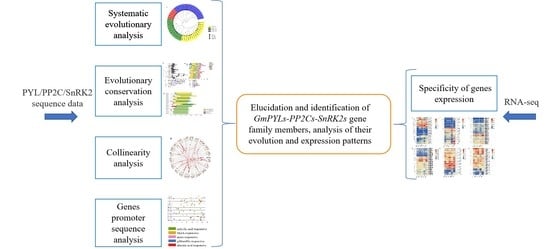
Identification, Evolutionary and Expression Analysis of PYL-PP2C-SnRK2s Gene Families in Soybean
Abstract
1. Introduction
2. Results
2.1. Identification and Nomenclature of GmPYL-PP2C-SnRK2s Gene Families
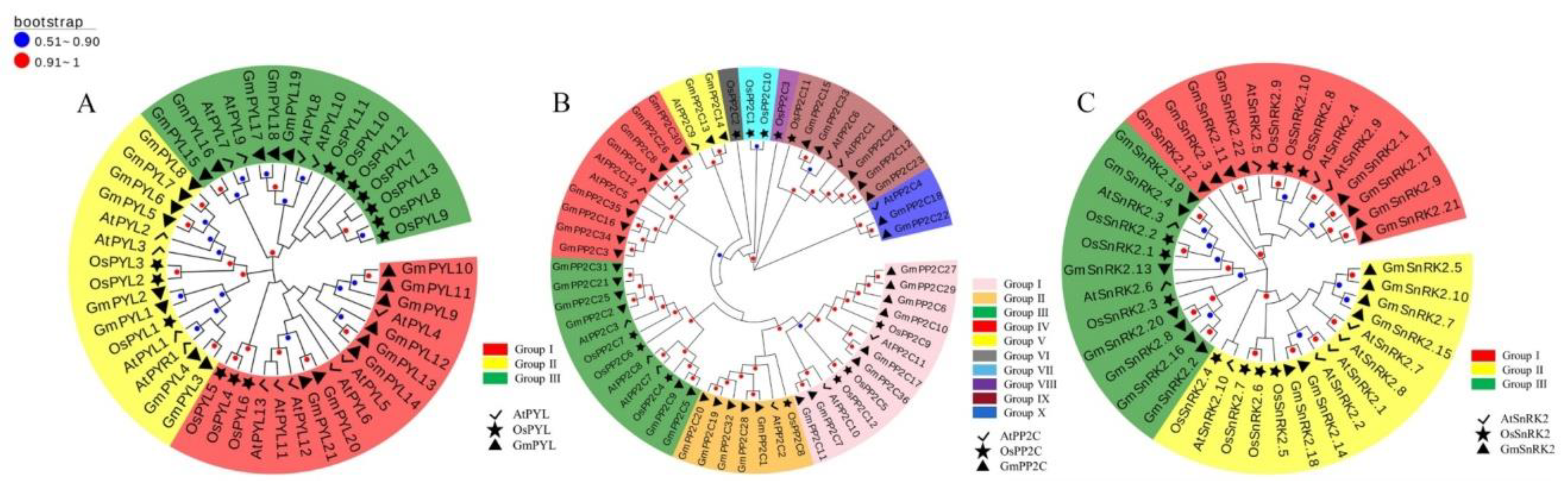
2.2. Phylogenetic Analysis of GmPYL-PP2C-SnRK2s Gene Families
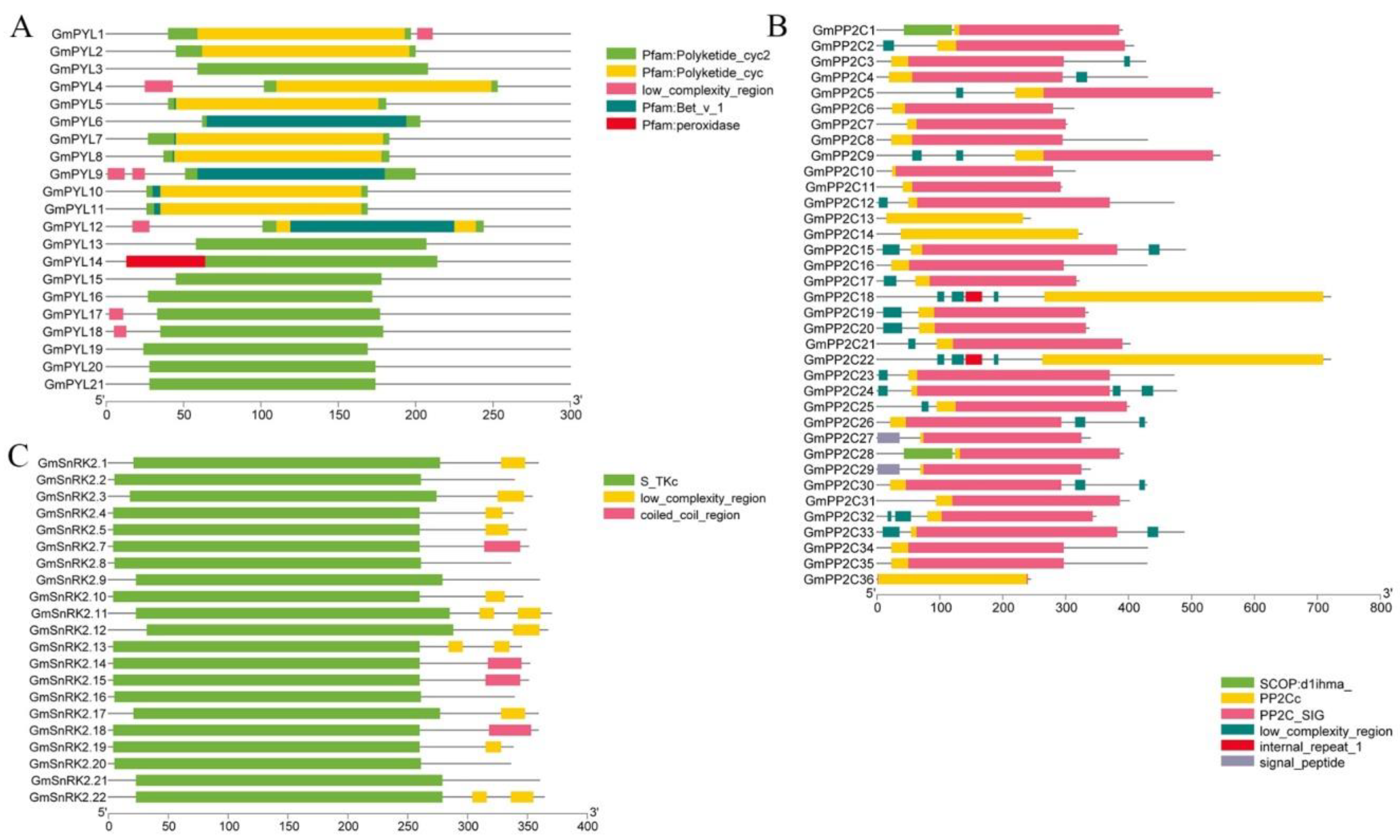
2.3. Evolutionary Conservation and Gene Structure of GmPYL-PP2C-SnRK2s
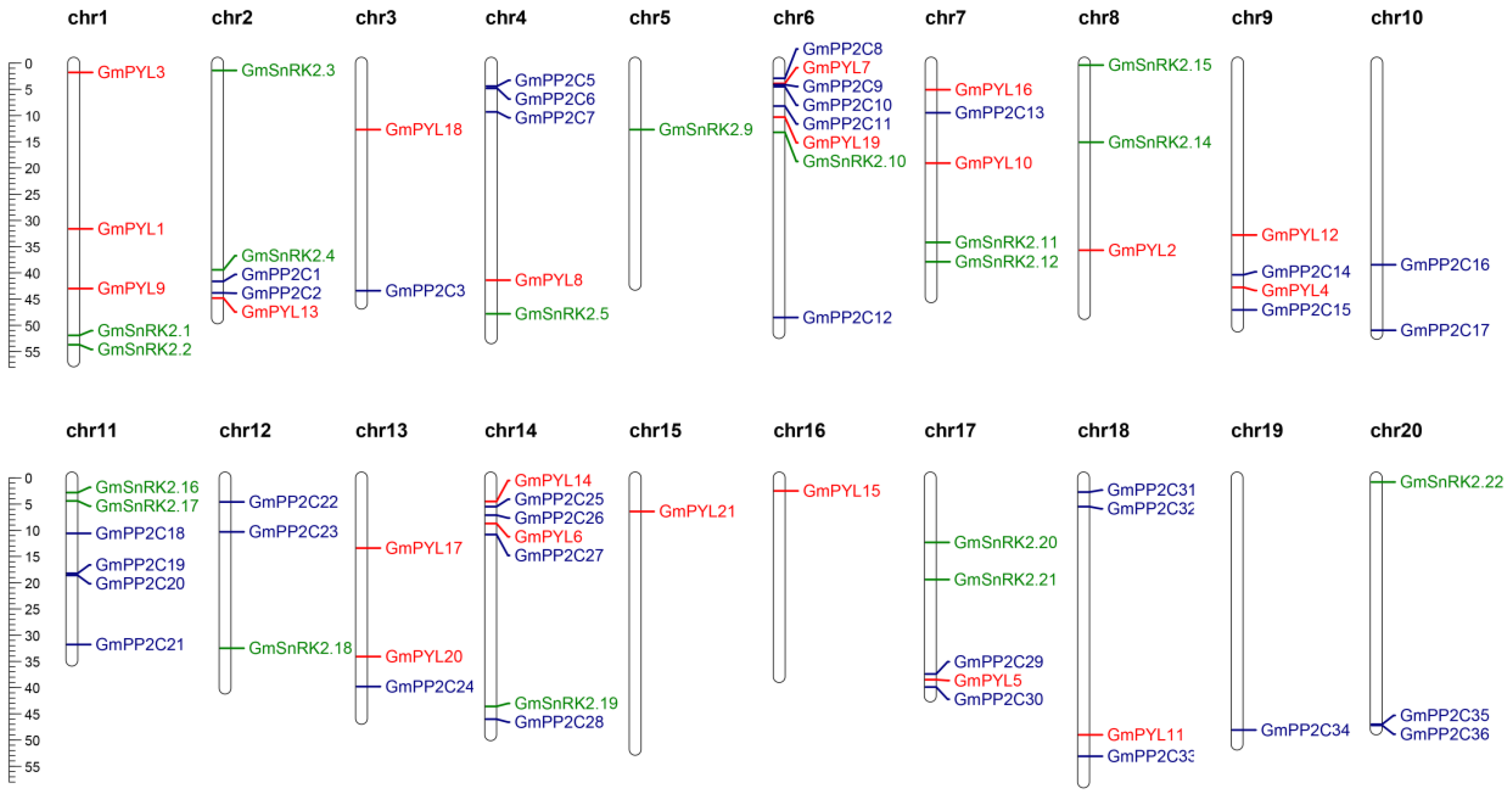
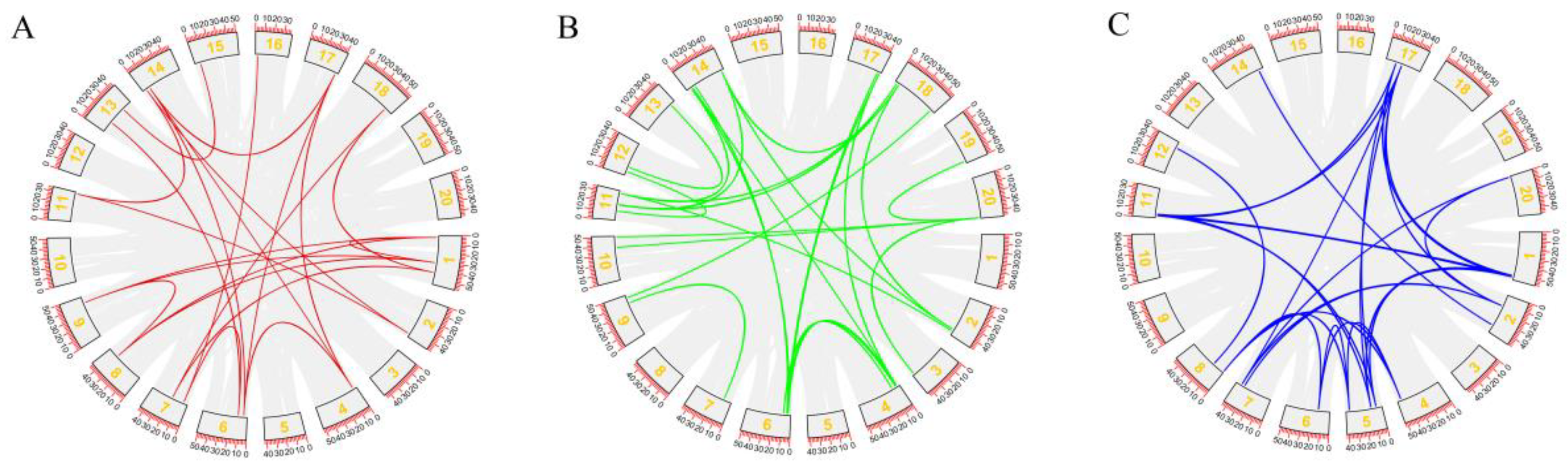
2.4. Chromosomal Localization and Collinearity Analysis of GmPYL-PP2C-SnRK2s Gene Families
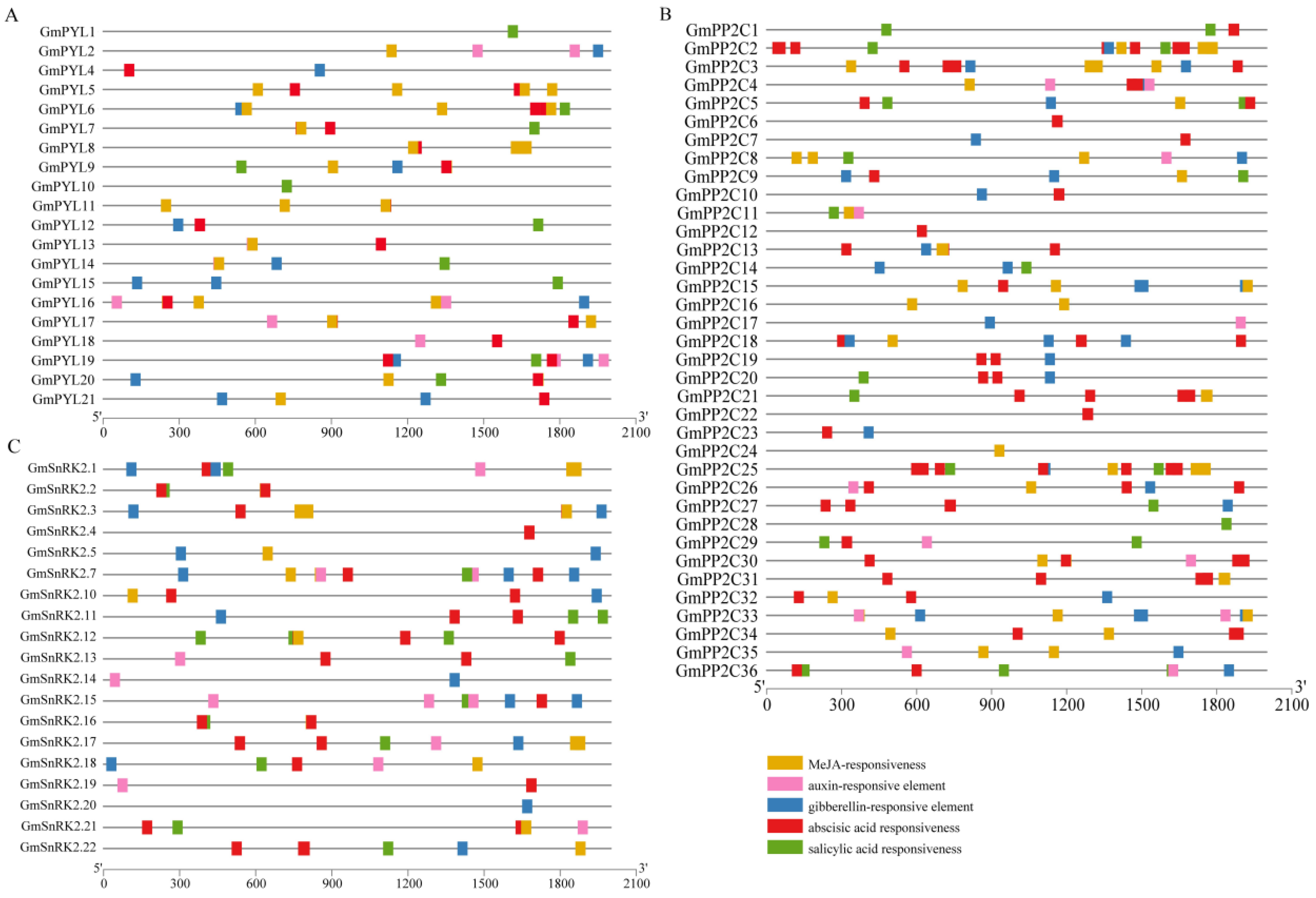
2.5. Analysis of GmPYLs, GmPP2Cs, GmSnRK2s Genes Promoter Sequence
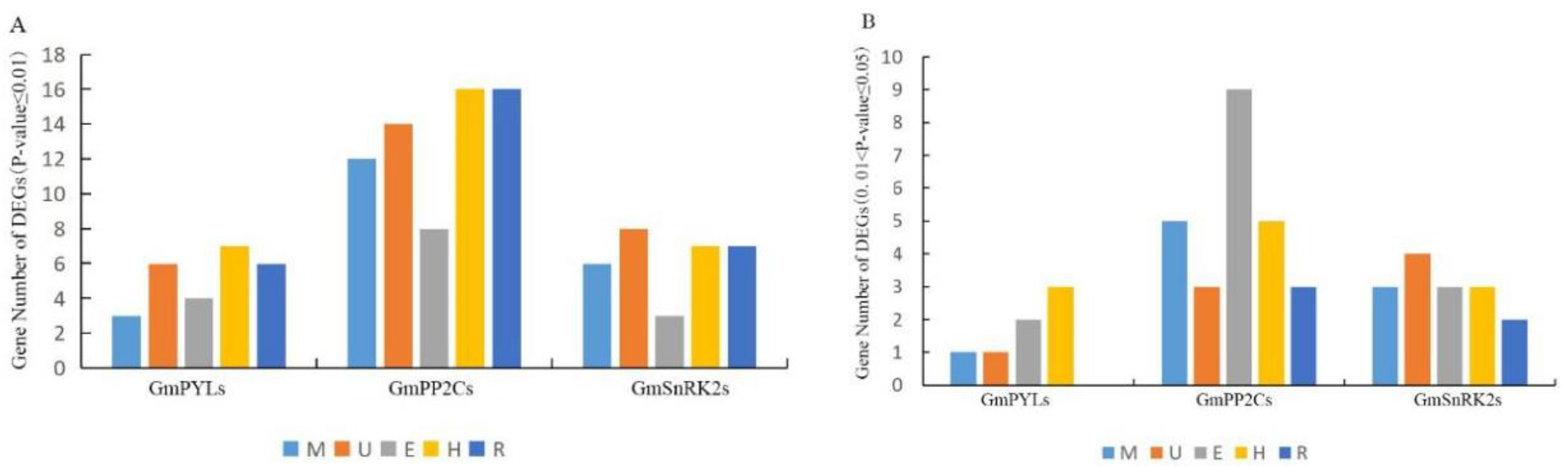
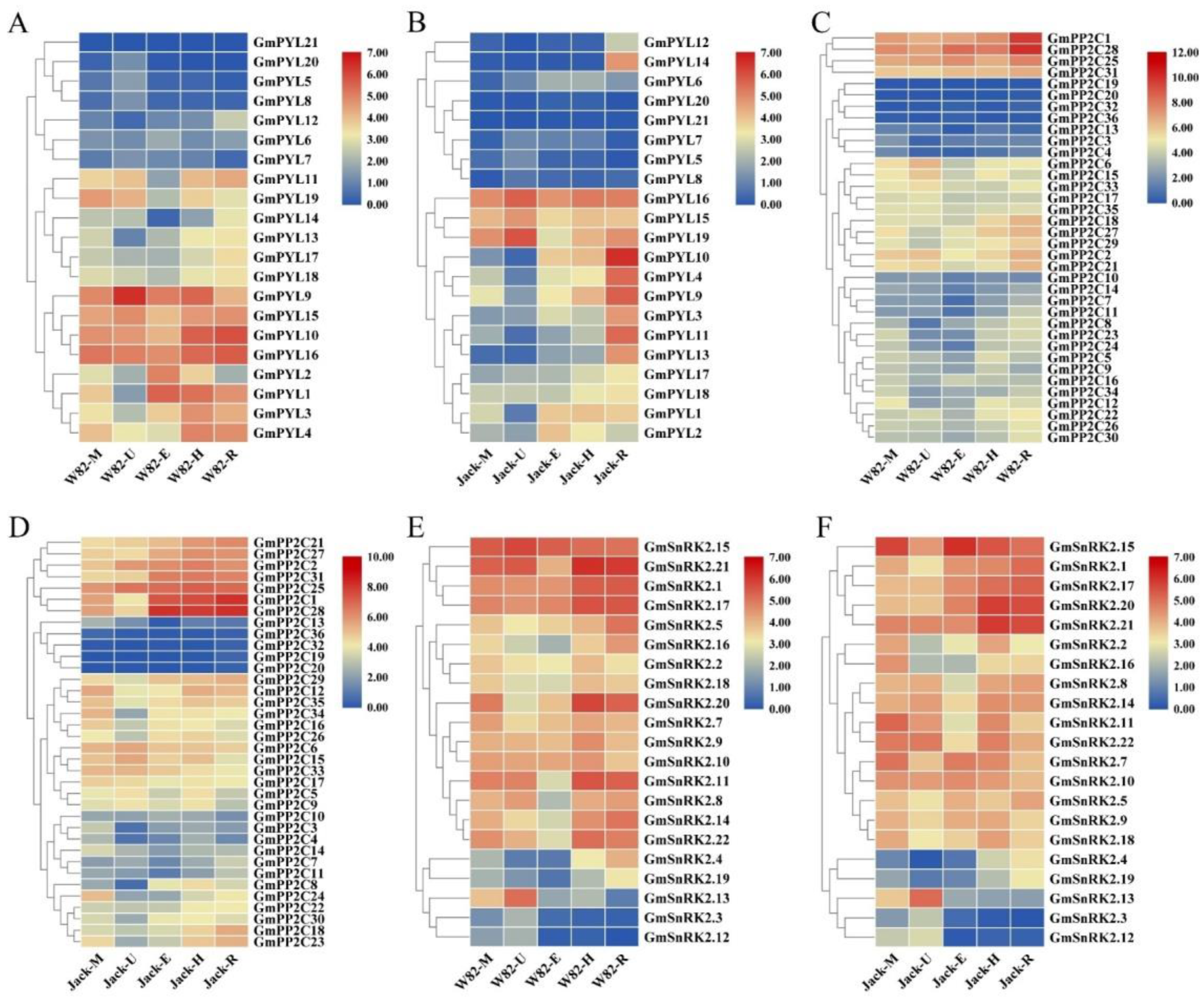
2.6. Tissue-Specificity of GmPYL-PP2C-SnRK2s Genes Expression
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Identification of GmPYL-PP2C-SnRK2s Gene Families
4.3. Phylogenetic Analysis
4.4. Analysis of Gene Structure and Their Conservation
4.5. Chromosomal Location and Collinearity Analysis
4.6. Analysis of Promoter Sequence
4.7. Analysis of Expression Patterns
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Koberg, M.; Abu-Much, R.; Gedanken, A. Optimization of Bio-Diesel Production from Soybean and Wastes of Cooked Oil: Combining Dielectric Microwave Irradiation and a SrO Catalyst. Bioresour. Technol. 2011, 102, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Lam, H.-M.; Nguyen, H.T.; Siddique, K.H.M.; Varshney, R.K.; Colmer, T.D.; Cowling, W.; Bramley, H.; Mori, T.A.; Hodgson, J.M.; et al. Neglecting Legumes Has Compromised Human Health and Sustainable Food Production. Nat. Plants 2016, 2, 16112. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, H.J.; Gong, Q.; Li, P.; Ma, S. Unraveling Abiotic Stress Tolerance Mechanisms—Getting Genomics Going. Curr. Opin. Plant Biol. 2006, 9, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wang, Z.; Ren, Z.; Zhi, L.; Yao, B.; Su, C.; Liu, L.; Li, X. SCFAtPP2-B11 Modulates ABA Signaling by Facilitating SnRK2.3 Degradation in Arabidopsis Thaliana. PLoS Genet. 2017, 13, e1006947. [Google Scholar] [CrossRef]
- Park, S.-Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.-F.F.; et al. Abscisic Acid Inhibits Type 2C Protein Phosphatases via the PYR/PYL Family of START Proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef]
- Melcher, K.; Ng, L.-M.; Zhou, X.E.; Soon, F.-F.; Xu, Y.; Suino-Powell, K.M.; Park, S.-Y.; Weiner, J.J.; Fujii, H.; Chinnusamy, V.; et al. A Gate–Latch–Lock Mechanism for Hormone Signalling by Abscisic Acid Receptors. Nat. Cell Biol. 2009, 462, 602–608. [Google Scholar] [CrossRef]
- Miyazono, K.-I.; Miyakawa, T.; Sawano, Y.; Kubota, K.; Kang, H.-J.; Asano, A.; Miyauchi, Y.; Takahashi, M.; Zhi, Y.; Fujita, Y.; et al. Structural Basis of Abscisic Acid Signalling. Nat. Cell Biol. 2009, 462, 609–614. [Google Scholar] [CrossRef]
- Soon, F.-F.; Ng, L.-M.; Zhou, X.E.; West, G.M.; Kovach, A.; Tan, M.H.E.; Suino-Powell, K.M.; He, Y.; Xu, Y.; Chalmers, M.J.; et al. Molecular Mimicry Regulates ABA Signaling by SnRK2 Kinases and PP2C Phosphatases. Science 2011, 335, 85–88. [Google Scholar] [CrossRef]
- Fujii, H.; Zhu, J.K. Arabidopsis Mutant Deficient in 3 Abscisic Acid-Activated Protein Kinases Reveals Criticalroles in Growth, Reproduction, and Stress. Proc. Natl. Acad. Sci. USA 2009, 106, 8380–8385. [Google Scholar] [CrossRef]
- Miao, Y.; Lu, D.; Wang, P.; Wang, X.C.; Chen, J.; Miao, C.; Song, C.P. An Arabidopsis Glutathione Peroxidasefunctions as Both a Redox Transducer and a Scavenger in Abscisic Acid and Drought Stress Responses. Plant Cell 2006, 18, 2749–2766. [Google Scholar] [CrossRef]
- Yu, Y.-T.; Wu, Z.; Lu, K.; Bi, C.; Liang, S.; Wang, X.-F.; Zhang, D.-P. Overexpression of the MYB37 Transcription Factor Enhances Abscisic Acid Sensitivity, and Improves Both Drought Tolerance and Seed Productivity in Arabidopsis Thaliana. Plant Mol. Biol. 2015, 90, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Szostkiewicz, I.; Korte, A.; Moes, D.; Yang, Y.; Christmann, A.; Grill, E. Regulators of PP2C Phosphatase Activity Function as Abscisic Acid Sensors. Science 2009, 324, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, G.; Li, Y.; Kong, X.; Zhang, L.; Wang, J.; Li, X.; Yang, L. ABA Receptor Subfamily III Enhances Abscisicacid Sensitivity and Improves the Drought Tolerance of Arabidopsis. Int. J. Mol. Sci. 2018, 19, 1938. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, M.; Mueller, H.M.; Bauer, H.; Peirats-Llobet, M.; Rodriguez, P.L.; Geilfus, C.-M.; Carpentier, S.C.; Al Rasheid, K.A.S.; Kollist, H.; Merilo, E.; et al. The Role of Arabidopsis ABA Receptors from the PYR/PYL/RCAR Family in Stomatal Acclimation and Closure Signal Integration. Nat. Plants 2019, 5, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Hu, Y.; Mu, Z.; Shi, H.; Chan, Z. Overexpression of Atpyl5 Under the Control of Guard Cell Specific Promoter Improves Drought Stress Tolerance in Arabidopsis. Plant Physiol. Biochem. 2018, 129, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Pizzio, G.A.; Rodriguez, L.; Antoni, R.; Gonzalez-Guzman, M.; Yunta, C.; Merilo, E.; Kollist, H.; Albert, A.; Rodriguez, P.L. The PYL4 A194T Mutant Uncovers a Key Role of PYR1-LIKE4 / Protein Phosphatase 2CA Interaction for Abscisic Acid Signaling and Plant Drought Resistance. Plant Physiol. Biochem. 2017, 163, 441–455. [Google Scholar] [CrossRef]
- Santiago, J.; Rodrigues, A.; Saez, A.; Rubio, S.; Antoni, R.; Dupeux, F.; Park, S.-Y.; Márquez, J.A.; Cutler, S.R.; Rodriguez, P.L. Modulation of Drought Resistance by the Abscisic Acid Receptor PYL5 Through Inhibition of Clade A PP2Cs. Plant J. 2009, 60, 575–588. [Google Scholar] [CrossRef]
- Fuchs, S.; Tischer, S.V.; Wunschel, C.; Christmann, A.; Grill, E. Abscisic Acid Sensor RCAR7/PYL13, Specific Regulator of Protein Phosphatase Coreceptors. Proc. Natl. Acad. Sci. USA 2014, 111, 5741–5746. [Google Scholar] [CrossRef]
- Ali, S.; Hayat, K.; Iqbal, A.; Xie, L. Implications of Abscisic Acid in the Drought Stress Tolerance of Plants. Agronomy 2020, 10, 1323. [Google Scholar] [CrossRef]
- Bai, G.; Yang, D.-H.; Zhao, Y.; Ha, S.; Yang, F.; Ma, J.; Gao, X.-S.; Wang, Z.; Zhu, J.-K. Interactions Between Soybean ABA Receptors and Type 2C Protein Phosphatases. Plant Mol. Biol. 2013, 83, 651–664. [Google Scholar] [CrossRef]
- Boudsocq, M.; Barbier-Brygoo, H.; Laurière, C. Identification of Nine Sucrose Nonfermenting 1-related Protein Kinases 2 Activated by Hyperosmotic and Saline Stresses in Arabidopsis Thaliana. J. Biol. Chem. 2004, 279, 41758–41766. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yamamoto, S.; Minami, H.; Kagaya, Y.; Hattori, T. Differential Activation of the Rice Sucrose Nonfermenting1–Related Protein Kinase2 Family by Hyperosmotic Stress and Abscisic Acid. Plant Cell 2004, 16, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Saha, J.; Chatterjee, C.; Sengupta, A.; Gupta, K.; Gupta, B. Genome- Wide Analysis and Evolutionary Study of Sucrose Non-Fermenting 1-Related Protein Kinase 2 (SnRK2) Gene Family Members in Arabidopsis and Oryza. Comput. Biol. Chem. 2014, 49, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Mao, X.; Zhang, H.; Chen, S.; Zhai, C.; Yang, S.; Jing, R. Cloning and Characterization of TaSnRK2.3, a Novel SnRK2 Gene in Common Wheat. J. Exp. Bot. 2013, 64, 2063–2080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mao, X.; Jing, R.; Chang, X.; Xie, H. Characterization of a Common Wheat (Triticum Aestivum L.) TaSnRK2.7 Gene Involved in Abiotic Stress Responses. J. Exp. Bot. 2010, 62, 975–988. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Cheng, Y.-H.; Zhang, C.; Shen, X.-J.; You, Q.-B.; Guo, W.; Li, X.; Song, X.-J.; Zhou, X.; Jiao, Y. Genome-Wide Identification and Characterization of the GmSnRK2 Family in Soybean. Int. J. Mol. Sci. 2017, 18, 1834. [Google Scholar] [CrossRef]
- Schweighofer, A.; Hirt, H.; Meskiene, I. Plant PP2C Phosphatases: Emerging Functions in Stress Signaling. Trends Plant Sci. 2004, 9, 236–243. [Google Scholar] [CrossRef]
- Singh, A.; Pandey, A.; Srivastava, A.K.; Tran, L.-S.P.; Pandey, G.K. Plant Protein Phosphatases 2C: From Genomic Diversity to Functional Multiplicity and Importance in Stress Management. Crit. Rev. Biotechnol. 2015, 36, 1023–1035. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Bailey, T.L.; Bodén, M.; Buske, F.A.; Frith, M.C.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for Motif Discovery and Searching. Nucleic Acids Res. 2009, 37, w202–w208. [Google Scholar] [CrossRef]
- Yang, R.; Yang, T.; Zhang, H.; Qi, Y.; Xing, Y.; Zhang, N.; Li, R.; Weeda, S.; Ren, S.; Ouyang, B.; et al. Hormone Profiling and Transcription Analysis Reveal a Major Role of Aba in Tomato Salt Tolerance. Plant Physiol. Biochem. 2014, 77, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, M.; Su, L.; Ge, K.; Li, L.; Li, X.; Liu, X.; Li, L. Negative Feedback Regulation of ABA Biosynthesis in Peanut (Arachis Hypogaea): A Transcription Factor Complex Inhibits AhNCED1 Expression During Water Stress. Sci. Rep. 2016, 6, 37943. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.-J.; Zhang, Y.-L.; Liu, X.; Huang, H.; Zhou, X.E.; Wang, W.-L.; Zeng, A.; Zhao, C.-Z.; Si, T.; Du, J.; et al. Combining Chemical and Genetic Approaches to Increase Drought Resistance in Plants. Nat. Commun. 2017, 8, 1183. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.-Y.; Li, J.; Wang, P.-Y.; Qin, F.; et al. Plant Abiotic Stress Response and Nutrient Use Efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef] [PubMed]
- Dalal, M.; Inupakutika, M. Transcriptional Regulation of ABA Core Signaling Component Genes in Sorghum (Sorghum Bicolor L. Moench). Mol. Breed. 2014, 34, 1517–1525. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, L.; Wang, X.; Hou, Y.-J.; Gao, J.; Wang, P.; Duan, C.-G.; Zhu, X.; Zhu, J.-K. The ABA Receptor PYL8 Promotes Lateral Root Growth by Enhancing MYB77-Dependent Transcription of Auxin-Responsive Genes. Sci. Signal. 2014, 7, ra53. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chan, Z.; Gao, J.; Xing, L.; Cao, M.; Yu, C.; Hu, Y.; You, J.; Shi, H.; Zhu, Y.; et al. ABA Receptor PYL9 Promotes Drought Resistance and Leaf Senescence. Proc. Natl. Acad. Sci. USA 2016, 113, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, N.; Xie, L. Molecular and Hormonal Regulation of Leaf Morphogenesis in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 5132. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Wang, D.; Zhang, S.; Ehlting, J.; Ni, F.; Jakab, S.; Zheng, C.; Zhong, Y. Genome-Wide and Expression Analysis of Protein Phosphatase 2C in Rice and Arabidopsis. BMC Genom. 2008, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, N.; Sarkeshik, A.; Nito, K.; Park, S.-Y.; Wang, A.; Carvalho, P.C.; Lee, S.; Caddell, D.F.; Cutler, S.R.; Chory, J.; et al. PYR/PYL/RCAR Family Members Are Major In-Vivo ABI1 Protein Phosphatase 2C-Interacting Proteins in Arabidopsis. Plant J. 2009, 61, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Z.-H.; Zhang, B.; Hills, A.; Blatt, M.R. PYR/PYL/RCAR Abscisic Acid Receptors Regulate K+ and Cl- Channels through Reactive Oxygen Species-Mediated Activation of Ca2+ Channels at the Plasma Membrane of Intact Arabidopsis Guard Cells. Plant Physiol. 2013, 163, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Adachi, Y.; Ye, W.; Hayashi, M.; Nakamura, Y.; Kinoshita, T.; Mori, I.C.; Murata, Y. Difference in Abscisic Acid Perception Mechanisms between Closure Induction and Opening Inhibition of Stomata. Plant Physiol. 2013, 163, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Hobo, T.; Ichimura, K.; Mizoguchi, T.; Takahashi, F.; Aronso, J.; Ecker, J.R.; Shinozaki, K. ABA-Activated SnRK2 Protein Kinase is Required for Dehydration Stress Signaling in Arabidopsis. Plant Cell Physiol. 2002, 43, 1473–1483. [Google Scholar] [CrossRef]
- Nakashima, K.; Fujita, Y.; Kanamori, N.; Katagiri, T.; Umezawa, T.; Kidokoro, S.; Maruyama, K.; Yoshida, T.; Ishiyama, K.; Kobayashi, M.; et al. Three Arabidopsis SnRK2 Protein Kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, Involved in ABA Signaling are Essential for the Control of Seed Development and Dormancy. Plant Cell Physiol. 2009, 50, 1345–1363. [Google Scholar] [CrossRef]
- Bhaskara, G.B.; Nguyen, T.T.; E Verslues, P. Unique Drought Resistance Functions of the Highly ABA-Induced Clade A Protein Phosphatase 2Cs. Plant Physiol. 2012, 160, 379–395. [Google Scholar] [CrossRef]
- Ali, S.; Xie, L. Plant Growth Promoting and Stress Mitigating Abilities of Soil Born Microorganisms. Recent Pat. Food Nutr. Agric. 2019, 10, 1. [Google Scholar] [CrossRef]
- Iyer, L.; Koonin, E.; Aravind, L. Adaptations of the Helix-Grip Fold for Ligand Binding and Catalysis in the START Domain Superfamily. Proteins 2011, 43, 134–144. [Google Scholar] [CrossRef]
- Fujii, H.; Chinnusamy, V.; Rodrigues, A.; Rubio, S.; Antoni, R.; Park, S.-Y.; Cutler, S.R.; Sheen, J.; Rodriguez, P.L.; Zhu, J.-K. In Vitro Reconstitution of an Abscisic Acid Signalling Pathway. Nat. Cell Biol. 2009, 462, 660–664. [Google Scholar] [CrossRef]
- Kulik, A.; Wawer, I.; Krzywińska, E.; Bucholc, M.; Dobrowolska, G. SnRK2 Protein Kinases—Key Regulators of Plant Response to Abiotic Stresses. OMICS 2011, 15, 859–872. [Google Scholar] [CrossRef]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the Physiological and Biochemical Impacts of Salt Stress on Plant Growth and Development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Khan, N.; Zandi, P.; Ali, S.; Mehmood, A.; Shahid, M.A.; Yang, J. Impact of Salicylic Acid and PGPR on the Drought Tolerance and Phytoremediation Potential of Helianthus annus. Front. Microbiol. 2018, 9, 2507. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Li, X.; Li, X.; Tan, W.; You, A.; Wu, S.; Tao, Y.; Chen, C.; Wang, J.; Zhang, D.; et al. OsPP2C09, a Negative Regulatory Factor in Abscisic Acid Signaling, Plays an Essential Role in Balancing Plant Growth and Drought Tolerance in Rice. New Phytol. 2020, 227, 1417–1433. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Jing, Y.; Shi, P.; Liu, J.; Chen, J.; Yan, J.; Chu, J.; Chen, K.-M.; Sun, J. JAZ proteins modulate seed germination through interaction with ABI 5 in bread wheat and Arabidopsis. New Phytol. 2019, 223, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Mustilli, A.-C.; Merlot, S.; Vavasseur, A.; Fenzi, F.; Giraudat, J. Arabidopsis OST1 Protein Kinase Mediates the Regulation of Stomatal Aperture by Abscisic Acid and Acts Upstream of Reactive Oxygen Species Production. Plant Cell 2002, 14, 3089–3099. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, A. Crosstalk Amongst Phytohormones From Planta and PGPR Under Biotic and Abiotic Stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Libault, M.; Farmer, A.; Joshi, T.; Takahashi, K.; Langley, R.J.; Franklin, L.D.; He, J.; Xu, D.; May, G.; Stacey, G. An Integrated Transcriptome Atlas of the Crop Model Glycine Max, and Its Use in Comparative Analyses in Plants. Plant J. 2010, 63, 86–99. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A Comparative Platform for Green Plant Genomics. Nucleic Acids Res. 2011, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 Years of the SMART Protein Domain Annotation Resource. Nucleic Acids Res. 2017, 46, D493–D496. [Google Scholar] [CrossRef] [PubMed]
- Voorrips, R.E. MapChart: Software for the Graphical Presentation of Linkage Maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Rombauts, S.; Déhais, P.; Van Montagu, M.; Rouzé, P. PlantCARE, a Plant Cis-Acting Regulatory Element Database. Nucleic Acids Res. 1999, 27, 295–296. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Ali, S.; Zhang, T.; Wang, W.; Xie, L. Identification, Evolutionary and Expression Analysis of PYL-PP2C-SnRK2s Gene Families in Soybean. Plants 2020, 9, 1356. https://doi.org/10.3390/plants9101356
Zhang Z, Ali S, Zhang T, Wang W, Xie L. Identification, Evolutionary and Expression Analysis of PYL-PP2C-SnRK2s Gene Families in Soybean. Plants. 2020; 9(10):1356. https://doi.org/10.3390/plants9101356
Chicago/Turabian StyleZhang, Zhaohan, Shahid Ali, Tianxu Zhang, Wanpeng Wang, and Linan Xie. 2020. "Identification, Evolutionary and Expression Analysis of PYL-PP2C-SnRK2s Gene Families in Soybean" Plants 9, no. 10: 1356. https://doi.org/10.3390/plants9101356
APA StyleZhang, Z., Ali, S., Zhang, T., Wang, W., & Xie, L. (2020). Identification, Evolutionary and Expression Analysis of PYL-PP2C-SnRK2s Gene Families in Soybean. Plants, 9(10), 1356. https://doi.org/10.3390/plants9101356