Chia, Quinoa, and Their Coproducts as Potential Antioxidants for the Meat Industry
Abstract
1. Introduction
2. Antioxidants Compounds in Chia, Quinoa, and Their Coproducts
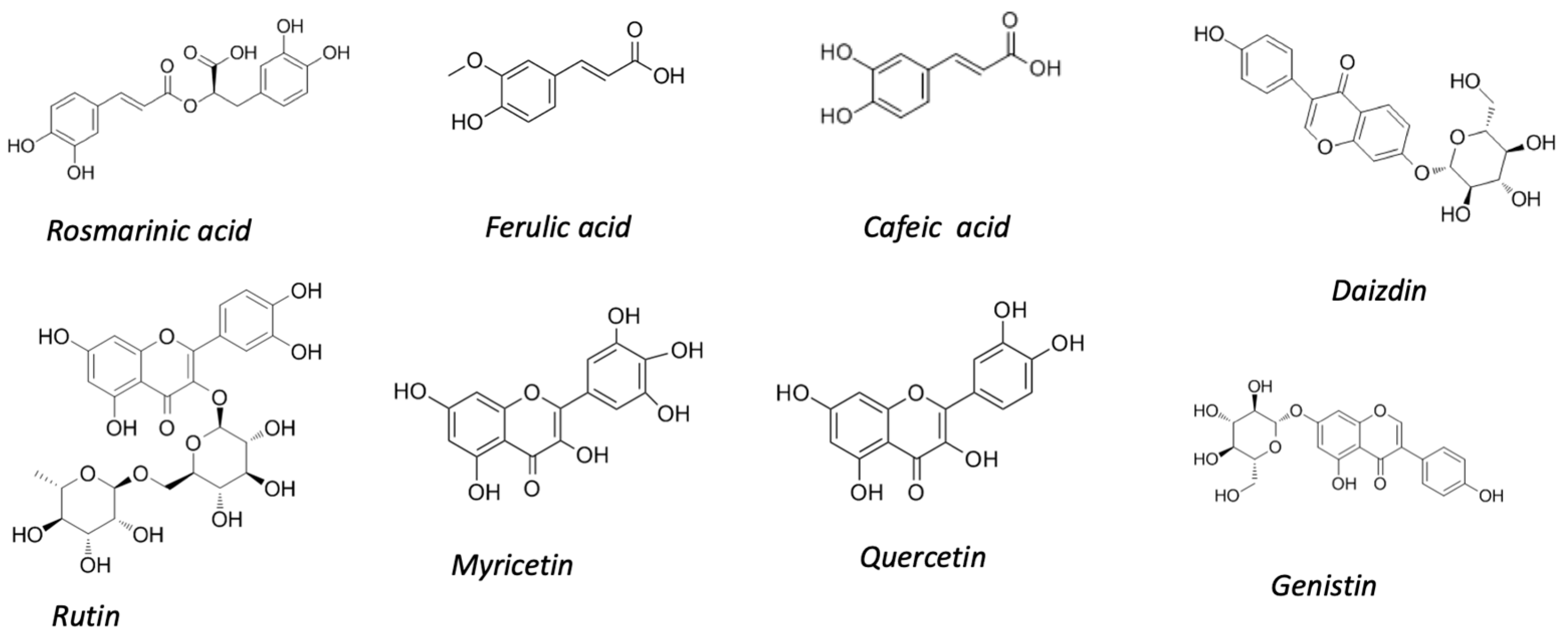
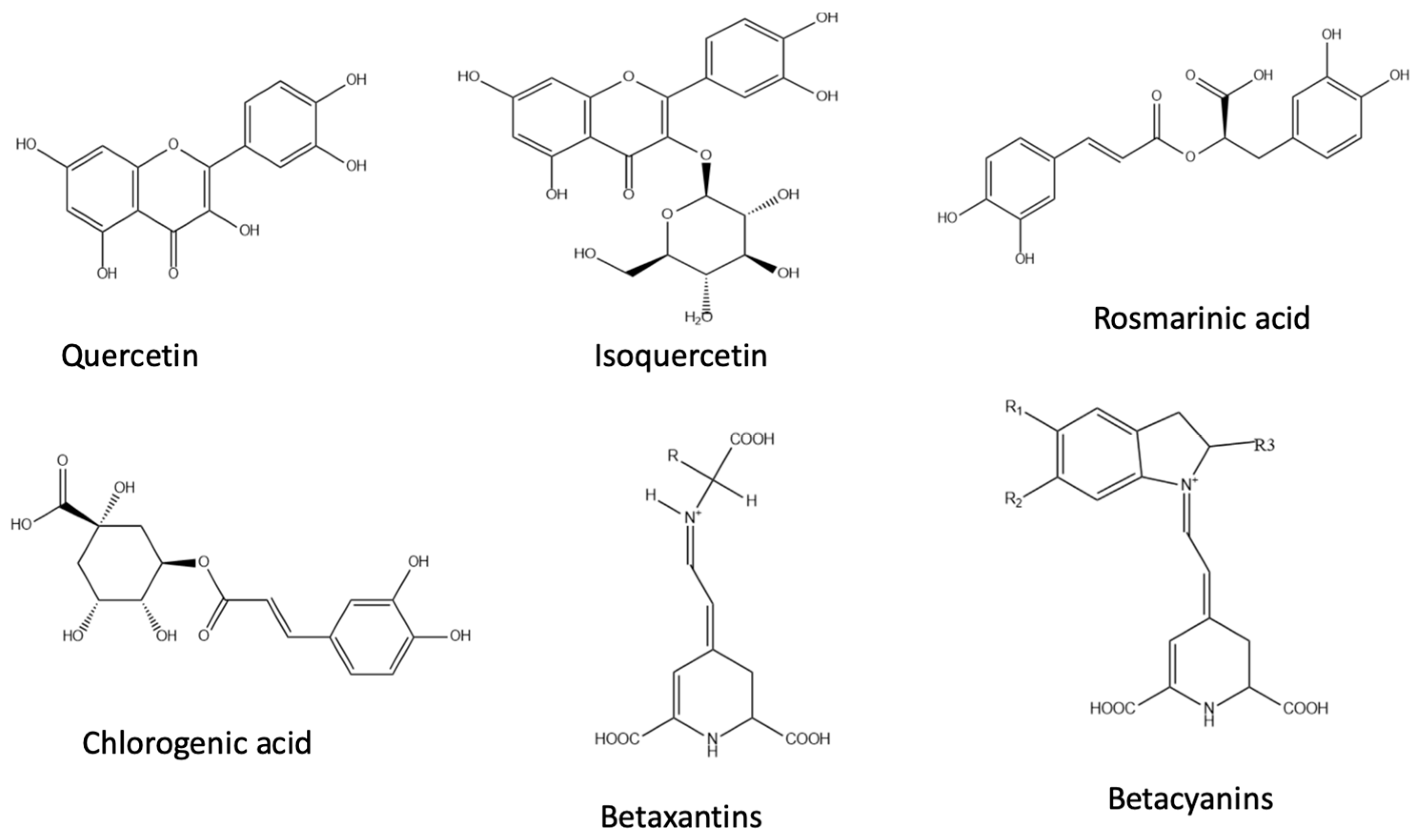
2.1. Chia and Its Coproducts
2.2. Quinoa and Its Coproducts
3. Antioxidant Activity in Chia, Quinoa, and Their Coproducts
3.1. Chia and Its Coproducts
3.2. Quinoa and Its Coproducts
4. Oxidation Stability of Meat Products Containing Chia, Quinoa, or Their Coproducts
4.1. Chia Related Meat Products
4.2. Quinoa Related Meat Products
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, V.; Chandra, S.; Dwivedi, P.; Parturkar, M. Quinoa (Chenopodium quinoa Willd.): A nutritional healthy grain. Intern. J. Adv. Res. 2015, 3, 725–736. [Google Scholar]
- Gordillo-Bastida, E.; Díaz-Rizzolo, D.A.; Roura, E.; Massanés, T.; Gomis, R. Quinoa (Chenopodium quinoa Willd), from nutritional value to potential health benefits: An integrative review. J. Nutr. Food Sci. 2016, 6, 1000497. [Google Scholar]
- Pellegrini, M.; Lucas-González, R.; Sayas-Barberá, E.; Fernández-López, J.; Pérez-Alvarez, J.A.; Viuda-Martos, M. Bioaccessibility of phenolic compounds and antioxidant capacity of chia seeds. Plant Foods Hum. Nutr. 2018, 73, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Lucas-González, R.; Ricci, A.; Fontecha, J.; Fernández-López, J.; Pérez-Alvarez, J.A.; Viuda-Martos, M. Chemical, fatty acid, polyphenolic profile, techno-functional and antioxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd) seeds. Ind. Crops Prod. 2018, 111, 38–46. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F. Formulation and quality attributes of quinoa food products. Food Bioprocess Technol. 2016, 9, 49–68. [Google Scholar] [CrossRef]
- Zettel, V.; Hitzmann, B. Applications of chia (Salvia hispanica L.) in food products. Trends Food Sci. Technol. 2018, 80, 43–50. [Google Scholar] [CrossRef]
- Ceyhun-Sezgin, A.; Sanlier, N. A new generation plant for the conventional cuisine: Quinoa (Chenopodium quinoa Willd). Trends Food Sci. Technol. 2019, 86, 51–58. [Google Scholar] [CrossRef]
- Pereira, E.; Encina-Zelada, C.; Barros, L.; Gonzales-Barron, U.; Cadavez, V.; Ferreira, I.C.F.R. Chemical and nutritional characterization of Chenopodium quinoa Willd (quinoa) grains: A good alternative to nutritious food. Food Chem. 2019, 280, 110–114. [Google Scholar] [CrossRef]
- Fernández-López, J.; Viuda-Martos, M.; Pérez-Alvarez, J.A. Quinoa and chia products as ingredients for healthier processed meat products: Technological strategies for their application and effects on the final product. Curr. Opin. Food Sci. 2020, 40, 26–32. [Google Scholar] [CrossRef]
- Ballester-Sánchez, J.; Fernández-Espinar, M.T.; Haros, C.M. Isolation of red quinoa fibre by wet and dry milling and application as a potential functional bakery ingredient. Food Hydrocoll. 2020, 101, 105513. [Google Scholar] [CrossRef]
- De Falco, B.; Amato, M.; Lanzotti, V. Chia seeds products: An overview. Phytochem. Rev. 2017, 16, 745–760. [Google Scholar] [CrossRef]
- Ballester-Sánchez, J.; Vicente-Gil, J.; Haros, C.M.; Fernández-Espinar, M.T. Effect of incorporating white, red or black quinoa flours on free and bound polyphenol content, antioxidant activity and colour of bread. Plant Foods Hum. Nutr. 2019, 74, 185–191. [Google Scholar]
- Aranibar, C.; Pigni, N.B.; Martinez, M.; Aguirre, A.; Ribotta, P.; Wunderlin, D.; Borneo, R. Utilization of a partially-deoiled chia flour to improve the nutritional and antioxidant properties of wheat pasta. LWT Food Sci. Technol. 2018, 89, 381–387. [Google Scholar] [CrossRef]
- Atwa, E.H.; Ghada, M.E.A. Effect of chia and quinoa seeds extract as natural antioxidant on the oxidative stability of fermented cream analogue. J. Food Dairy Sci. 2020, 11, 51–57. [Google Scholar] [CrossRef]
- Navruz-Varli, S.; Sanlier, N. Nutritional and health benefits of quinoa (Chenopodium quinoa Willd). J. Cereal Sci. 2019, 69, 371–376. [Google Scholar] [CrossRef]
- Nowak, V.; Du, J.; Charrondière, U.R. Assesment of the nutritional composition of quinoa (Chenopodium quinoa Willd). Food Chem. 2016, 193, 47–54. [Google Scholar] [CrossRef]
- Vilacundo, R.; Hernández-Ledesma, B. Nutritional and biological value of quinoa (Chenopodium quinoa Willd). Curr. Opin. Food Sci. 2017, 14, 1–6. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Zhang, B.; Chen, P.X.; Liu, R.; Tsao, R. Characterization of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 166, 380–388. [Google Scholar] [CrossRef]
- Escribano, J.; Cabanes, J.; Jiménez-Atiénzar, M.; Ibañez-Tremolada, M.; Gómez-Pando, L.R.; García-Carmona, F.; Gandía-Herrero, F. Characterization of betalains, saponins and antioxidant power in differently colored quinoa (Chenopodium quinoa) varieties. Food Chem. 2017, 234, 285–294. [Google Scholar] [CrossRef]
- Muñoz, I.A.; Cobos, A.; Díaz, O.; Aguilera, J.M. Chia seed (Salvia hispanica): An ancient grain and a new functional food. Food Rev. Intern. 2013, 29, 394–408. [Google Scholar] [CrossRef]
- Ding, Y.; Lin, H.W.; Lin, Y.L.; Yang, D.J.; Yu, Y.S.; Chen, W., Jr.; Wang, S.Y.; Chen, Y.C. Nutritional composition in the chia seed and its processing properties on restructured ham-like products. J. Food Drug Anal. 2018, 26, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, J.; Lucas-González, R.; Viuda-Martos, M.; Sayas-Barberá, E.; Pérez-Alvarez, J.A. Chia oil extraction coproduct as a potential new ingredient for the food industry: Chemical, physicochemical, techno-functional and antioxidant properties. Plant Foods Hum. Nutr. 2018, 73, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Caudillo, E.; Tecante, A.; Valdivia-López, M.A. Dietary fibre content and antioxidant activity of phenolic compounds present in Mexican chia (Salvia hispanica L.) seeds. Food Chem. 2008, 107, 656–663. [Google Scholar] [CrossRef]
- Pintado, T.; Ruiz-Capillas, C.; Herrero, A.M. New lipid materials based on chia emulsion gels. Application in meat products. Biomed. J. Sci. Tech. Res. 2019, 18, 13215–13218. [Google Scholar] [CrossRef]
- Martínez-Cruz, O.; Paredes-López, O. Phytochemical profile and nutraceutical potential of chia sedes (Salvia hispanica L) by ultra high performance liquid chromatography. J. Chromatogr. A 2014, 1346, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Abderrahim, F.; Huanatico, E.; Segura, R.; Arribas, S.; Gonzalez, M.C.; Condezo-Hoyos, L. Physical features, phenolic compounds, betalains and total antioxidant capacity of coloured quinoa seeds (Chenopodium quinoa Willd.) from Peruvian Altiplano. Food Chem. 2015, 183, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Noratto, G.D.; Murphy, K.; Chew, B.P. Quinoa intake reduces plasma and liver cholesterol, lessens obesity associated inflammation, and helps to prevent hepatic steatosis in obese db/db mouse. Food Chem. 2019, 287, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Repo-Carrasco-Valencia, R. Dietary fibre and bioactive compounds of kernels. In Pseudocereals: Chemistry and technology; Haros, C.M., Schoenlechner, R., Eds.; John Wiley & Sons, Ltd.: Oxfork, UK, 2017; pp. 71–93. [Google Scholar]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Chaijan, M.; Panpipat, W. Mechanism of oxidation in foods of animal origin. Chapter 1. In Natural Antioxidants: Applications in Foods of Animal Origin; Banerjee, R., Verma, A.K., Siddiqui, M.W., Eds.; CRC Press: Oakville, ON, Canada, 2017; pp. 1–50. [Google Scholar]
- Maqsood, S.; Benjakul, S. Comparative studies on molecular changes and pro-oxidative activity of haemoglobin from different fish species as influenced by pH. Food Chem. 2011, 124, 875–883. [Google Scholar] [CrossRef]
- Naveena, B.M.; Sen, A.R.; Vaithiyanathan, S.; Babji, Y.; Kondaiah, N. Comparative efficacy of pomegranate juice, pomegranate rind powder extract and BHT as antioxidants in cooked chicken patties. Meat Sci. 2008, 80, 304–308. [Google Scholar] [CrossRef]
- Karre, L.; Lopez, K.; Getty, K.J.K. Natural antioxidants in meat and poultry products. Meat Sci. 2013, 94, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Hang, D.; Mcmillin, K.W.; Godber, J.S. Hemoglobin, myoglobin and total pigments in beef and chicken muscles: Chromatographic determination. J. Food Sci. 1994, 59, 1279–1282. [Google Scholar]
- Richards, M.P.; Dettmann, M.A. Comparative analysis of different hemoglobins: Autoxidation, reaction with peroxide and lipid oxidation. J. Agric. Food Chem. 2003, 51, 3886–3891. [Google Scholar] [CrossRef] [PubMed]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V. Mechanisms of oxidative processes in meat and toxicity induced by postprandial degradation products: Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 96–123. [Google Scholar] [CrossRef]
- Love, J.D.; Pearson, A.M. Lipid oxidation in meat and meat products. A review. J. Am. Oil Chem. Soc. 1971, 48, 547–549. [Google Scholar] [CrossRef]
- Cheng, J.; Ockerman, H.W. Effect of phosphate with tumbling on lipid oxidation of precooked roast beef. Meat Sci. 2003, 65, 1353–1359. [Google Scholar] [CrossRef]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent Trends in the use of natural antioxidants for meat and meat products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef]
- Branen, A.L. Toxicology and biochemistry of butylated hydroxy anisole and butylated hidroxy toluene. J. Am. Oil Chem Soc. 1975, 52, 59–63. [Google Scholar] [CrossRef]
- Lindenschmidt, R.C.; Tryka, A.F.; Goad, M.E.; Witschi, H.P. The effects of dietary butylated hydroxytoluene on liver and colon tumor development in mice. Toxicology 1986, 38, 151–160. [Google Scholar] [CrossRef]
- Cieslik, E.; Geda, A.; Adamus, W. Contents of polyphenols in fruit and vegetables. Food Chem. 2006, 94, 135–142. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Alvarez, J.A. Spices as functional foods. Crit. Rev. Food Sci. Nutr. 2010, 51, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Chávez, L.M.; Valdivia-López, M.A.; Aburto-Juárez, M.L.; Tecante, A. Chemical characterization of the lipid fraction of Mexican chia seed (Salvia hispanica L.). Intern. J. Food Prop. 2008, 11, 687–697. [Google Scholar] [CrossRef]
- Ixtaina, V.Y.; Martínez, M.L.; Spotorno, V.; Mateo, C.M.; Maestri, D.M.; Diehl, B.W.K. Characterization of chia seed oils obtained by pressing and solvent extraction. J. Food Compos. Anal. 2011, 24, 166–174. [Google Scholar] [CrossRef]
- Capitani, M.I.; Spotorno, V.; Nolasco, S.M.; Tomás, M.C. Physicochemical and functional characterization of by-products from chia (Salvia hispanica L.) seeds of Argentina. LWT-Food Sci. Technol. 2012, 45, 94–102. [Google Scholar] [CrossRef]
- Marineli, R.S.; Moraes, E.A.; Lenquiste, S.A.; Godoy, A.T.; Eberlin, M.N.; Marostica Junior, M.R. Chemical characterization and antioxidant potential of Chilean chia seeds and oil (Salvia hispanica L.). LWT Food Sci. Technol. 2014, 59, 1304–1310. [Google Scholar] [CrossRef]
- Dini, I.; Tenore, G.C.; Dini, A. Antioxidant compounds content and antioxidant activity before and after cooking in sweet and bitter Chenopodium quinoa seeds. LWT-Food Sci. Technol. 2010, 43, 447–451. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, K.; Yao, Y.; Chen, Y.; Guo, H.; Ren, G.; Yang, X.; Li, J. Antioxidan, anti-inflamatory, and antitumor activities of phenolic compounds from white, red, and black Chenopodium quinoa seed. Cereal Chem. 2020, 97, 703–713. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, E.C.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Ayerza, R.; Coates, W. Influence of environment on growing period and yield, protein, oil, and α−linolenic content of three chia (Salvia hispanica L.) selections. Ind. Crops Prod. 2009, 30, 321–324. [Google Scholar] [CrossRef]
- Mezadri, T.; Villaño, D.; Fernández-Pachón, M.S.; García-Parrilla, M.C.; Troncoso, A.M. Antioxidant compounds and antioxidant activity in acerola (Malpighia emarginata DC.) fruits and derivatives. J. Food Compos. Anal. 2008, 21, 282–290. [Google Scholar] [CrossRef]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- Cho, K.M.; Ha, T.J.; Lee, Y.B.; Seo, W.D.; Kim, J.Y.; Ryu, H.W.; Jeong, S.H.; Kang, Y.M.; Lee, J.H. Soluble phenolics and antioxidant properties of soybean (Glycine max L.) cultivars with varying seed coat colours. J. Funct. Foods 2013, 5, 1065–1076. [Google Scholar] [CrossRef]
- Alcântara, M.A.; Polari, I.L.B.; Meireles, B.R.L.A.; Alcântara de Lima, A.E.; da Silva, J.C., Jr.; Vieira, E.A.; dos Santos, N.A.; Cordeiro, A.M.T.M. Effect of the solvent composition on the profile of phenolic compounds extracted from chia seeds. Food Chem. 2019, 275, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.P.; Anunciaçao, P.C.; Matyelka, J.C.S.; Lucia, C.M.D.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Chemical composition of Brazilian chia seeds grown in different places. Food Chem. 2017, 221, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Serpen, A.; Capuano, E.; Fogliano, V.; Gökmen, V. A new procedure to measure the antioxidant activity of insoluble food components. J. Agric. Food Chem. 2007, 55, 7676–7681. [Google Scholar] [CrossRef] [PubMed]
- Serpen, A.; Gökmen, V.; Fogliano, V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Sargy, S.C.; Silva, B.C.; Santos, H.M.C.; Montanher, P.F.; Boeing, J.S.; Santos, O.O., Jr.; Souza, N.E.; Visentainer, J.V. Antioxidant capacity and chemical composition in seeds rich in omega-3: Chia, flax and perilla. Food Sci. Technol. 2013, 33, 541–548. [Google Scholar] [CrossRef]
- Oliveira-Alves, S.C.; Vendramini-Costa, D.B.; Cazarin, C.B.B.; Maróstica Júnior, M.B.; Ferrerira, J.P.B.; Silva, A.B.; Prado, M.A.; Bronze, M.R. Characterization of phenolic compounds in chia (Salvia hispanica L.) seeds, fiber flour and oil. Food Chem. 2017, 232, 295–305. [Google Scholar] [CrossRef]
- Brend, Y.; Galili, I.; Badani, H.; Hovav, R.; Galili, S. Total phenolic content and antioxidant activity or red and yellow quinoa (Chenopodium quinoa Willd.) seeds as affected by baking and cooking conditions. Food Nutr. Sci. 2012, 3, 1150–1155. [Google Scholar] [CrossRef]
- Pasko, P.; Barton, H.; Zagrodzki, P.; Gorinstein, S.; Folta, M.; Zachwieja, Z. Anthocyanins, total polyphenols and antioxidant activity capacity of coloured quinoa seeds (Chenopodium quinoa Willd.) from Peruvian Altiplano. Food Chem. 2009, 115, 994–998. [Google Scholar]
- Valencia, Z.; Cámara, F.; Ccapa, K.; Catacora, P.; Quispe, F. Bioactive compounds and antioxidant activity from peruvian quinoa seeds (Chenopodium quinoa W.). Rev. Soc. Quim. Perú 2017, 83, 16–29. [Google Scholar]
- Sobota, A.; Swieca, M.; Gesinski, K.; Wirkijowska, A.; Bochnak, J. Yellow-coated quinoa (Chenopodium quinoa Willd) - physicochemical, nutritional and antioxidant properties. J. Sci. Food Agric. 2020, 100, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Repo-Carrasco-Valencia, R.; Hellström, J.K.; Pihlava, J.M.; Matilla, P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus). Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Repo-Carrasco-Valencia, R.; Zelada, C.R.E. Determinación de la capacidad antioxidante y compuestos fenólicos de cereales andinos: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicause) y kiwicha (Amaranths caudatus). Rev. Soc. Quim. Perú 2008, 74, 85–99. [Google Scholar]
- Díaz-Valencia, Y.K.; Alca, J.A.; Calori-Domingues, M.A.; Zanabria-Galvez, S.J.; Da Cruz, S.H. Nutritional composition, total phenolic compounds and antioxidant activity of quinoa (Chenopodium quinoa Willd.) of different colours. Nova Biotechnol. Chi. 2018, 17, 74–85. [Google Scholar] [CrossRef]
- Farajzadeht, Z.; Shakerian, A.; Rahimi, E.; Bagheri, M. Chemical, antioxidant, total phenolic and flavonoid components and antimicrobial effects of different species of quinoa seeds. Egypt. J. Vet. Sci. 2020, 51, 43–54. [Google Scholar] [CrossRef]
- Vázquez-Luna, A.; Fuentes, F.; Rivadeneyra, E.; Hernández, C.; Díaz-Sobac, R. Nutrimental content and functional properties of quinoa flour from Chile and Mexico. Int. J. Agric. Nat. Resour. 2019, 46, 144–153. [Google Scholar] [CrossRef]
- Sohaimy, S.A.; Mohamed, S.E.; Shehata, M.G.; Mehany, T.; Zaitoun, M.A. Compositional analysis and functional characteristics of quinoa flour. Ann. Res. Rev. Biol. 2018, 22, 1–11. [Google Scholar] [CrossRef]
- Aguilar, J.; Miano, A.C.; Obregón, J.; Soriano-Colchado, J.; Barraza-Jáuregui, G. Malting process as an alternative to obtain high nutritional quality quinoa flour. J Cereal Sci. 2019, 90, 102858. [Google Scholar] [CrossRef]
- Caruso, M.C.; Favati, F.; Di Cairano, M.; Galgano, F.; Labella, R.; Scarpa, R.; Condelli, N. Shelf-life evaluation and nutraceutical properties of chia seeds from a recent long-day flowering genotype cultivated in Mediterranean área. LWT-Food Sci. Technol. 2018, 87, 400–405. [Google Scholar] [CrossRef]
- Porras-Loaiza, P.; Jiménez-Munguía, M.T.; Sosa-Morales, M.E.; Palou, E.; López-Malo, A. Physical properties, chemical characterization and fatty acid composition of Mexican chia (Salvia hispánica L.) seeds. Int. J. Food Sci. Technol. 2014, 49, 571–577. [Google Scholar] [CrossRef]
- Tunçil, Y.E.; Çelik, O.F. Total phenolic contents, antioxidant and antibacterial activities of chia seeds (Salvia hispanica L.) having different coat color. Akademik Ziraat Dergisi 2019, 8, 113–120. [Google Scholar] [CrossRef]
- Antonini, E.; Torri, L.; Piochi, M.; Cabrino, G.; Meli, M.A.; De Bellis, R. Nutritional, antioxidant and sensory properties of functional beef burgers formulated with chia seeds and goji puree, before and after in vitro digestion. Meat Sci. 2020, 161, 108021. [Google Scholar] [CrossRef]
- Dick, M.; Pagno, C.H.; Haas-Costa, T.M.; Gomaa, A.; Subirade, M.; de Oliveira Rios, A.; Hickmann-Flîres, S. Edible films base on chia flour: Development and characterization. Polym. Sci. 2016, 133, 42455. [Google Scholar]
- Xuan, T.D.; Ganggiang, G.; Minh, T.N.; Quy, T.N.; Dang, T. An overview of chemical profiles, antioxidant and antimicrobial activities of commercial vegetable edible oils marketed in Japan. Foods 2018, 7, 21. [Google Scholar] [CrossRef]
- Guiotto, E.N.; Ixtaina, V.Y.; Nolasco, S.M.; Tomás, M.C.M. Effect of storage conditions and antioxidants on the oxidative stability of sunflower-chia oil blends. J. Am. Oil Chem. Soc. 2014, 91, 767–776. [Google Scholar] [CrossRef]
- Tuberoso, C.; Kowalczyk, A.; Sarritzu, E.; Cabras, P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 2007, 103, 1494–1501. [Google Scholar] [CrossRef]
- Dąbrowski, G.; Konopka, I.; Czaplicki, S. Supercritical CO2 extraction in chia oils production: Impact of process duration and co-solvent addition. Food Sci. Biotechnol. 2018, 27, 677–686. [Google Scholar] [CrossRef]
- Stikic, R.I.; Milincic, D.D.; Kostic, A.Z.; Jovanovic, Z.B.; Gasic, U.M.; Tesic, Z.L.; Djordjevic, N.Z.; Savic, S.K.; Czekus, B.G.; Pesic, M.B. Polyphenolic profiles, antioxidant, and in vitro anticancer activities of the seeds of Puno and Titicaca quinoa cultivars. Cereal Chem. 2020, 97, 626–633. [Google Scholar] [CrossRef]
- Gao, K.; Zhang, W.T.; Tang, Y.; Li, X.H.; Rong, T. Phenolic composition and antioxidant activities in Canada’s quinoa. Food Ferment. Ind. 2016, 42, 107–113. [Google Scholar]
- Lin, M.; Han, P.; Li, Y.; Wang, W.; Lai, D.; Zhou, L. Quinoa secondary metabolites and their biological activities or functions. Molecules 2019, 24, 2512. [Google Scholar] [CrossRef]
- Tang, Y.; Tsao, R. Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: A review. Mol. Nutr. Food Res. 2017, 61, 1600767. [Google Scholar] [CrossRef] [PubMed]
- Chacaliaza-Rodríguez, L.; Espinoza-Begazo, G.; Ramos-Escudero, F.; Servan, K. Proximate chemical composition and content of biologically active components in leaves of two quinoa cultivars (Salcedo and Altiplano) produced in Peru. Res. J. Med. Plants. 2016, 10, 450–456. [Google Scholar]
- Carciochi, R.A.; Manrique, G.D.; Dimitrov, K. Changes in phenolic composition and antioxidant activity during germination of quinoa seeds (Chenopodium quinoa Willd.). Intern. Food Res. J. 2014, 21, 767–773. [Google Scholar]
- Ng, S.C.; Anderson, A.; Coker, J.; Ondrus, M. Characterization of lipid oxidantion proudcts in quinoa (Chenopodium quinoa). Food Chem. 2007, 101, 185–192. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Liu, R.; Hernández, M.; Draves, J.; Marcone, M.F.; Tsao, R. Assessing the fatty acid, carotenoid, and tocopherol compositions of amaranth and quinoa seeds grown in Ontario and their overall contribution to nutritional quality. J. Agric. Food Chem. 2016, 64, 1103–1110. [Google Scholar] [CrossRef]
- Gandía-Herrerro, F.; Escribano, J.; García-Carmona, F. The role of phenokic hydroxy groups in the free radical scavenging activity of betalains. J. Nat. Prod. 2009, 72, 1142–1146. [Google Scholar] [CrossRef]
- Gandía-Herrerro, F.; Escribano, J.; García-Carmona, F. Biological activities of plant pigments betalains. Crit. Rev. Food Sci. Nutr. 2016, 56, 937–945. [Google Scholar] [CrossRef]
- Nsimba, R.Y.; Kikizaki, H.; Konishi, Y. Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. seeds. Food Chem. 2008, 106, 760–766. [Google Scholar] [CrossRef]
- 7Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar]
- Pekkarinen, S.S.; Heinonen, I.M.; Hopia, A.I. Flavonoid quercetin, myrcetin, kaemferol and (+)-catechin as antioxidants in methyl linoleate. J. Sci. Food Agric. 1999, 79, 499–506. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals and antioxidants: A personal view. Nutr. Rev. 1994, 52, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Ovando, A.; Rosado-Rubio, G.; Chel-Guerrero, L.; Betancur-Ancona, D. Physicochemical properties of a fibrous fraction from chia (Salvia hispánica L.). LWT-Food Sci. Technol. 2009, 42, 168–173. [Google Scholar]
- Souza, A.H.P.; Gohara, A.K.; Rotta, E.M.; Chaves, M.A.; Silva, C.M.; Dias, L.F.; Gomes, S.T.M.; Souza, N.E.; Matsushita, M. Effect of the addition of chia’s by-product on the composition of fatty acids in hamburgers through chemometric methods. J. Sci. Food Agric. 2015, 95, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Zambrano, P.; Riera-González, G.; Cruz-Viera, L. Quinoa as gelling agent in a mortadela formulation. Int. Food Res. J. 2019, 26, 1069–1077. [Google Scholar]
- Fernández-López, J.; Lucas-González, R.; Viuda-Martos, M.; Sayas-Barberá, M.E.; Ballester-Sánchez, J.; Haros, C.M.; Martínez-Mayoral, A.; Pérez-Alvarez, J.A. Chemical and technological properties of bologna-type sausages with added black quinoa wet-milling coproducts as binder replacer. Food Chem. 2020, 310, 125936. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, J.; Lucas-González, R.; Roldán-Verdú, A.; Viuda-Martos, M.; Sayas-Barberá, E.; Ballester-Sánchez, J.; Haros, C.M.; Pérez-Alvarez, J.A. Effects of black quinoa wet-milling coproducts on the quality properties of bologna-type sausages during cold storage. Foods 2020, 9, 274. [Google Scholar] [CrossRef]
- Fernández-López, J.; Pérez-Alvarez, J.A.; Fernández-López, J.A. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem. 1997, 59, 345–353. [Google Scholar] [CrossRef]
- Pintado, T.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Strategies for incorporation of chia (Salvia hispánica L.) in frankfurters as a health-promoting ingredient. Meat Sci. 2016, 114, 75–84. [Google Scholar] [CrossRef]
- Herrero, A.M.; Ruiz-Capillas, C.; Pintado, T.; Carmona, P.; Jiménez-Colmenero, F. Infrared spectroscopy used to determine effects of chia and olive oil incorporation strategies on lipid structure of reduced-fat frankfurters. Food Chem. 2017, 221, 1333–1339. [Google Scholar] [CrossRef]
- de Oliveira-Paula, M.M.; Gonçalves-Silva, J.R.; de Oliveira, K.L.; Massingue, A.; Mendes-Ramos, E.; Benevenuto, A.A., Jr.; Louzalda-Silva, M.H.; Olmi-Silva, V.R. Technological and sensory characteristics of hamburgers added with chia seed as fat replacer. Ciência Rural. 2019, 49, 320190090. [Google Scholar]
- Heck, R.T.; Lucas, B.N.; Dos Santos, D.J.P.; Pinton, M.B.; Fagundes, M.B.; Etchepare, M.A.; Cichoski, A.J.; de Menezes, C.R.; Barin, J.S.; Wagner, R.; et al. Oxidative stability of burgers containing chia oil microparticles enriched with rosemary by green-extraction techniques. Meat Sci. 2018, 146, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Lucas-González, R.; Rodán-Verdú, A.; Sayas-Barberá, E.; Fernández-López, J.; Pérez-Alvarez, J.A.; Viuda-Martos, M. Assessment of emulsion gels formulated with chestnut (Castanea sativa M.) flour and chia (Salvia hispanica L.) oil as partial fat replacers in pork burger formulation. J. Sci. Food Agric. 2020, 100, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Heck, R.T.; Saldaña, E.; Lorenzo, J.M.; Correa, L.P.; Fagundes, M.B.; Cichoski, A.J.; de Menezes, C.R.; Wagner, R.; Campagnol, P.C.B. Hydrogelled emulsion from chia and linseed oils: A promising strategy to produce low-fat burgers with a healthier lipid profile. Meat Sci. 2019, 156, 174–182. [Google Scholar] [CrossRef]
- de Carvalho, F.A.L.; Lorenzo, J.M.; Pateiro, M.; Bermúdez, R.; Purriños, L.; Trindade, M.A. Effect of guarana (Paullinia cupana) seed and pitanga (Eugenia uniflora L.) leaf extracts on lamb burgers with fat replacement by chia oil emulsion during shelf life storage at 2 °C. Food Res. Int. 2019, 125, 108554. [Google Scholar] [CrossRef] [PubMed]
- Shokry, A.M. The usage of quinoa flour as a potential ingredient in production of meat burger with functional properties. Middle East J. Appl. Sci. 2016, 6, 1128–1137. [Google Scholar]
- Özer, C.O.; Seçen, S.M. Effects of quinoa flour on lipid and protein oxidation in raw and cooked beef burger during long term frozen storage. Food Sci. Technol. 2018, 38, 221–227. [Google Scholar] [CrossRef]
- Baioumy, A.A.; Bobreneva, I.V.; Tvorogova, A.A.; Shobanova, T.V. Possibility of using quinoa seeds (Chenopodium quinoa) in meat products and its impact on nutritional and organoleptic characteristics. Biosci. Res. 2018, 15, 3307–3315. [Google Scholar]
- Bagdatli, A. The influence of quinoa (Chenopodium quinoa Willd.) flour on the physicochemical, textural and sensorial properties of beef meatball. Ital. J. Food Sci. 2018, 30, 280–288. [Google Scholar]
- Pintado, T.; Herrero, A.M.; Jiménez-Colmenero, F.; Pasqualin-Cavalheiro, C.; Ruiz-Capillas, C. Chia and oat emulsion gels as new animal fat replacers and healthy bioactive sources in fresh sausage formulation. Meat Sci. 2018, 135, 6–13. [Google Scholar] [CrossRef]
- de Carvalho, F.A.L.; Munekata, P.E.S.; Pateiro, M.; Campagnol, P.C.B.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Effect of replacing backfat with vegetable oils during the shelf-life of cooked lamb sausages. LWT-Food Sci. Technol. 2020, 122, 109052. [Google Scholar] [CrossRef]
- Barros, J.C.; Munekata, P.E.S.; Pires, M.A.; Rodrigues, I.; Andaloussi, O.S.; Rodrigues, C.E.; Trindade, M.A. Omega-3- and fibre-enriched chicken nuggets by replacement of chicken skin with chia (Salvia hispanica L) flour. LWT-Food Sci. Technol. 2018, 90, 283–289. [Google Scholar] [CrossRef]
- Verma, A.K.; Rakjumar, V.; Kumar, S. Effect of amaranth and quinoa seed flour on rheological and physicochemical properties of goat meat nuggets. J. Food Sci. Technol. 2019, 56, 5027–5035. [Google Scholar] [CrossRef] [PubMed]
- Pires, M.A.; Barros, J.C.; Rodrigues, I.; Munekata, P.E.S.; Trindade, M.A. Improving the lipid profile of bologna type sausages with Echium (Echium plantagineum L.) oil and chia (Salvia hispanica L.) flour. LWT Food Sci. Technol. 2020, 119, 108907. [Google Scholar] [CrossRef]
- de Souza-Paglarini, C.; de Figueiredo-Furtado, G.; Honório, A.R.; Mokarzel, L.; da Silva-Vidal, V.A.; Badan-Ribeiro, A.P.; Lopes Cunha, R.; Rodrigues-Pollonio, M.A. Functional emulsion gels as pork back fat replacers in Bologna sausage. Food Struct. 2019, 20, 100105. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-González, R.; Sayas-Barberá, E.; Fernández-López, J.; Pérez-Alvarez, J.A.; Viuda-Martos, M. Quinoa (Chenopodium quinoa Willd) paste as partial fat replacer in the development of reduced fat cooked meat product type pâté: Effect on quality and safety. CyTA-J. Food 2018, 16, 1079–1088. [Google Scholar] [CrossRef]
- Fernández-Diez, A.; Caro, I.; Castro, A.; Salvá, B.K.; Ramos, D.D.; Mateo, J. Partial fat replacement by boiled quinoa on the quality characteristics of a dry-cured sausage. J. Food Sci. 2016, 81, C1891–C1898. [Google Scholar]
- Fernández-López, J.; Lucas-González, R.; Viuda-Martos, M.; Sayas-Barberá, M.E.; Navarro, C.; Haros, C.M.; Pérez-Alvarez, J.A. Chia (Salvia hispánica L.) products as ingredients for reformulating frankfurters: Effects on quality properties and shelf-life. Meat Sci. 2019, 156, 139–145. [Google Scholar] [CrossRef]
- Scapin, G.; Schimdt, M.M.; Prestes, R.C.; Ferreira, S.; Silva, A.F.C.; da Rosa, C.S. Effect of extract of chia seed (Salvia hispanica) as an antioxidant in fresh pork sausage. Intern. Food Res. J. 2015, 3, 1195–1202. [Google Scholar]
- Ayerza, R.; Coates, W. Ground chia seeds and chia oil effects on plasma lipids and fatty acids in the rat. Nutr. Res. 2005, 25, 995–1003. [Google Scholar] [CrossRef]
- Cofrades, S.; Santos-López, J.A.; Freire, M.; Benedí, J.; Sánchez-Muniz, F.J. Oxidative stability of meat systems made with W1/O/W2 emulsions prepared with hydroxytyrosol and chia oil as lipid phase. LWT Food Sci. Technol. 2014, 59, 941–947. [Google Scholar] [CrossRef]
- Gorinstein, S.; Lojek, A.; Číž, M.; Pawelzik, E.; Delgado-Licon, E.; Medina, O.J.; Moreno, M.; Salas, I.A.; Goshew, I. Comparison of composition and antioxidant capacity of some cereals and pseudocereals. Intern. J. Food Sci. Technol. 2008, 43, 627–629. [Google Scholar] [CrossRef]
- Haros, C.M.; Wronkowska, M. Pseudocereal dry and wet milling: Processes products and applications. In Pseudocereals: Chemistry and Technology; Haros, C.M., Schoenlechner, R., Eds.; John Wiley & Sons, Ltd.: Oxfork, UK, 2017; pp. 163–183. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
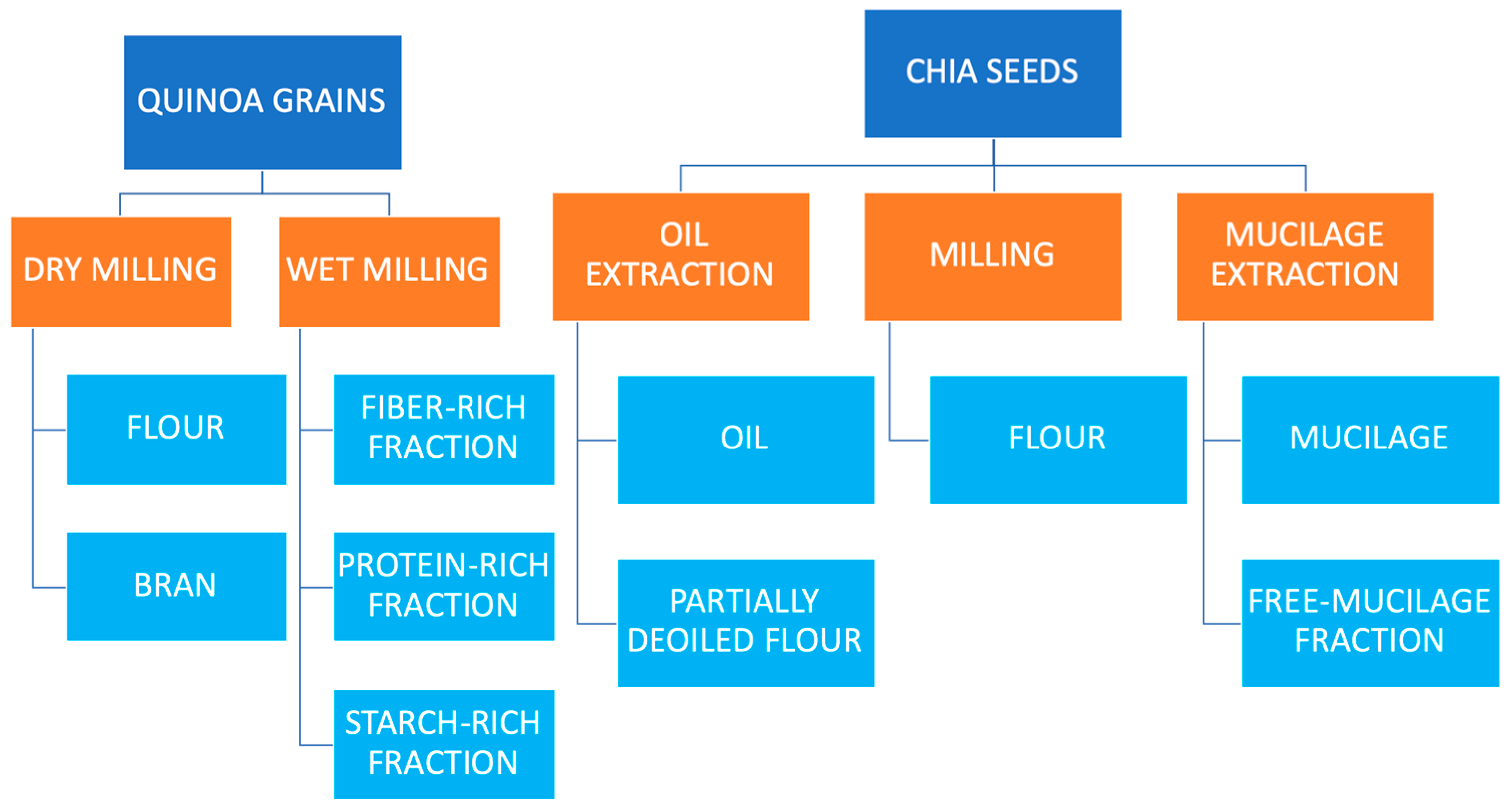
| Origin | Total Phenol Content (TPC) | Antioxidant Activity | Extraction | References | ||
|---|---|---|---|---|---|---|
| (mg GAE/g) | DPPH (mg TE/g) | FRAP (mg TE/g) | Protocol | |||
| QUINOA | ||||||
| seeds (white, red, and black) | Bolivia | 5.0–6.6 | 3.4 | 2.7 | double extraction (methanol/water and acetone/water) | Ballester-Sánchez et al. [12] |
| seeds (colored) | Peru | 1.2–3.4 | - | - | double extraction (methanol/water and acetone/water) | Abderrahim et al. [26] |
| seeds (red and yellow) | - | 1.4–2.1 | - | 6–9 | double extraction (methanol/water and acetone/water) | Brend et al. [61] |
| seeds | Bolivia | 3.8 | 9.7 | - | double extraction (methanol/water and acetone/water) | Pasko et al. [62] |
| seeds (white and colored) | Bolivia and Perú | 3.9–4.2 | 1.9–5.0 | 2.4–4.6 | double extraction (methanol/water and acetone/water) | Pellegrini et al. [4] |
| seeds (red) | Bolivia | 4.9 | 6.0 | - | double extraction (methanol/water and acetone/water) | Ballester Sánchez et al. [10] |
| seeds (white, red, and black) | Andean Region and Canada | 2.0–5.2 | 1.3–2.8 | 2.0–8.8 | one extraction (acetone/water) | Tang et al. [18] |
| seeds | Peru | 0.8–3.4 | 1.6–2.2 | - | double extraction (ethanol/water and methanol/water) | Valencia et al. [63] |
| seeds (white, red, and black) | Bolivia | 5.1–6.1 | 1.8–3.5 | - | double extraction (methanol/water) | Liu et al. [49] |
| seeds (yellow) | Poland, Denmark, Chile and Argentine | 7.1–10.6 | - | - | triple extraction (methanol/water, methanol/water and acetone/water) | Sobota et al. [64] |
| seeds | Peru | 1.4–1.9 | 0.1–2.4 | - | one extraction (ethanol/water) | Repo-Carrasco-Valencia et al. [65] Repo-Carrasco-Valencia and Celada [66] |
| seeds (white, red, and black) | Peru | 0.6–1.0 | 0.5–1.5 | - | one extraction (ethanol/water) | Díaz-Valencia et al. [67] |
| seeds | Iran | 0.2–0.4 | - | - | one extraction (ethanol/water) | Farajzadeh et al. [68] |
| seeds | Ecuador and Peru | 7.7–8.6 | 0.7–1.7 | 0.7–2.2 | triple extraction (methanol/water) | Dini et al. [48] |
| flour (white) | Mexico | 1.8–3.2 | - | - | one extraction (methanol/water) | Vazquez-Luna et al. [69] |
| flour (white) | Egypt | 0.2 | - | - | one extraction (methanol/water) | Sohaimy et al. [70] |
| flour (white, red, and black) | Peru | 0.6–1.0 | - | - | one extraction (ethanol/water) | Aguilar et al. [71] |
| malted flour (white, red, and black) | Peru | 0.9–1.5 | - | - | one extraction (ethanol/water) | Aguilar et al. [71] |
| fiber-rich fraction by wet-milling (red) | Bolivia | 3.8 | 3.6 | 3.6 | double extraction (methanol/water and acetone/water) | Ballester Sánchez et al. [10] |
| fiber-rich fraction by dry-milling (red) | Bolivia | 6.6 | 5.2 | 6.9 | double extraction (methanol/water and acetone/water) | Ballester Sánchez et al. [10] |
| CHIA | ||||||
| seeds | Bolivia and Peru | 4.1 | 5.6 | 70.1 | double extraction (methanol/water and acetone/water) | Fernández-López et al. [22] |
| seeds | Australia | 2.4 | - | - | ethanol/water | Ding et al. [21] |
| seeds | Bolivia and Peru | 3.9 | 5.4 | 71.8 | double extraction (methanol/water and acetone/water) | Pellegrini et al. [3] |
| seeds | Bolivia and Chile | 1.2 | - | 18.5 | double extraction (methanol/water) | Oliveira-Alves et al. [60] |
| seeds | Chile | 0.94 | 109.2 | - | ethanol | Marinelli et al. [47] |
| seeds | Mexico | 0.88–0.92 | - | - | ethanol | Reyes-Caudillo et al. [23] |
| seeds | Italian | 1.8 | - | - | acetonitrile/acetic acid solution | Caruso et al. [72] |
| seeds | Mexico | 0.5–0.7 | - | - | ethanol | Porras-Loaiza et al. [73] |
| seeds | Mexico | 1.64 | - | - | triple (methanol/water) | Martínez-Cruz and Paredes-López [25] |
| seeds | Argentine | 3.4 | 49.37 | methanol/water | Tuncil and Celik [74] | |
| seeds | Italy | 1.5 | 1.6 | - | ethanol/water | Antonini et al. [75] |
| seeds | Brazil | 10.1–60.9 | 2.5–95.1 | 5.1–247.6 | water/ethanol/acetone (alone and different mixtures) | Alcântara et al. [55] |
| flour | Brazil | 4.8 | - | - | acetonitrile/acetic acid solution | Dick et al. [76] |
| flour | Mexico | 7.9 | - | - | acetonitrile/acetic acid solution | Dick et al. [76] |
| oil | Bolivia and Chile | 0.02 | - | 0.2 | methanol/water | Oliveira-Alves et al. [60] |
| oil | Japan | 4.9 | 6.1 IC50 (mg/L) | - | methanol | Xuan et al. [77] |
| partially-deioled flour | Bolivia and Chile | 1.1 | - | 17.2 | double extraction (methanol/water) | Oliveira-Alves et al. [60] |
| partially-deioled flour | Argentine | 2.2 | - | - | double extraction (acetone/water) | Aranibar et al. [13] |
| partially-deioled flour | Bolivia and Peru | 5.0 | 7.0 | 81.0 | double extraction (methanol/water and acetone/water) | Fernández-López et al. [22] |
| partially-deioled flour | Bolivia and Peru | 4.9 | 7.2 | 80.9 | double extraction (methanol/water and acetone/water) | Pellegrini et al. [3] |
| Meat Product | Chia/Quinoa | Effect on Lipid Oxidation (TBARs Value) | References |
|---|---|---|---|
| Frankfurter | chia seeds | 35% reduction after 21 days refrigerated storage | Fernández-López et al. [9] |
| chia flour | 35% reduction after 21 days refrigerated storage | Fernández-López et al. [9] | |
| chia flour | 300% increase (values < 0.2 mg MA/kg) | Pintado et al. [101] | |
| chia flour | not evaluated | Herrero et al. [102] | |
| deioled chia flour | 35% reduction after 21 days refrigerated storage | Fernández-López et al. [9] | |
| chia oil (O/W emulsion) | 250% increase (values < 0.2 mg MA/kg) | Pintado et al. [101] | |
| chia oil (O/W emulsion) | not evaluated | Herrero et al. [102] | |
| chia oil (emulsion gel) | 275% increase (values < 0.2 mg MA/kg) | Pintado et al. [101] | |
| chia oil (emulsion gel) | not evaluated | Herrero et al. [102] | |
| Ham-like product | chia seeds | 30% reduction | Ding et al. [21] |
| Burgers | chia seeds | up to 68% reduction | de Oliveira-Paula et al. [103] |
| chia seeds | 50% reduction | Antonini et al. [75] | |
| deffated chia flour | increase TBARs | Souza et al. [96] | |
| chia oil | 25% increase in raw burgers, 11% after cooking | Heck et al. [104] | |
| chia oil (microencapsulated) | 50% increase in raw burgers, 70% after cooked | Heck et al. [104] | |
| chia oil (emulsion gel) | 30% increase | Lucas-González et al. [105] | |
| chia oil (hydrogel emulsion) | up to 350% increase (at 100% substitution level) | Heck et al. [106] | |
| chia oil (O/W emulsion) | not evaluated | De Carvalho et al. [107] | |
| quinoa flour | not evaluated | Shokry [108] | |
| quinoa flour | 20% reduction at 90 d frozen storage | Özer and Seçen [109] | |
| quinoa flour | not evaluated | Baioumy et al. [110] | |
| Meatballs | quinoa flour | not evaluated | Bagdatli [111] |
| Fresh sausages | chia emulsion gel | not evaluated | Pintado et al. [112] |
| Cooked sausages | chia oil (O/W emulsion) | 28% increase | De Carvalho et al. [113] |
| Nuggets | chia flour | not evaluated | Barros et al. [114] |
| quinoa flour | not evaluated | Verma et al. [115] | |
| Bolognas | chia flour | not evaluated | Pires et al. [116] |
| chia emulsion gel | without variation | de Souza-Paglarini et al. [117] | |
| quinoa grains | 50% reduction | Fernández-López et al. [98,99] | |
| quinoa flour | not evaluated | Vargas-Zambrano et al. [97] | |
| quinoa (fiber-rich fraction) | 15% reduction | Fernández-López et al. [98,99] | |
| Pâté | quinoa flour | 20% reduction | Pellegrini et al. [118] |
| Dry-cured sausages | quinoa grains (boiled) | decrease hexanal content | Fernández-Díaz et al. [119] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-López, J.; Viuda-Martos, M.; Sayas-Barberá, M.E.; Navarro-Rodríguez de Vera, C.; Lucas-González, R.; Roldán-Verdú, A.; Botella-Martínez, C.; Pérez-Alvarez, J.A. Chia, Quinoa, and Their Coproducts as Potential Antioxidants for the Meat Industry. Plants 2020, 9, 1359. https://doi.org/10.3390/plants9101359
Fernández-López J, Viuda-Martos M, Sayas-Barberá ME, Navarro-Rodríguez de Vera C, Lucas-González R, Roldán-Verdú A, Botella-Martínez C, Pérez-Alvarez JA. Chia, Quinoa, and Their Coproducts as Potential Antioxidants for the Meat Industry. Plants. 2020; 9(10):1359. https://doi.org/10.3390/plants9101359
Chicago/Turabian StyleFernández-López, Juana, Manuel Viuda-Martos, María Estrella Sayas-Barberá, Casilda Navarro-Rodríguez de Vera, Raquel Lucas-González, Alba Roldán-Verdú, Carmen Botella-Martínez, and Jose Angel Pérez-Alvarez. 2020. "Chia, Quinoa, and Their Coproducts as Potential Antioxidants for the Meat Industry" Plants 9, no. 10: 1359. https://doi.org/10.3390/plants9101359
APA StyleFernández-López, J., Viuda-Martos, M., Sayas-Barberá, M. E., Navarro-Rodríguez de Vera, C., Lucas-González, R., Roldán-Verdú, A., Botella-Martínez, C., & Pérez-Alvarez, J. A. (2020). Chia, Quinoa, and Their Coproducts as Potential Antioxidants for the Meat Industry. Plants, 9(10), 1359. https://doi.org/10.3390/plants9101359








