Future Implications. Front. Plant. Sci. 2018, 9, 1361. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef] [PubMed]
- Sorek, R.; Lawrence, C.M.; Wiedenheft, B. CRISPR-Mediated Adaptive Immune Systems in Bacteria and Archaea. Annu. Rev. Biochem. 2013, 82, 237–266. [Google Scholar] [CrossRef] [PubMed]
- Datsenko, K.A.; Pougach, K.; Tikhonov, A.; Wanner, B.L.; Severinov, K.; Semenova, E. Molecular memory of prior infections activates the CRISPR/Cas adaptive bacterial immunity system. Nat. Commun. 2012, 3, 945. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Alkhnbashi, O.S.; Costa, F.; Shah, S.A.; Saunders, S.J.; Barrangou, R.; Brouns, S.J.; Charpentier, E.; Haft, D.H.; et al. An updated evolutionary classification of CRISPR–Cas systems. Nat. Rev. Genet. 2015, 13, 722–736. [Google Scholar] [CrossRef]
- Bolotin, A.; Quinquis, B.; Sorokin, A.; Ehrlich, S.D. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology 2005, 151, 2551–2561. [Google Scholar] [CrossRef]
- Xie, K.; Zhang, J.; Yang, Y. Genome-Wide Prediction of Highly Specific Guide RNA Spacers for CRISPR–Cas9-Mediated Genome Editing in Model Plants and Major Crops. Mol. Plant. 2014, 7, 923–926. [Google Scholar] [CrossRef]
- Sternberg, S.H.; Redding, S.; Jinek, M.; Greene, E.C.; Doudna, J.A. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature 2014, 507, 62–67. [Google Scholar] [CrossRef]
- Jinek, M.; Jiang, F.; Taylor, D.W.; Sternberg, S.H.; Kaya, E.; Ma, E.; Anders, C.; Hauer, M.; Zhou, K.; Lin, S.; et al. Structures of Cas9 Endonucleases Reveal RNA-Mediated Conformational Activation. Science 2014, 343, 1247997. [Google Scholar] [CrossRef]
- Belhaj, K.; Chaparro-Garcia, A.; Kamoun, S.; Patron, N.; Nekrasov, V. Editing plant genomes with CRISPR/Cas9. Curr. Opin. Biotechnol. 2015, 32, 76–84. [Google Scholar] [CrossRef]
- Nishimasu, H.; Ran, F.A.; Hsu, P.D.; Konermann, S.; Shehata, S.I.; Dohmae, N.; Ishitani, R.; Zhang, F.; Nureki, O. Crystal Structure of Cas9 in Complex with Guide RNA and Target DNA. Cell 2014, 156, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R. RNA-mediated programmable DNA cleavage. Nat. Biotechnol. 2012, 30, 836–838. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.J.; Qiu, J.-L.; et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef]
- Voytas, D.F. Plant Genome Engineering with Sequence-Specific Nucleases. Annu. Rev. Plant. Biol. 2013, 64, 327–350. [Google Scholar] [CrossRef]
- Knoll, A.; Fauser, F.; Puchta, H. DNA recombination in somatic plant cells: Mechanisms and evolutionary consequences. Chromosome Res. 2014, 22, 191–201. [Google Scholar] [CrossRef]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and Applications of CRISPR-Cas9 for Genome Engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef]
- Svitashev, S.; Schwartz, C.; Lenderts, B.; Young, J.K.; Cigan, A.M. Genome editing in maize directed by CRISPR–Cas9 ribonucleoprotein complexes. Nat. Commun. 2016, 7, 13274. [Google Scholar] [CrossRef]
- Qi, L.; Larson, M.H.; Gilbert, L.A.; Doudna, J.A.; Weissman, J.S.; Arkin, A.P.; Lim, W.A. Repurposing CRISPR as an RNA-Guided Platform for Sequence-Specific Control of Gene Expression. Cell 2013, 152, 1173–1183. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A• T to G• C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; Van Der Oost, J.; Regev, A.; et al. Cpf1 Is a Single RNA-Guided Endonuclease of a Class 2 CRISPR-Cas System. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Arazoe, T.; Yachie, N.; Banno, S.; Kakimoto, M.; Tabata, M.; Mochizuki, M.; Miyabe, A.; Araki, M.; Hara, K.Y.; et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 2016, 353, aaf8729. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Tsai, S.Q.; Prew, M.S.; Nguyen, N.T.; Welch, M.M.; Lopez, J.M.; McCaw, Z.R.; Aryee, M.J.; Joung, J.K. Genome-wide specificities of CRISPR-Cas Cpf1 nucleases in human cells. Nat. Biotechnol. 2016, 34, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, J.; Hur, J.K.; Been, K.W.; Yoon, S.-H.; Kim, J.-S. Genome-wide analysis reveals specificities of Cpf1 endonucleases in human cells. Nat. Biotechnol. 2016, 34, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Zetsche, B.; Heidenreich, M.; Mohanraju, P.; Fedorova, I.; Kneppers, J.; DeGennaro, E.M.; Winblad, N.; Choudhury, S.R.; Abudayyeh, O.O.; Gootenberg, J.S.; et al. Multiplex gene editing by CRISPR–Cpf1 using a single crRNA array. Nat. Biotechnol. 2016, 35, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Scheben, A.; Wolter, F.; Batley, J.; Puchta, H.; Edwards, D. Towards CRISPR/Cas crops—Bringing together genomics and genome editing. New Phytol. 2017, 216, 682–698. [Google Scholar] [CrossRef]
- Zhao, H.; Wolt, J.D. Risk associated with off-target plant genome editing and methods for its limitation. Emerg. Top. Life Sci. 2017, 1, 231–240. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.-S. Unexpected CRISPR on-target effects. Nat. Biotechnol. 2018, 36, 703–704. [Google Scholar] [CrossRef]
- Ouagrham-Gormley, S.B.; Fye-Marnien, S.R. Is CRISPR a security threat? In Defense against Biological Attacks; Springer: Berlin, Germany, 2019; pp. 233–251. [Google Scholar]
- Caplan, A.L.; Parent, B.; Shen, M.; Plunkett, C. No time to waste—The ethical challenges created by CRISPR. EMBO Rep. 2015, 16, 1421–1426. [Google Scholar] [CrossRef]
- Noble, C.; Olejarz, J.; Esvelt, K.M.; Church, G.M.; Nowak, M.A. Evolutionary dynamics of CRISPR gene drives. Sci. Adv. 2017, 3, e1601964. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Guo, D.; Zhang, J.; Huang, Q.; Qin, G.; Zhang, X.; Wan, J.; Gu, H.; Qu, L.-J. Targeted mutagenesis in rice using CRISPR-Cas system. Cell Res. 2013, 23, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yang, B. A High-Efficiency CRISPR Platform for Maize Improvement. Available online: https://vtechworks.lib.vt.edu/handle/10919/78865 (accessed on 2 October 2020).
- Mao, Y.; Zhang, H.; Xu, N.; Zhang, B.; Gou, F.; Zhu, J.-K. Application of the CRISPR–Cas System for Efficient Genome Engineering in Plants. Mol. Plant. 2013, 6, 2008–2011. [Google Scholar] [CrossRef] [PubMed]
- Scheben, A.; Yuan, Y.; Edwards, D. Advances in genomics for adapting crops to climate change. Curr. Plant. Biol. 2016, 6, 2–10. [Google Scholar] [CrossRef]
- Liu, X.R.; Zhang, Q.W.; Yang, G.Q.; Zhang, C.L.; Dong, H.X.; Liu, Y.; Yin, R.H.; Lin, L. Pivotal roles of Tomato photoreceptor SIUVR8 in seedling development and UV-B stress tolerance. Biochem. Biophys. Res. Commun. 2020, 522, 177–183. [Google Scholar] [CrossRef]
- Ortigosa, A.; Gimenez-Ibanez, S.; Leonhardt, N.; Solano, R. Design of a bacterial speck resistant tomato by CRISPR/Cas9-mediated editing of SlJAZ2. Plant. Biotechnol. J. 2018, 17, 665–673. [Google Scholar] [CrossRef]
- Zsögön, A.; Čermák, T.; Naves, E.R.; Notini, M.M.; Edel, K.H.; Weinl, S.; Freschi, L.; Voytas, D.F.; Kudla, J.; Peres, L.E.P. De novo domestication of wild tomato using genome editing. Nat. Biotechnol. 2018, 36, 1211–1216. [Google Scholar] [CrossRef]
- Prihatna, C.; Barbetti, M.J.; Barker, S.J. A Novel Tomato Fusarium Wilt Tolerance Gene. Front. Microbiol. 2018, 9, 1226. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Chen, S.; Tian, H.; Fu, D.; Zhu, B.; Luo, Y.; Zhu, H. Lycopene Is Enriched in Tomato Fruit by CRISPR/Cas9-Mediated Multiplex Genome Editing. Front. Plant. Sci. 2018, 9, 559. [Google Scholar] [CrossRef]
- Tashkandi, M.; Ali, Z.; Aljedaani, F.; Shami, A.; Mahfouz, M.M. Engineering resistance against Tomato yellow leaf curl virus via the CRISPR/Cas9 system in tomato. Plant. Signal. Behav. 2018, 13, e1525996. [Google Scholar] [CrossRef]
- Yu, Q.-H.; Wang, B.; Li, N.; Tang, Y.; Yang, S.; Yang, T.; Xu, J.; Guo, C.; Yan, P.; Wang, Q.; et al. CRISPR/Cas9-induced Targeted Mutagenesis and Gene Replacement to Generate Long-shelf Life Tomato Lines. Sci. Rep. 2017, 7, 11874. [Google Scholar] [CrossRef]
- Nekrasov, V.; Wang, C.; Win, J.; Lanz, C.; Weigel, D.; Kamoun, S. Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion. Sci. Rep. 2017, 7, 482. [Google Scholar] [CrossRef] [PubMed]
- Zsögön, A.; Cermak, T.; Voytas, D.; Peres, L.E.P. Genome editing as a tool to achieve the crop ideotype and de novo domestication of wild relatives: Case study in tomato. Plant. Sci. 2017, 256, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Soyk, S.; Müller, N.A.; Park, S.J.; Schmalenbach, I.; Jiang, K.; Hayama, R.; Zhang, L.; Van Eck, J.; Jiménez-Gómez, J.M.; Lippman, Z.B. Variation in the flowering gene SELF PRUNING 5G promotes day-neutrality and early yield in tomato. Nat. Genet. 2016, 49, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Borovsky, Y.; Monsonego, N.; Mohan, V.; Shabtai, S.; Kamara, I.; Faigenboim, A.; Hill, T.; Chen, S.; Stoffel, K.; Van Deynze, A.; et al. The zinc-finger transcription factor Cc LOL 1 controls chloroplast development and immature pepper fruit color in Capsicum chinense and its function is conserved in tomato. Plant. J. 2019, 99, 41–55. [Google Scholar] [CrossRef]
- Dunemann, F. New strategies for the development of haploid crop plants via genome elimination. Jul.-Kühn-Arch. 2016, 457, 40–45. [Google Scholar]
- González, M.N.; Massa, G.A.; Andersson, M.; Turesson, H.; Olsson, N.; Fält, A.-S.; Storani, L.; Oneto, C.A.D.; Hofvander, P.; Feingold, S.E. Reduced Enzymatic Browning in Potato Tubers by Specific Editing of a Polyphenol Oxidase Gene via Ribonucleoprotein Complexes Delivery of the CRISPR/Cas9 System. Front. Plant. Sci. 2020, 10. [Google Scholar] [CrossRef]
- Ye, M.; Peng, Z.; Tang, D.; Yang, Z.; Li, D.; Xu, Y.; Zhang, C.; Huang, S. Generation of self-compatible diploid potato by knockout of S-RNase. Nat. Plants 2018, 4, 651–654. [Google Scholar] [CrossRef]
- Enciso-Rodriguez, F.; Manrique-Carpintero, N.C.; Nadakuduti, S.S.; Buell, C.R.; Zarka, D.; Douches, D. Overcoming Self-Incompatibility in Diploid Potato Using CRISPR-Cas9. Front. Plant. Sci. 2019, 10, 376. [Google Scholar] [CrossRef]
- Nakayasu, M.; Akiyama, R.; Lee, H.J.; Osakabe, K.; Osakabe, Y.; Watanabe, B.; Sugimoto, Y.; Umemoto, N.; Saito, K.; Muranaka, T.; et al. Generation of α-solanine-free hairy roots of potato by CRISPR/Cas9 mediated genome editing of the St16DOX gene. Plant. Physiol. Biochem. 2018, 131, 70–77. [Google Scholar] [CrossRef]
- Andersson, M.; Turesson, H.; Olsson, N.; Fält, A.-S.; Ohlsson, P.; Gonzalez, M.N.; Samuelsson, M.; Hofvander, P. Genome editing in potato via CRISPR-Cas9 ribonucleoprotein delivery. Physiol. Plant. 2018, 164, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Q.; Zhai, H.; Gao, S.; Yang, L.; Wang, Z.; Xu, Y.; Huo, J.; Ren, Z.; Zhao, N.; et al. IbBBX24 Promotes the Jasmonic Acid Pathway and Enhances Fusarium Wilt Resistance in Sweet Potato. Plant. Cell 2020, 32, 1102–1123. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, H.; Zong, M.; Guo, S.; Ren, Z.; Zhao, J.; Li, M.; Zhang, J.; Tian, S.; Wang, J.; et al. Localization shift of a sugar transporter contributes to phloem unloading in sweet watermelons. New Phytol. 2020, 227, 1858–1871. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, S.; Ji, G.; Zhao, H.; Sun, H.; Ren, Y.; Tian, S.; Li, M.; Gong, G.; Zhang, H.; et al. A unique chromosome translocation disrupting ClWIP1 leads to gynoecy in watermelon. Plant. J. 2019, 101, 265–277. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Q.; Yang, X.; Xu, J.; Liu, G.; Yao, X.; Ren, R.; Xu, J.; Lou, L. CRISPR/Cas9-mediated mutagenesis of Clpsk1 in watermelon to confer resistance to Fusarium oxysporum f.sp. niveum. Plant. Cell Rep. 2020, 39, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhao, S.; Sun, H.; Wang, X.; Wu, S.; Lin, T.; Ren, Y.; Gao, L.; Deng, Y.; Zhang, J.; et al. Resequencing of 414 cultivated and wild watermelon accessions identifies selection for fruit quality traits. Nat. Genet. 2019, 51, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Jiang, L.; Cui, X.; Zhang, J.; Guo, S.; Li, M.; Zhang, H.; Ren, Y.; Gong, G.; Zong, M.; et al. Engineering herbicide-resistant watermelon variety through CRISPR/Cas9-mediated base-editing. Plant. Cell Rep. 2018, 37, 1353–1356. [Google Scholar] [CrossRef]
- Huang, Y.; Cao, H.; Yang, L.; Chen, C.; Shabala, L.; Xiong, M.; Niu, M.; Liu, J.; Zheng, Z.; Zhou, L.; et al. Tissue-specific respiratory burst oxidase homolog-dependent H2O2 signaling to the plasma membrane H+-ATPase confers potassium uptake and salinity tolerance in Cucurbitaceae. J. Exp. Bot. 2019, 70, 5879–5893. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Li, D.; Liu, X.; Qi, J.; Gao, D.; Zhao, S.; Huang, S.; Sun, J.; Yang, L. Engineering Non-transgenic Gynoecious Cucumber Using an Improved Transformation Protocol and Optimized CRISPR/Cas9 System. Mol. Plant. 2017, 10, 1575–1578. [Google Scholar] [CrossRef] [PubMed]
- Stajič, E.; Kiełkowska, A.; Murovec, J.; Bohanec, B. Deep sequencing analysis of CRISPR/Cas9 induced mutations by two delivery methods in target model genes and the CENH3 region of red cabbage (Brassica oleracea var. capitata f. rubra). Plant. Cell Tissue Organ. Cult. 2019, 139, 227–235. [Google Scholar] [CrossRef]
- Park, S.-C.; Park, S.; Jeong, Y.J.; Lee, S.B.; Pyun, J.W.; Kim, S.; Kim, T.H.; Kim, S.W.; Jeong, J.C.; Kim, C.Y. DNA-free mutagenesis of GIGANTEA in Brassica oleracea var. capitata using CRISPR/Cas9 ribonucleoprotein complexes. Plant. Biotechnol. Rep. 2019, 13, 483–489. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Ahn, H.; Ryu, J.; Oh, Y.; Sivanandhan, G.; Won, K.-H.; Park, Y.D.; Kim, J.-S.; Kim, H.; Lim, Y.P.; et al. Generation of early-flowering Chinese cabbage (Brassica rapa spp. pekinensis) through CRISPR/Cas9-mediated genome editing. Plant. Biotechnol. Rep. 2019, 13, 491–499. [Google Scholar] [CrossRef]
- Ma, C.; Zhu, C.; Zheng, M.; Liu, M.; Zhang, D.; Liu, B.; Li, Q.; Si, J.; Ren, X.; Song, H. CRISPR/Cas9-mediated multiple gene editing in Brassica oleracea var. capitata using the endogenous tRNA-processing system. Hortic. Res. 2019, 6, 1–15. [Google Scholar] [CrossRef]
- Bertier, L.D.; Ron, M.; Huo, H.; Bradford, K.J.; Britt, A.B.; Michelmore, R.W. High-Resolution Analysis of the Efficiency, Heritability, and Editing Outcomes of CRISPR/Cas9-Induced Modifications ofNCED4in Lettuce (Lactuca sativa). G3 Genes Genomes Genet. 2018, 8, 1513–1521. [Google Scholar] [CrossRef]
- Gomez, M.A.; Lin, Z.D.; Moll, T.; Luebbert, C.; Chauhan, R.D.; Vijayaraghavan, A.; Kelley, R.; Beyene, G.; Taylor, N.J.; Carrington, J. Simultaneous CRISPR/Cas9-mediated editing of cassava eIF4E isoforms nCBP-1 and nCBP-2 confers elevated resistance to cassava brown streak disease. bioRxiv 2017, 209874. [Google Scholar] [CrossRef]
- Martín-Pizarro, C.; Triviño, J.C.; Posé, D. Functional analysis of the TM6 MADS-box gene in the octoploid strawberry by CRISPR/Cas9-directed mutagenesis. J. Exp. Bot. 2019, 70, 885–895. [Google Scholar] [CrossRef]
- Feng, J.; Dai, C.; Luo, H.; Han, Y.; Liu, Z.; Kang, C. Reporter gene expression reveals precise auxin synthesis sites during fruit and root development in wild strawberry. J. Exp. Bot. 2018, 70, 563–574. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, Y.; Orbović, V.; Xu, J.; White, F.F.; Jones, J.B.; Wang, N. Genome editing of the disease susceptibility gene CsLOB1 in citrus confers resistance to citrus canker. Plant. Biotechnol. J. 2017, 15, 817–823. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Peng, A.; Xie, Z.; He, Y.; Zou, X. CRISPR/Cas9-mediated editing of CsWRKY22 reduces susceptibility to Xanthomonas citri subsp. citri in Wanjincheng orange (Citrus sinensis (L.) Osbeck). Plant. Biotechnol. Rep. 2019, 13, 501–510. [Google Scholar] [CrossRef]
- Pompili, V.; Costa, L.D.; Piazza, S.; Pindo, M.; Malnoy, M. Reduced fire blight susceptibility in apple cultivars using a high-efficiency CRISPR/Cas9-FLP/FRT-based gene editing system. Plant. Biotechnol. J. 2019, 18, 845–858. [Google Scholar] [CrossRef]
- Tripathi, J.N.; Ntui, V.O.; Ron, M.; Muiruri, S.K.; Britt, A.; Tripathi, L. CRISPR/Cas9 editing of endogenous banana streak virus in the B genome of Musa spp. overcomes a major challenge in banana breeding. Commun. Biol. 2019, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Wu, S.; Dou, T.; Zhu, H.; Hu, C.; Huo, H.; He, W.; Deng, G.; Sheng, O.; Bi, F.; et al. Using CRISPR/Cas9 genome editing system to create MaGA20ox2 gene-modified semi-dwarf banana. Plant. Biotechnol. J. 2019, 18, 17–19. [Google Scholar] [CrossRef]
- Ren, F.; Ren, C.; Zhang, Z.; Duan, W.; Lecourieux, D.; Li, S.; Liang, Z. Efficiency Optimization of CRISPR/Cas9-Mediated Targeted Mutagenesis in Grape. Front. Plant. Sci. 2019, 10, 612. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tu, M.; Wang, D.; Liu, J.; Li, Y.; Li, Z.; Wang, Y.; Wang, X. CRISPR/Cas9-mediated efficient targeted mutagenesis in grape in the first generation. Plant. Biotechnol. J. 2017, 16, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Gumtow, R.; Wu, D.; Uchida, J.Y.; Tian, M. A Phytophthora palmivora Extracellular Cystatin-Like Protease Inhibitor Targets Papain to Contribute to Virulence on Papaya. Mol. Plant.-Microbe Interact. 2018, 31, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Fister, A.S.; Landherr, L.; Maximova, S.N.; Guiltinan, M.J. Transient Expression of CRISPR/Cas9 Machinery Targeting TcNPR3 Enhances Defense Response in Theobroma cacao. Front. Plant. Sci. 2018, 9, 9. [Google Scholar] [CrossRef]
- Breitler, J.-C.; Dechamp, E.; Campa, C.; Rodrigues, L.A.Z.; Guyot, R.; Marraccini, P.; Etienne, H. CRISPR/Cas9-mediated efficient targeted mutagenesis has the potential to accelerate the domestication of Coffea canephora. Plant. Cell Tissue Organ. Cult. 2018, 134, 383–394. [Google Scholar] [CrossRef]
- Xu, J.; Kang, B.; Naing, A.H.; Bae, S.; Kim, J.; Kim, H.; Kil Kim, C. CRISPR /Cas9-mediated editing of 1-aminocyclopropane-1-carboxylate oxidase1 enhances Petunia flower longevity. Plant. Biotechnol. J. 2019, 18, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Wu, F.; Yuan, Y.; Chen, Y.; Lin, C. High-efficiency CRISPR /Cas-based editing of Phalaenopsis orchid MADS genes. Plant. Biotechnol. J. 2019, 18, 889–891. [Google Scholar] [CrossRef]
- Kishi-Kaboshi, M.; Aida, R.; Sasaki, K. Generation of Gene-Edited Chrysanthemum morifolium Using Multi-Copy Transgenes as Targets and Markers. Plant. Cell Physiol. 2017, 58, 216–226. [Google Scholar] [CrossRef]
- Shibuya, K.; Watanabe, K.; Ono, M. CRISPR/Cas9-mediated mutagenesis of the EPHEMERAL1 locus that regulates petal senescence in Japanese morning glory. Plant. Physiol. Biochem. 2018, 131, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Oda-Yamamizo, C.; Sage-Ono, K.; Ohmiya, A.; Ono, M. Alteration of flower colour in Ipomoea nil through CRISPR/Cas9-mediated mutagenesis of carotenoid cleavage dioxygenase 4. Transgenic Res. 2017, 27, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Lu, L.; Liu, H.-Y.; Li, S.; Xing, F.; Chen, L.-L. CRISPR-P: A Web Tool for Synthetic Single-Guide RNA Design of CRISPR-System in Plants. Mol. Plant. 2014, 7, 1494–1496. [Google Scholar] [CrossRef]
- Naito, Y.; Hino, K.; Bono, H.; Ui-Tei, K. CRISPRdirect: Software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 2015, 31, 1120–1123. [Google Scholar] [CrossRef]
- Labun, K.; Montague, T.G.; Gagnon, J.A.; Thyme, S.B.; Valen, E. CHOPCHOP v2: A web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res. 2016, 44, W272–W276. [Google Scholar] [CrossRef] [PubMed]
- Benchling Quick and Easy CRISPR Designs. Available online: https://benchling.com/crispr (accessed on 2 October 2020).
- Addgene CRISPR Plasmids: Plants. Available online: http://www.addgene.org/crispr/plant/ (accessed on 2 October 2020).
- Xing, H.-L.; Dong, L.; Wang, Z.-P.; Zhang, H.-Y.; Han, C.-Y.; Liu, B.; Wang, X.-C.; Chen, Q.-J. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant. Biol. 2014, 14, 327. [Google Scholar] [CrossRef] [PubMed]
- Čermák, T.; Baltes, N.J.; Čegan, R.; Zhang, Y.; Voytas, D.F. High-frequency, precise modification of the tomato genome. Genome Biol. 2015, 16, 232. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Q.; Zhu, Q.; Liu, W.; Chen, Y.; Qiu, R.; Wang, B.; Yang, Z.; Li, H.; Lin, Y.; et al. A Robust CRISPR/Cas9 System for Convenient, High-Efficiency Multiplex Genome Editing in Monocot and Dicot Plants. Mol. Plant. 2015, 8, 1274–1284. [Google Scholar] [CrossRef]
- Xie, K.; Minkenberg, B.; Yang, Y. Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc. Natl. Acad. Sci. USA 2015, 112, 3570–3575. [Google Scholar] [CrossRef]
- Khan, A.A.; El-Sayed, A.; Akbar, A.; Mangravita-Novo, A.; Bibi, S.; Afzal, Z.; Norman, D.J.; Ali, G.S. A highly efficient ligation-independent cloning system for CRISPR/Cas9 based genome editing in plants. Plant. Methods 2017, 13, 86. [Google Scholar] [CrossRef]
- Snapgene Tutorial Videos. Available online: https://www.snapgene.com/support/tutorial-videos/ (accessed on 2 October 2020).
- Jiang, W.; Zhou, H.; Bi, H.; Fromm, M.; Yang, B.; Weeks, D.P. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res. 2013, 41, e188. [Google Scholar] [CrossRef] [PubMed]
- Mikami, M.; Toki, S.; Endo, M. Comparison of CRISPR/Cas9 expression constructs for efficient targeted mutagenesis in rice. Plant. Mol. Biol. 2015, 88, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Butler, N.M.; Atkins, P.A.; Voytas, D.F.; Douches, D.S. Generation and Inheritance of Targeted Mutations in Potato (Solanum tuberosum L.) Using the CRISPR/Cas System. PLoS ONE 2015, 10, e0144591. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Mikami, M.; Toki, S. Multigene knockout utilizing off-target mutations of the CRISPR/Cas9 system in rice. Plant. Cell Physiol. 2015, 56, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Ye, L.; Qin, L.; Liu, X.; He, Y.; Wang, J.; Chen, L.; Lu, G. CRISPR/Cas9-mediated efficient and heritable targeted mutagenesis in tomato plants in the first and later generations. Sci. Rep. 2016, 6, 24765. [Google Scholar] [CrossRef]
- Char, S.N.; Neelakandan, A.K.; Nahampun, H.; Frame, B.; Main, M.; Spalding, M.H.; Becraft, P.W.; Meyers, B.C.; Walbot, V.; Wang, K.; et al. AnAgrobacterium-delivered CRISPR/Cas9 system for high-frequency targeted mutagenesis in maize. Plant. Biotechnol. J. 2017, 15, 257–268. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, R.; Song, G.; Gao, J.; Li, W.; Han, X.; Chen, M.; Li, Y.; Li, G. Targeted mutagenesis using the Agrobacterium tumefaciens-mediated CRISPR-Cas9 system in common wheat. BMC Plant. Biol. 2018, 18, 302. [Google Scholar] [CrossRef]
- Osakabe, Y.; Liang, Z.; Ren, C.; Nishitani, C.; Osakabe, K.; Wada, M.; Komori, S.; Malnoy, M.; Velasco, R.; Poli, M.; et al. CRISPR–Cas9-mediated genome editing in apple and grapevine. Nat. Protoc. 2018, 13, 2844–2863. [Google Scholar] [CrossRef]
- Lee, K.; Zhu, H.; Yang, B.; Wang, K. An Agrobacterium-mediated CRISPR/Cas9 platform for genome editing in maize. In Plant Genome Editing with CRISPR Systems; Springer: Berlin, Germany, 2019; pp. 121–143. [Google Scholar]
- Reem, N.T.; Van Eck, J. Application of CRISPR/Cas9-mediated gene editing in tomato. In Plant Genome Editing with CRISPR Systems; Springer: Berlin, Germany, 2019; pp. 171–182. [Google Scholar]
- Sun, Y.; Zhang, X.; Wu, C.; He, Y.; Ma, Y.; Hou, H.; Guo, X.; Du, W.; Zhao, Y.; Xia, L. Engineering Herbicide-Resistant Rice Plants through CRISPR/Cas9-Mediated Homologous Recombination of Acetolactate Synthase. Mol. Plant. 2016, 9, 628–631. [Google Scholar] [CrossRef]
- Hamada, H.; Liu, Y.; Nagira, Y.; Miki, R.; Taoka, N.; Imai, R. Biolistic-delivery-based transient CRISPR/Cas9 expression enables in planta genome editing in wheat. Sci. Rep. 2018, 8, 14422. [Google Scholar] [CrossRef]
- Ali, Z.; Abulfaraj, A.; Idris, A.; Ali, S.; Tashkandi, M.; Mahfouz, M.M. CRISPR/Cas9-mediated viral interference in plants. Genome Biol. 2015, 16, 238. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Han, T.; Liu, G.; Chen, T.; Wang, Y.; Yu, A.Y.L.; Liu, Y. A geminivirus-based guide RNA delivery system for CRISPR/Cas9 mediated plant genome editing. Sci. Rep. 2015, 5, 14926. [Google Scholar] [CrossRef] [PubMed]
- Demirer, G.S.; Zhang, H.; Goh, N.S.; González-Grandío, E.; Landry, M.P. Carbon nanotube-mediated DNA delivery without transgene integration in intact plants. Nat. Protoc. 2019, 14, 2954–2971. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, R.; Rajiv, P.; Abd-Elsalam, K.A. Carbon nanotubes: Plant gene delivery and genome editing. In Carbon Nanomaterials for Agri-Food and Environmental Applications; Elsevier BV: Amsterdam, The Netherlands, 2020; pp. 279–296. [Google Scholar]
- Tian, S.; Jiang, L.; Gao, Q.; Zhang, J.; Zong, M.; Zhang, H.; Ren, Y.; Guo, S.; Gong, G.; Liu, F.; et al. Efficient CRISPR/Cas9-based gene knockout in watermelon. Plant. Cell Rep. 2016, 36, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.M.; Gunn, H.; Moretti, N.; Zemetra, R.S. A Streamlined Protocol for Wheat (Triticum aestivum) Protoplast Isolation and Transformation with CRISPR-Cas Ribonucleoprotein Complexes. Front. Plant. Sci. 2020, 11, 769. [Google Scholar] [CrossRef]
- Xu, R.-F.; Li, H.; Qin, R.-Y.; Li, J.; Qiu, C.-H.; Yang, Y.-C.; Ma, H.; Li, L.; Wei, P.-C.; Yang, J.-B. Generation of inheritable and “transgene clean” targeted genome-modified rice in later generations using the CRISPR/Cas9 system. Sci. Rep. 2015, 5, 11491. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Z.; Zong, Y.; Wang, Y.; Liu, J.; Chen, K.; Qiu, J.-L.; Gao, C. Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat. Commun. 2016, 7, 12617. [Google Scholar] [CrossRef]
- Hua, Y.; Wang, C.; Huang, J.; Wang, K. A simple and efficient method for CRISPR/Cas9-induced mutant screening. J. Genet. Genom. 2017, 44, 207–213. [Google Scholar] [CrossRef]
- Zhang, Z.; Ge, X.; Luo, X.; Wang, P.; Fan, Q.; Hu, G.; Xiao, J.; Li, F.; Wu, J. Simultaneous Editing of Two Copies of Gh14-3-3d Confers Enhanced Transgene-Clean Plant Defense Against Verticillium dahliae in Allotetraploid Upland Cotton. Front. Plant. Sci. 2018, 9, 842. [Google Scholar] [CrossRef]
- Kim, D.; Alptekin, B.; Budak, H. CRISPR/Cas9 genome editing in wheat. Funct. Integr. Genom. 2017, 18, 31–41. [Google Scholar] [CrossRef]
- Brinkman, E.K.; Chen, T.; Amendola, M.; Van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 2014, 42, e168. [Google Scholar] [CrossRef]
- Braatz, J.; Harloff, H.-J.; Mascher, M.; Stein, N.; Himmelbach, A.; Jung, C. CRISPR-Cas9 Targeted Mutagenesis Leads to Simultaneous Modification of Different Homoeologous Gene Copies in Polyploid Oilseed Rape (Brassica napus). Plant. Physiol. 2017, 174, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Su, H.; Bai, H.; Wang, R.; Liu, Y.; Guo, X.; Liu, C.; Zhang, J.; Yuan, J.; Birchler, J.A.; et al. High-efficiency genome editing using a dmc1 promoter-controlled CRISPR/Cas9 system in maize. Plant. Biotechnol. J. 2018, 16, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, G.; Zhou, J.; Ren, Q.; You, Q.; Tian, L.; Xin, X.; Zhong, Z.; Liu, B.; Zheng, X.; et al. A large-scale whole-genome sequencing analysis reveals highly specific genome editing by both Cas9 and Cpf1 (Cas12a) nucleases in rice. Genome Biol. 2018, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Nadakuduti, S.S.; Buell, C.R.; Voytas, D.F.; Starker, C.G.; Douches, D.S. Genome Editing for Crop Improvement—Applications in Clonally Propagated Polyploids With a Focus on Potato (Solanum tuberosum L.). Front. Plant. Sci. 2018, 9, 1607. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yang, Y.; Qin, R.; Li, H.; Qiu, C.; Li, L.; Wei, P.; Yang, J. Rapid improvement of grain weight via highly efficient CRISPR/Cas9-mediated multiplex genome editing in rice. J. Genet. Genom. 2016, 43, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, H.; Wang, H.; Lafitte, H.R.; Archibald, R.L.; Yang, M.; Hakimi, S.M.; Mo, H.; Habben, J.E. ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant. Biotechnol. J. 2016, 15, 207–216. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Mohamed, S.; Tanoi, K.; Kobayashi, N.I.; Takagi, K.; Vernet, A.; Guiderdoni, E.; Périn, C.; Sentenac, H.; Véry, A.-A. Production of low-Cs+ rice plants by inactivation of the K+ transporter Os HAK 1 with the CRISPR -Cas system. Plant. J. 2017, 92, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Taniguchi, H.; Hassan, M.; Bhowmik, P.; Karim, M.R.; Śmiech, M.; Zhao, K.; Rahman, M.; Islam, T. Application of CRISPR/Cas9 Genome Editing Technology for the Improvement of Crops Cultivated in Tropical Climates: Recent Progress, Prospects, and Challenges. Front. Plant. Sci. 2018, 9, 617. [Google Scholar] [CrossRef]
- Woo, J.W.; Kim, J.; Kwon, S.I.; Corvalán, C.; Cho, S.W.; Kim, H.; Kim, S.-G.; Kim, S.-T.; Choe, S.; Kim, J.-S. DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat. Biotechnol. 2015, 33, 1162–1164. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.-S. Bypassing GMO regulations with CRISPR gene editing. Nat. Biotechnol. 2016, 34, 1014–1015. [Google Scholar] [CrossRef]
- Hilscher, J.; Bürstmayr, H.; Stoger, E. Targeted modification of plant genomes for precision crop breeding. Biotechnol. J. 2016, 12, 1600173. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Araki, M. A future scenario of the global regulatory landscape regarding genome-edited crops. GM Crop. Food 2017, 8, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Waltz, E. CRISPR-Edited Crops Free to Enter. Market., Skip Regulation; Nature Publishing Group: Berlin, Germany, 2016. [Google Scholar]
- Sander, J.; Jeschke, M. CRISPR-Cas Advanced Plant Breeding. Crop Insights 2016, 26, 18. Available online: https://crisprcas.pioneer.com/wp-content/uploads/2017/01/CRISPR-Cas_Advanced_Plant_Breeding_CI161215.pdf (accessed on 1 September 2020).
- Wieczorek, A.; Wright, M.G. History of agricultural biotechnology: How crop development has evolved. Nat. Educ. Knowl. 2012, 3, 9. [Google Scholar]
- Kim, H.; Kim, J.-S. A guide to genome engineering with programmable nucleases. Nat. Rev. Genet. 2014, 15, 321–334. [Google Scholar] [CrossRef]
- Ishii, T.; Araki, M. Consumer acceptance of food crops developed by genome editing. Plant. Cell Rep. 2016, 35, 1507–1518. [Google Scholar] [CrossRef]
- Globus, R.; Qimron, U. A technological and regulatory outlook on CRISPR crop editing. J. Cell. Biochem. 2017, 119, 1291–1298. [Google Scholar] [CrossRef]
- Liu, X.; Xie, C.; Si, H.; Yang, J. CRISPR/Cas9-mediated genome editing in plants. Methods 2017, 121, 94–102. [Google Scholar] [CrossRef]
- USDA Re: Confirmation of Regulatory Status of Waxy Com Developed by CRISPR-Cas Technology. Available online: https://www.aphis.usda.gov/biotechnology/downloads/reg_loi/15-352-01_air_response_signed.pdf (accessed on 2 October 2020).
- USDA Re: Request for confirmation that transgene-free, CRISPR-edited mushroom is not a regulated article. Available online: https://www.aphis.usda.gov/biotechnology/downloads/reg_loi/15-321-01_air_response_signed.pdf (accessed on 2 October 2020).
- Songstad, D.D.; Petolino, J.F.; Voytas, D.F.; Reichert, N.A. Genome Editing of Plants. Crit. Rev. Plant. Sci. 2017, 36, 1–23. [Google Scholar] [CrossRef]
- USDA Secretary Perdue Issues USDA Statement on Plant Breeding Innovation. Available online: https://www.usda.gov/media/press-releases/2018/03/28/secretary-perdue-issues-usda-statement-plant-breeding-innovation (accessed on 2 October 2020).
- USDA-APHIS Regulated Article Letters of Inquiry. Available online: https://www.aphis.usda.gov/aphis/ourfocus/biotechnology/am-i-regulated/Regulated_Article_Letters_of_Inquiry (accessed on 2 October 2020).
- Barrangou, R. Finding SECURE Ground: USDA Edits the Biotechnology Regulatory Framework; Mary Ann Liebert, Inc.: New Rochelle, NY, USA, 2020. [Google Scholar]
- USDA-APHIS about the SECURE Rule. Available online: https://www.aphis.usda.gov/aphis/ourfocus/biotechnology/biotech-rule-revision (accessed on 2 October 2020).
- Agency, C.F.I. Regulatory Oversight of Plant Products Developed using Biotechnology. Available online: https://www.inspection.gc.ca/plant-varieties/plants-with-novel-traits/gene-editing-techniques/eng/1541800629219/1541800629556 (accessed on 2 October 2020).
- Wight, A.J. Strict EU ruling on gene-edited crops squeezes science. Nature 2018, 563, 15–16. [Google Scholar] [CrossRef]
- Callaway, E. CRISPR plants now subject to tough GM laws in European Union. Nature 2018, 560, 16. [Google Scholar] [CrossRef] [PubMed]
- Mallapaty, S. Australian gene-editing rules adopt ’middle ground’. Nature 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Metje-Sprink, J.; Sprink, T.; Hartung, F. Genome-edited plants in the field. Curr. Opin. Biotechnol. 2020, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
- MOEF Rules for the Manufacture, Use/Import/Export and Storage of Hazardous Micro Organisms/ genetically Engineered Organisms or Cells. Available online: https://geacindia.gov.in/resource-documents/biosafety-regulations/acts-and-rules/Rules-for-the-manufacture-use-import-export-and-storage-1989.pdf (accessed on 2 October 2020).
- Pillay, S.; Thaldar, D. CRISPR: Challenges to South African biotechnology law. S. Afr. J. Bioeth. Law 2018, 11, 89–92. [Google Scholar] [CrossRef]
- MALF Resolution 173/2015. Available online: http://servicios.infoleg.gob.ar/infolegInternet/anexos/245000-249999/246978/norma.htm (accessed on 2 October 2020).
- Synthego CRISPR in Agriculture: An Era of Food Evolution. Available online: https://www.synthego.com/blog/crispr-agriculture-foods#crops (accessed on 2 October 2020).
- Calyxt, I. First Commercial Sale of Calyxt High Oleic Soybean Oil on the U.S. Market. Available online: https://calyxt.com/first-commercial-sale-of-calyxt-high-oleic-soybean-oil-on-the-u-s-market/ (accessed on 2 October 2020).
- Bartkowski, B.; Theesfeld, I.; Pirscher, F.; Timaeus, J. Snipping around for food: Economic, ethical and policy implications of CRISPR/Cas genome editing. Geoforum 2018, 96, 172–180. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Zhu, H. CRISPR technology is revolutionizing the improvement of tomato and other fruit crops. Hortic. Res. 2019, 6, 77. [Google Scholar] [CrossRef]
- Bain, C.; Lindberg, S.; Selfa, T. Emerging sociotechnical imaginaries for gene edited crops for foods in the United States: Implications for governance. Agric. Hum. Values 2019, 37, 265–279. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
| 1987–1995 Short direct repeats observed in Escherichia coli, Haloferax mediterranei, and Haloferax volcanii [25,26,27] |
| 2002 The term “CRISPR” coined, CRISPR components identified/named [28] |
| 2005 CRISPR speculated as a defense mechanism in bacteria [29] |
| 2007–2008 CRISPR/Cas genes confirmed to provide resistance to phages/ explanation of antiviral defense mechanism [30,31] |
| 2010 CRISPR/Cas system can specifically cleave double-strand DNA [32] |
| 2012 Cas9 endonuclease guided by RNA for gene editing [7] |
| 2013 Human genome-edited by CRISPR/Cas9 system [33,34] First use of CRISPR/Cas9 in plants [14,35,36,37] |
| 2014– Routine application of CRISPR/Cas9 for crop improvement [38,39,40,41] |
| Crop. | Research Objective Met Using CRISPR/Cas9 | References |
|---|---|---|
| Tomato | Understand the role of a photoreceptor in seedling development/stress tolerance | [90] |
| Bacterial speck resistance | [91] | |
| Combine desired traits with useful traits present in wild type | [92] | |
| Confirm function of a gene involved in Fusarium wilt tolerance | [93] | |
| Improve lycopene content | [94] | |
| Develop Tomato Yellow Leaf Curl Virus resistance | [95] | |
| Long shelf life, generate parthenocarpy | [96] | |
| Transgene-free powdery mildew resistant plants | [97] | |
| Achieve ideotype | [98] | |
| Develop day-neutral and early yielding plants | [99] | |
| Capsicum | Understand the role of a transcription factor in chloroplast development and fruit color | [100] |
| Carrot | Generate haploid plants | [101] |
| Potato | Reduce enzymatic browning | [102] |
| Overcome self-incompatibility | [103,104] | |
| Reduce steroidal glycoalkaloids | [105] | |
| Develop amylopectin starch cultivars | [106] | |
| Sweet potato | Enhance Fusarium wilt resistance | [107] |
| Watermelon | Validate function of vacuolar sugar transporter gene | [108] |
| Obtain gynoecious genotypes | [109] | |
| Resistance to Fusarium oxysporum f. sp. niveum Race 1 | [110] | |
| Functional characterization of a gene in fruit flesh sugar accumulation | [111] | |
| Herbicide resistance | [112] | |
| Pumpkin | Understanding the role of root apex in salt tolerance | [113] |
| Cucumber | Transgene-free gynoecious plants | [114] |
| Broad virus resistance | [15] | |
| Cabbage | Compare delivery methods in model genes | [115] |
| Target flowering-time regulator gene | [116] | |
| Generate early flowering phenotype | [117] | |
| Multiplex gene editing to overcome self-incompatibility and produce male-sterile lines | [118] | |
| Lettuce | Generate seedlings capable of germinating at higher temperatures | [119] |
| Cassava | Brown streak resistance | [120] |
| Strawberry | Characterize a transcription factor involved in anther development | [121] |
| Identify genes involved in auxin accumulation and biosynthesis | [122] | |
| Citrus | Canker resistance | [18,123,124] |
| Apple | Reduce fire blight susceptibility | [125] |
| Banana | Inactivate banana streak virus | [126] |
| Basis of generating dwarf and semi-dwarf cultivars | [127] | |
| Grapes | Study editing efficiency | [128] |
| Obtain biallelic mutations in the first generation | [129] | |
| Papaya | Study the evolution of oomycetes in evading plant defense mechanism | [130] |
| Cacao | Edit gene involved in suppressing defense response | [131] |
| Coffee | Proof-of-concept to knock out genes of interest | [132] |
| Petunia | Understand genes involved in flower longevity and ethylene production | [133] |
| Orchid | Understand the MADS gene family expressed in floral organs | [134] |
| Chrysanthemum | First report of gene editing | [135] |
| Japanese morning glory | Flower longevity | [136] |
| Understand the role of a gene in petal coloration | [137] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatta, B.P.; Malla, S. Improving Horticultural Crops via CRISPR/Cas9: Current Successes and Prospects. Plants 2020, 9, 1360. https://doi.org/10.3390/plants9101360
Bhatta BP, Malla S. Improving Horticultural Crops via CRISPR/Cas9: Current Successes and Prospects. Plants. 2020; 9(10):1360. https://doi.org/10.3390/plants9101360
Chicago/Turabian StyleBhatta, Bed Prakash, and Subas Malla. 2020. "Improving Horticultural Crops via CRISPR/Cas9: Current Successes and Prospects" Plants 9, no. 10: 1360. https://doi.org/10.3390/plants9101360
APA StyleBhatta, B. P., & Malla, S. (2020). Improving Horticultural Crops via CRISPR/Cas9: Current Successes and Prospects. Plants, 9(10), 1360. https://doi.org/10.3390/plants9101360






