Transcriptome Analysis Unravels Metabolic and Molecular Pathways Related to Fruit Sac Granulation in a Late-Ripening Navel Orange (Citrus sinensis Osbeck)
Abstract
1. Introduction
2. Results
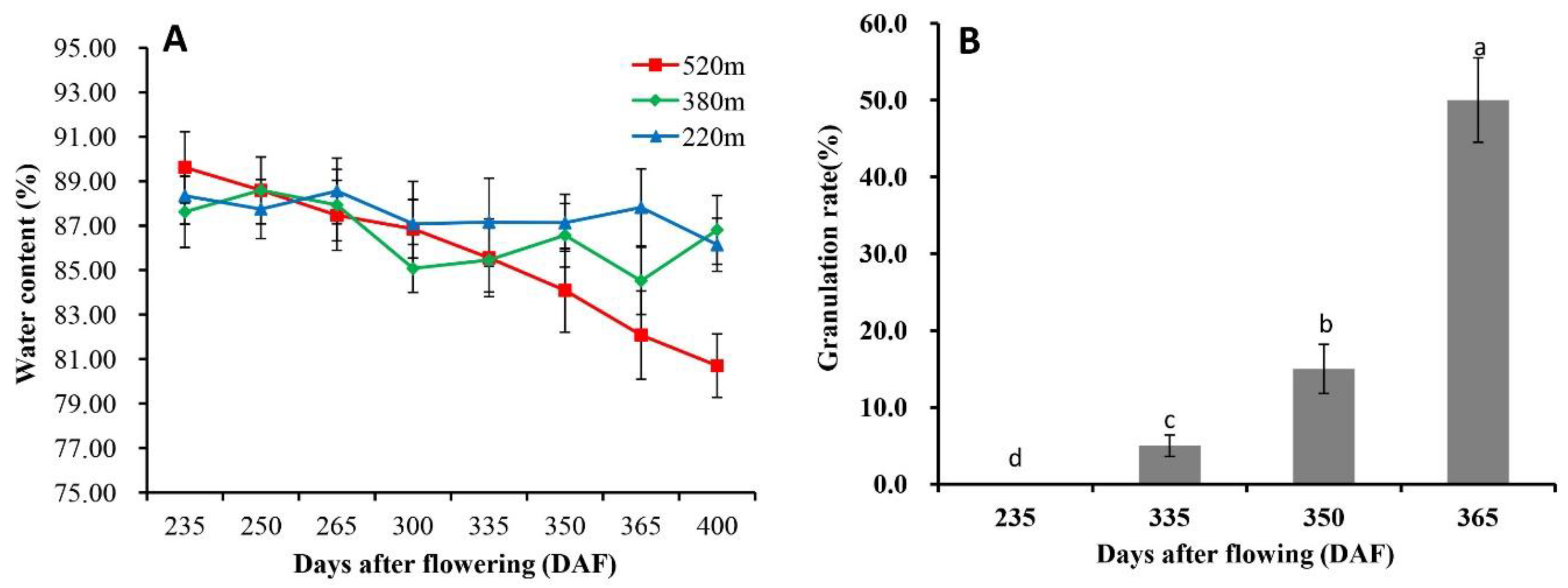
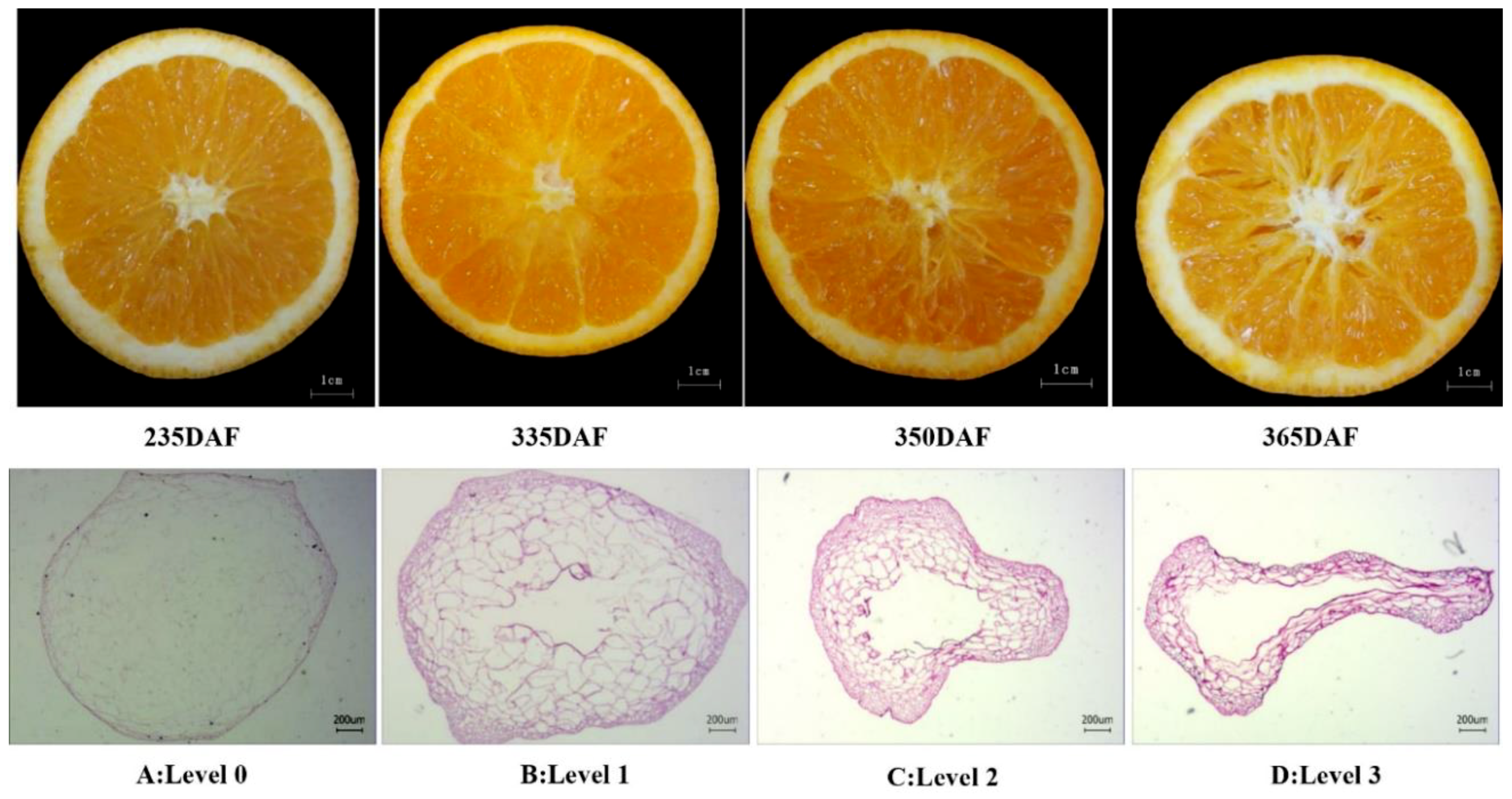
2.1. Occurrence of Granulation in Late-Ripening Navel Orange Fruit
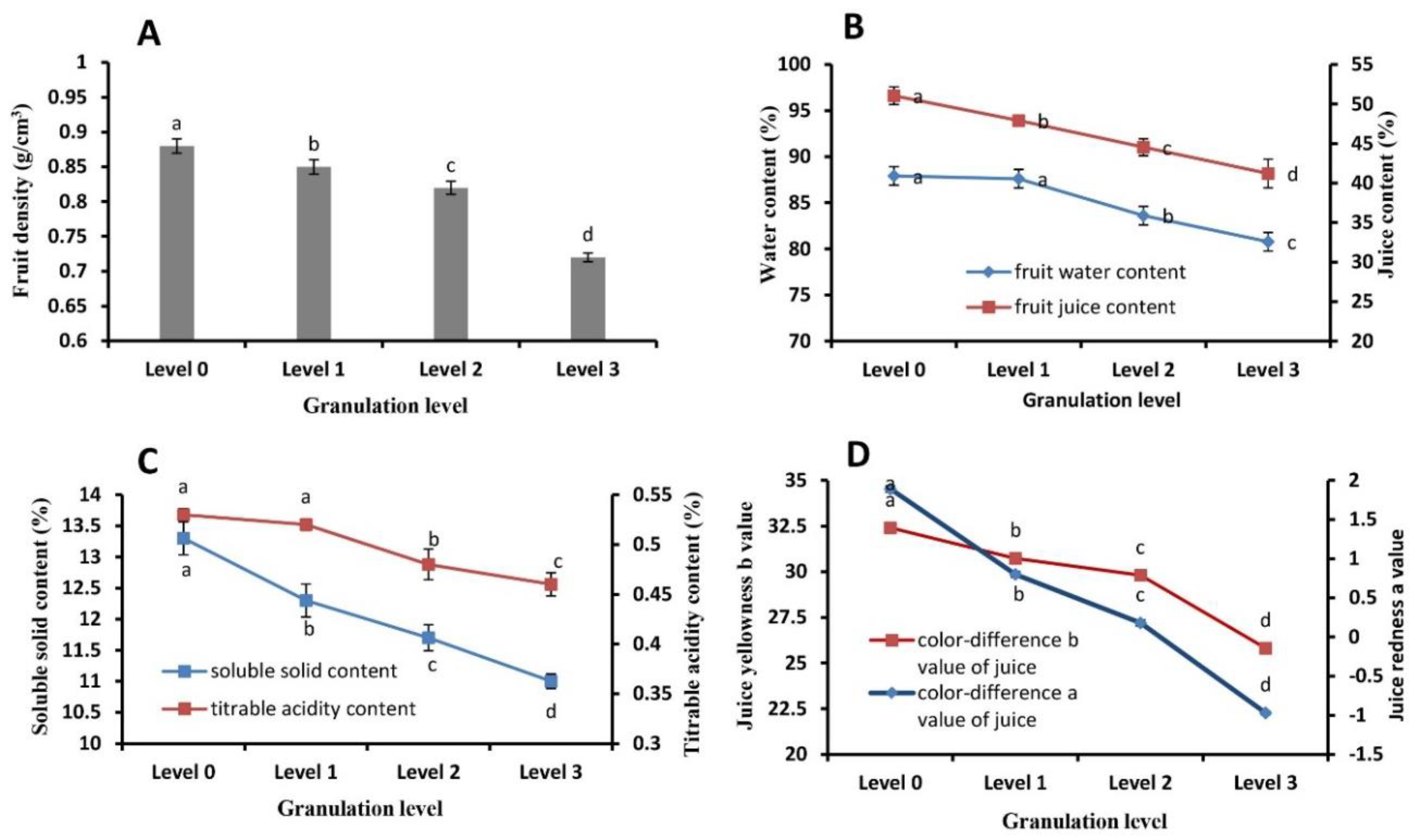
2.2. Juice Sac Quality and Color at Different Granulation Levels
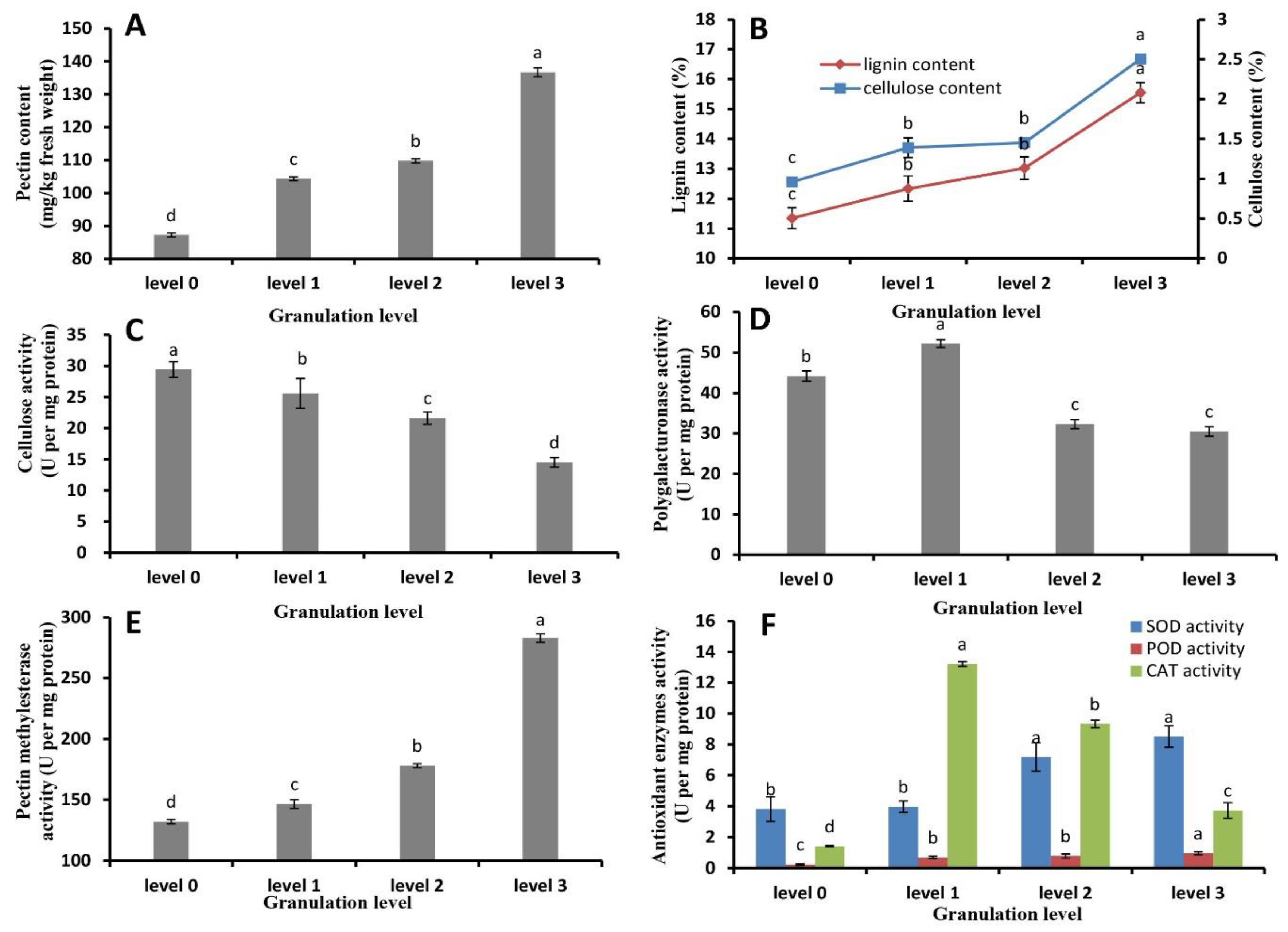
2.3. Pectin, Cellulose, and Lignin Content and Metabolic Enzyme Activity
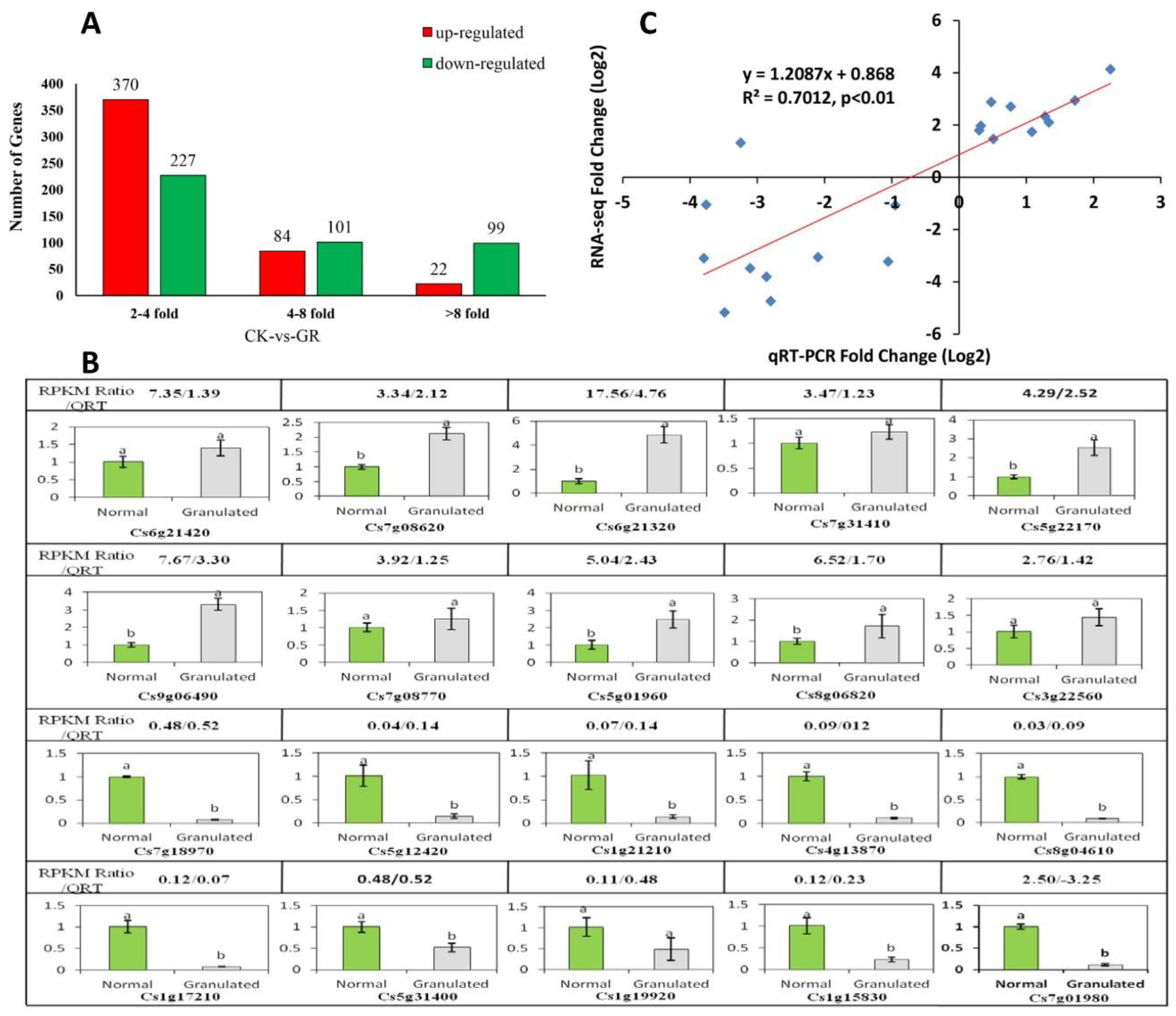
2.4. Sequencing and Analysis of Digital Gene Expression Profiles
2.4.1. Analysis of Late-Ripening Navel Orange Fruit Transcriptome
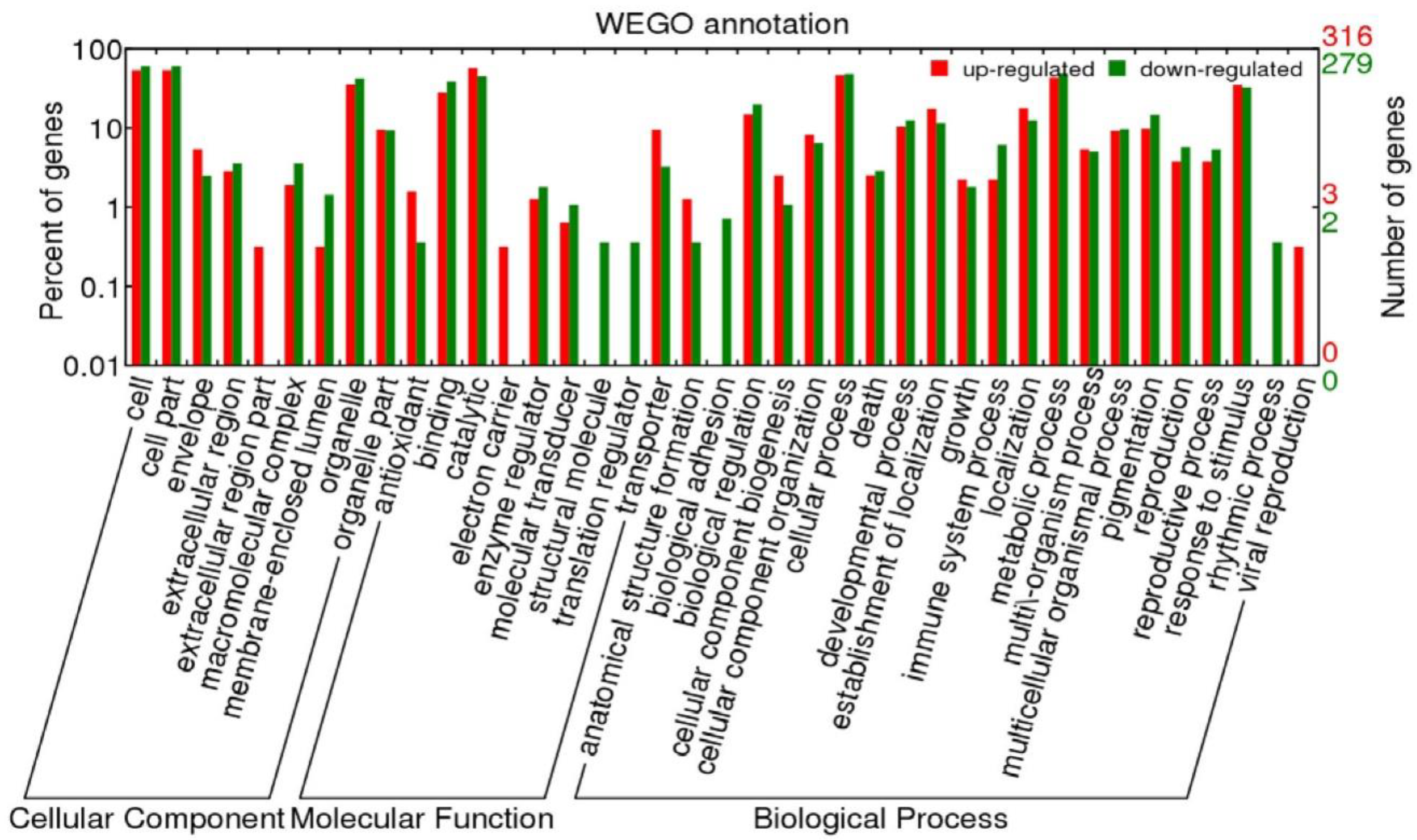
2.4.2. Functional Annotation of Differentially Expressed Genes
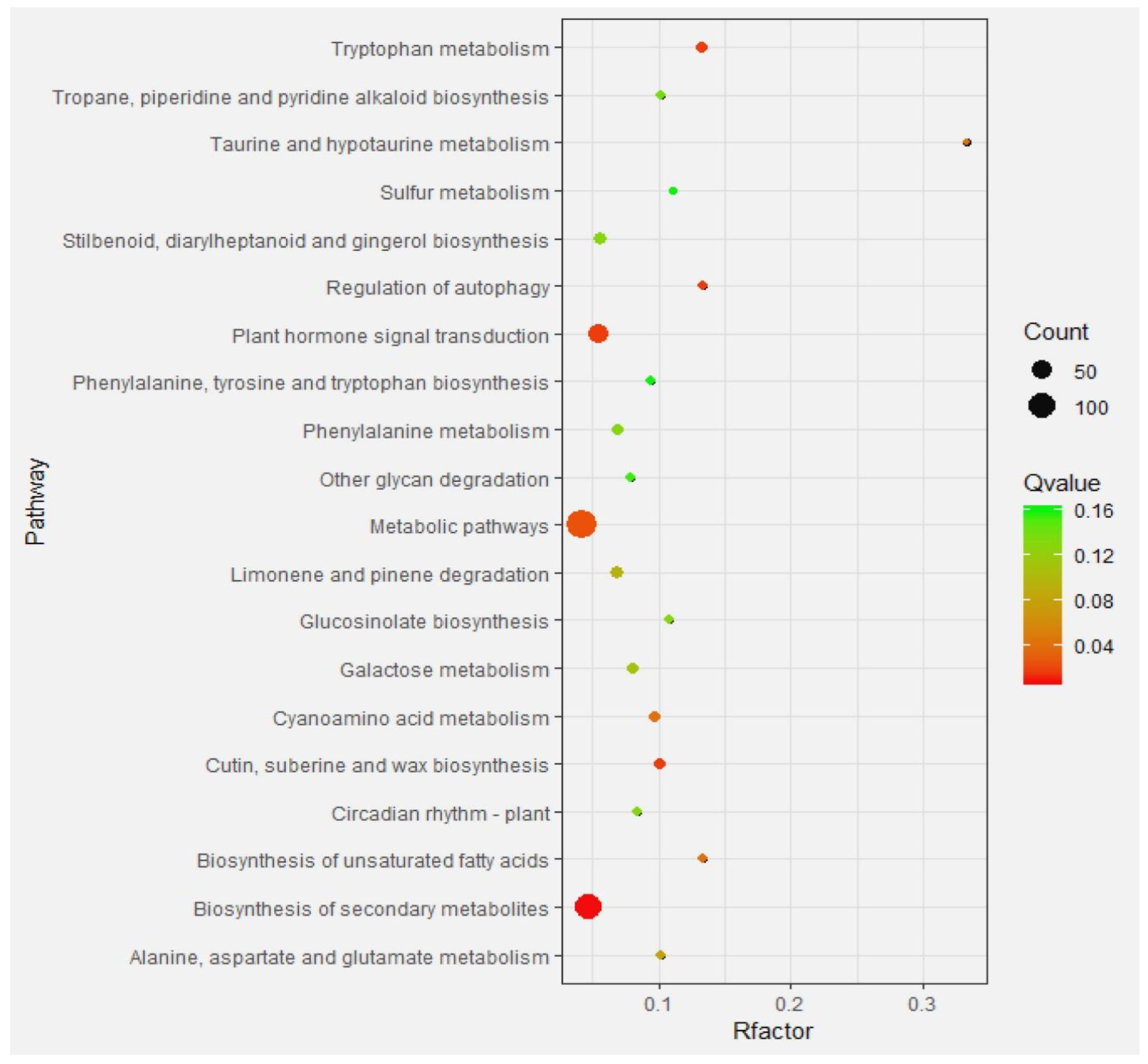
2.4.3. Enrichment Analysis of Differential Gene Pathways
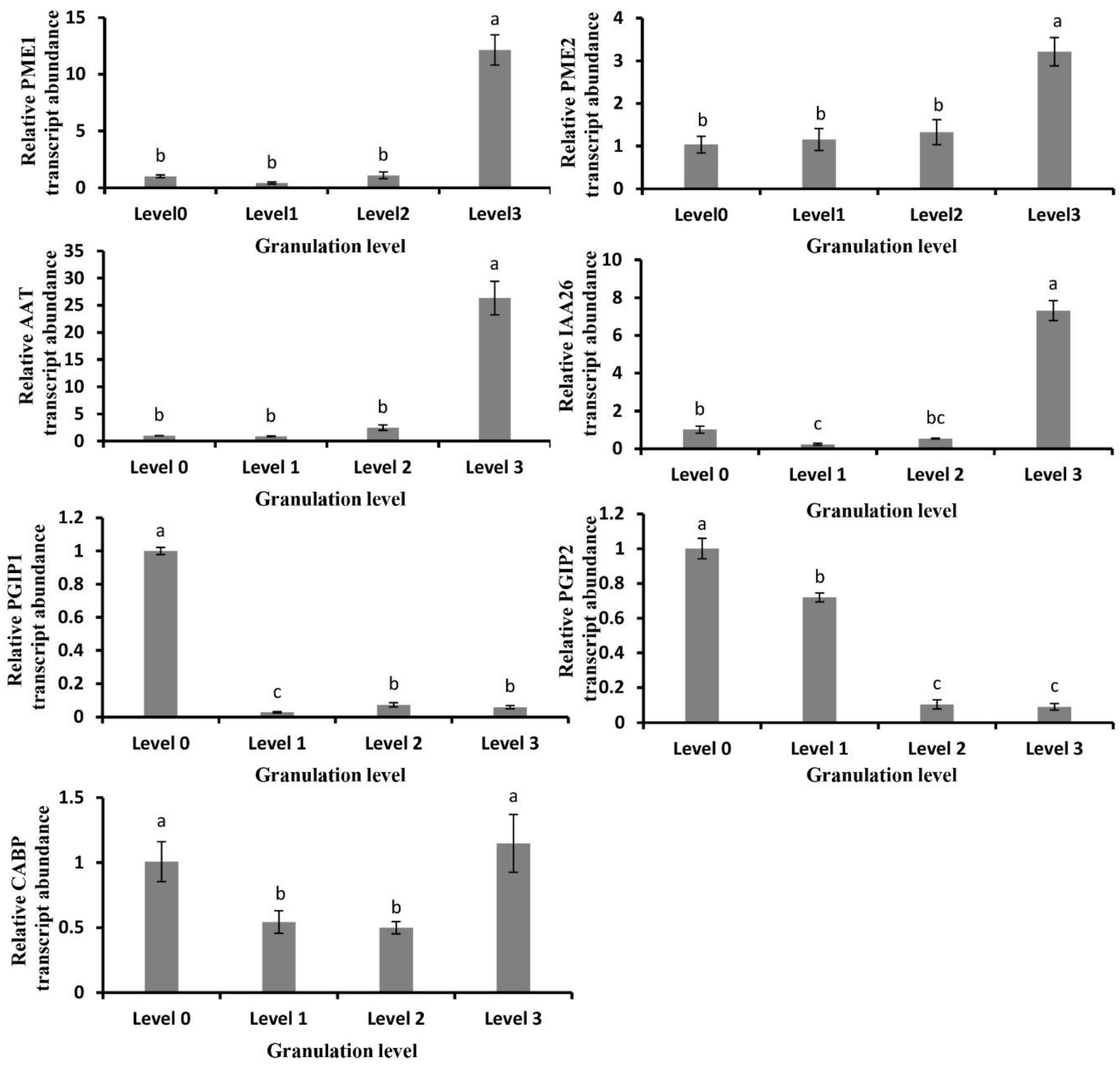
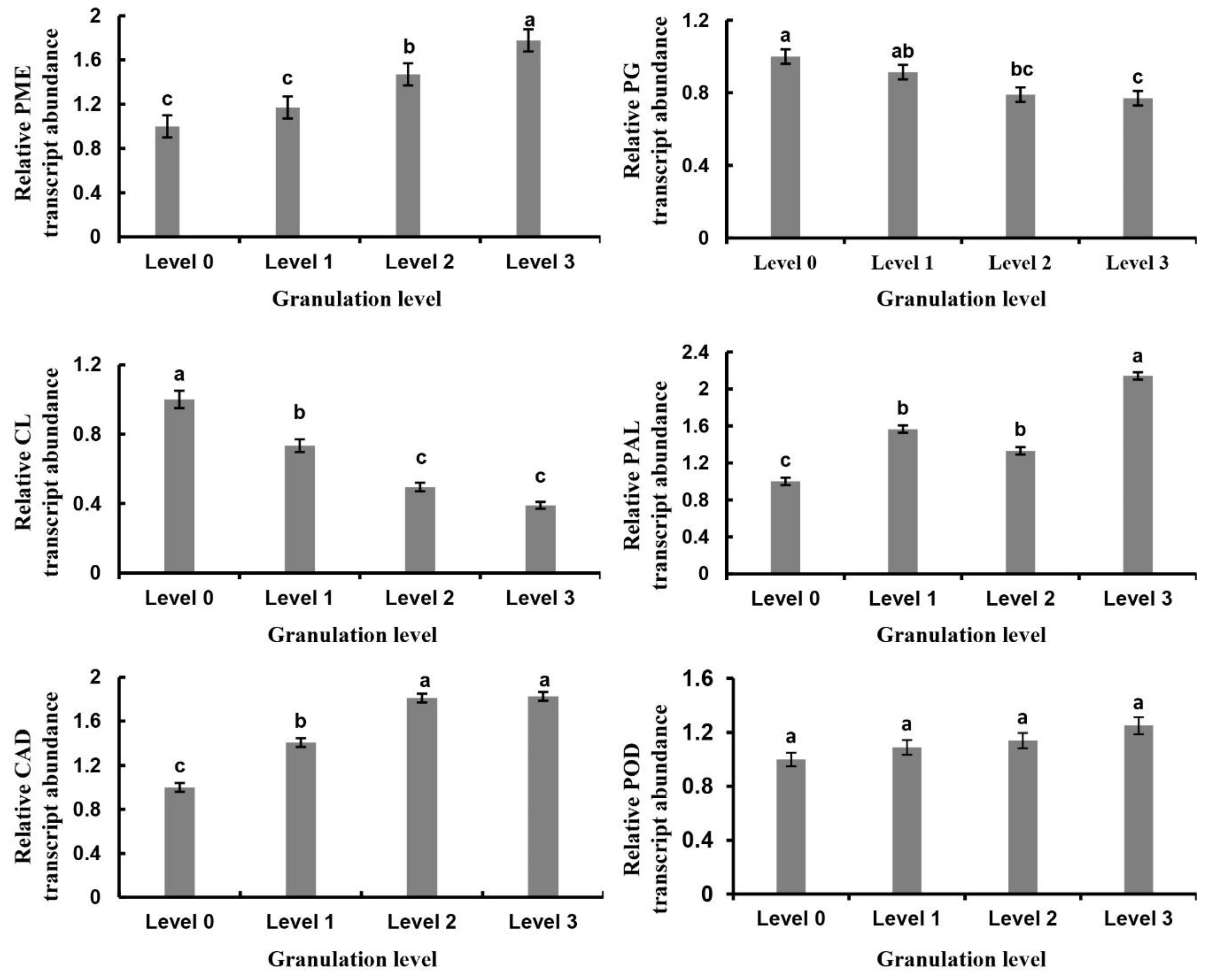
2.5. Analysis of Granulation-Related Genes and Their Expression Changes
2.6. Gene Expression Related to Cell Wall Metabolism in Granulated Fruit
2.7. Gene Expression of Metabolic Enzymes in Cell Wall of Normal Juice Sacs Treated at 4 °C
3. Discussion
3.1. Effects of Winter Low Temperature on Fruit Granulation
3.2. Gene Expression in Late-Ripening Navel Orange Fruit under Low Winter Temperature
3.3. Differences in Fruit Granulation between Overwintering Citrus Remained on the Tree and Post-Harvest Storage Citrus
4. Conclusions
5. Materials and Methods
5.1. Materials and Sample Collection
5.2. Determination of Juice Sac Granulation Level
5.3. Fruit Quality Analysis
5.4. Determination of Pectin, Cellulose, and Lignin Contents
5.5. Cross Section Preparation and Microscopic Observeation of Juice Sacs
5.6. RNA-Sequencing and Characterization, Sequence Assessment, and Screening of Differentially Expressed Genes (DEGs)
5.7. Validation of RNA-Sequencing Data by Quantitative Real-Time RT-PCR
5.8. Measurement of Enzyme Activity Related to Cell Wall Metabolism in Juice Sacs
5.9. Gene Expression in Juice Sacs Treated at 4 °C by RT-PCR
5.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Storey, R.; Treeby, M.T. Short-and long-term growth of navel orange fruit. J. Pomol. Hortic. Sci. 1999, 74, 464–471. [Google Scholar] [CrossRef]
- Mohammadian, M.A.; Largani, Z.K.; Sajedi, R.H. Quantitative and qualitative comparison of antioxidant activity in the flavedo tissue of three cultivars of citrus fruit under cold stress. Aust. J. Crop. Sci. 2012, 6, 402. [Google Scholar]
- Bartholomew, E.T.; Sinclair, W.B.; Raby, E.C. Granulation (crystallization) of ‘Valencia’ oranges. Calif. Citrogr. 1934, 19, 88–89. [Google Scholar]
- Noort, G.V. Dryness in navel fruit. Proc. Lst. Int. Citrus Symp. 1969, 3, 1333–1342. [Google Scholar]
- Gilfillan, I.M.; Stevenson, J.A. Postharvest development of granulation in South African export oranges. Proc. Intl Soc. Citric. 1977, 1, 299–303. [Google Scholar]
- Burns, J.K.; Achor, D.S. Cell wall changes in juice vesicles associated with “section drying” in stored late-harvested grapefruit. J. Am. Soc. Hortic. Sci. 1989, 114, 283–287. [Google Scholar]
- Hwang, Y.S.; Huber, D.J.; Albrigo, L.G. Comparison of cell wall components in normal and disordered juice vesicles of grapefruit. J. Am. Soc. Hortic. Sci. Am. Soc. Hortic. Sci. 1990, 115, 281–287. [Google Scholar] [CrossRef]
- Singh, R. 65-year research on citrus granulation. Indian J. Hortic. 2001, 58, 112–144. [Google Scholar]
- Ritenour, M.A.; Albrigo, L.G.; Burns, J.K.; Miller, W.M. Granulation in Florida citrus. Proc. Fla. State Hortic. Soc. 2004, 117, 358–361. [Google Scholar]
- Sharma, R.R.; Saxena, S.K. Rootstocks influence granulation in Kinnow mandarin (Citrus nobilis x C. deliciosa). Sci. Hortic. 2004, 101, 235–242. [Google Scholar] [CrossRef]
- Sharma, R.R.; Singh, R.; Saxena, S.K. Characteristics of citrus fruit in relation to granulation. Sci. Hortic. 2006, 111, 91–96. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, P.; Qi, Y.P.; Zhou, C.P.; Yang, L.T.; Liao, X.Y.; Wang, L.Q.; Zhu, D.H.; Chen, L.S. Effects of granulation on organic acid metabolism and its relation to mineral elements in citrus grandis juice sacs. Food Chem. 2014, 145, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.L.; Pan, T.F.; Guo, Z.X.; Pan, D.M. Specific Lignin Accumulation in Granulated Juice Sacs of Citrus maxima. J. Agric. Food Chem. 2014, 62, 50. [Google Scholar] [CrossRef] [PubMed]
- Shomer, I.; Chalutz, E.; Vasiliver, R.; Lomaniec, E.; Berman, M. Scierification of juice sacs in pummelo (Citrus grandis) fruit. Can. J. Bot. 1989, 67, 625–632. [Google Scholar] [CrossRef]
- Goto, A. Relationship between Pectic Substances and Calcium in Healthy, Gelated, and Granulated Juice Sacs of Sanbokan (Citrus sulcata hort. ex Takahashi) Fruit. Plant. Cell Physiol. 1989, 30, 801–806. [Google Scholar] [CrossRef]
- Jia, N.; Liu, J.; Tan, P.H.; Sun, Y.F.; Lv, Y.M.; Liu, J.M.; Sun, J.; Huang, Y.T.; Lu, J.; Jin, N.; et al. Citrus sinensis MYB transcription factor CsMYB85 induce fruit juice sacs lignification through interaction with other CsMYB transcription factors. Front. Plant Sci. 2019, 10, 213. [Google Scholar] [CrossRef]
- Theanjumpol, P.; Wongzeewasakun, K.; Muenmanee, N.; Wongsaipun, S.; Krongchai, C.; Changrue, V.; Boonyakiat, D.; Kittiwachana, S. Non-destructive identification and estimation of granulation in ‘Sai Num Pung’ tangerine fruit using near infrared spectroscopy and chemometrics. Postharvest Biol. Technol. 2019, 153, 13–20. [Google Scholar] [CrossRef]
- Awasthi, R.P.; Nauriyal, J.P. Studies on granulation in sweet orange. II. Differences in moisture, total soluble solids and ascorbic acid of juice vesicles in different stages of granulation. Punjab Hortic. J. 1972, 12, 203–211. [Google Scholar]
- Xu, Q.; Chen, L.L.; Ruan, X.A.; Chen, D.J.; Zhu, A.D.; Chen, C.L.; Bertrand, D.; Jiao, W.B.; Hao, B.H.; Lyon, M.P.; et al. The draft genome of sweet orange (Citrus sinensis). Nat. Genet. 2013, 45, 59–66. [Google Scholar] [CrossRef]
- Yuan, Y. BM-Map: An efficient software package for accurately allocating multireads of RNA-sequencing data. Bmc Genom. 2012, 13 (Suppl. 8), S9. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, S.; Duan, J.; Wang, J.; Chen, S.; Cheng, Z.; Zhang, Q.; Liang, X.; Li, Y. De novo assembly and Characterisation of the Transcriptome during seed development, and generation of genic-SSR markers in Peanut (Arachis hypogaea L.). BMC Genom. 2012, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Araki, C. Chemical compositions and internal anatomy of the gelated and granulated juice sacs of Sanbokan (Citrus sulcata hort. ex Takahashi) fruit. J. Jpn. Soc. Hortic. Sci. 1983, 52, 316–324. [Google Scholar] [CrossRef][Green Version]
- Burns, J.K.; Albrigo, G. Time of harvest and method of storage affect granulation in grapefruit. HortScience 1998, 33, 728–730. [Google Scholar] [CrossRef]
- Ding, J.; Deng, X.X.; Zhang, H.Y.; Cheng, Y.J.; Xu, Q.A.; Liu, Q. Somaclonal variation with early juice-sac granulation obtained by inter-specific protoplast fusion in citrus. J. Pomol. Hortic. Sci. 2009, 84, 567–573. [Google Scholar] [CrossRef]
- Burdon, J.; Lallu, N.; Yearsley, C.; Osman, S.; Billing, D.; Boldingh, H. Postharvest conditioning of Satsuma mandarins for reduction of acidity and skin puffiness. Postharvest Biol. Technol. 2007, 43, 102–114. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Cheng, F.S.; Dai, C.; Sun, Y.F.; Lu, J.; Huang, Y.T.; Li, M.M.; He, Y.; Wang, F.Z.; et al. Identification of microRNAs correlated with citrus granulation based on bioinformatics and molecular biology analysis. Postharvest Biol. Technol. 2016, 118, 59–67. [Google Scholar] [CrossRef]
- Cesarino, I.; Araújo, P.; Júnior, A.P.D.; Mazzafera, P. An overview of lignin metabolism and its effect on biomass recalcitrance. Rev. Bras. De Botânica 2012, 35, 303–311. [Google Scholar] [CrossRef]
- Vanholme, R.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin engineering. Curr. Opin. Plant. Biol. 2008, 11, 278–285. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, L.; Tu, L.; Liu, L.; Yuan, D.; Jin, L.; Long, L.; Zhang, X. Lignin metabolism has a central role in the resistance of cotton to the wilt fungus Verticillium dahliae as revealed by RNA-Seq-dependent transcriptional analysis and histochemistry. J. Exp. Bot. 2011, 62, 5607–5621. [Google Scholar] [CrossRef]
- Ibánez, A.M.; Martinelli, F.; Reagan, R.L.; Uratsu, S.L.; Vo, A.; Mario, A.; Tinoco, M.A.; Phu, M.L.; Chen, Y.; Rocke, D.M.; et al. Transcriptome and metabolome analysis of citrus fruit to elucidate puffing disorder. Plant. Sci. 2014, 217, 87–98. [Google Scholar] [CrossRef]
- Martinelli, F.; Ibánez, A.M.; Reagan, R.L.; Davino, S.; Dandekar, A.M. Stress responses in citrus peel: Comparative analysis of host responses to Huanglongbing disease and puffing disorder. Sci. Hortic. 2015, 192, 409–420. [Google Scholar] [CrossRef]
- Huang, X.S.; Wang, W.; Zhang, Q.; Liu, J.H. A basic helix-loop-helix transcription factor, PtrbHLH, of Poncirus trifoliata confers cold tolerance and modulates peroxidase-mediated scavenging of hydrogen peroxide. Plant. Physiol. 2013, 162, 1178–1194. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Cao, Q.; Xie, J.; Deng, L.L.; Zeng, K.F. Alteration of sugar and organic acid metabolism in postharvest granulation of Ponkan fruit revealed by transcriptome profiling. Postharvest Biol. Technol. 2018, 139, 2–11. [Google Scholar] [CrossRef]
- Ding, Y.; Chang, J.; Ma, Q.; Chen, L.L.; Liu, S.Z.; Jin, S.; Han, J.W.; Xu, R.W.; Zhu, A.D.; Guo, J.; et al. Network analysis of postharvest senescence process in citrus fruit revealed by transcriptomic and metabolomic profiling. Plant. Physiol. 2015, 168, 357–376. [Google Scholar] [CrossRef]
- Sheng, L.; Shen, D.D.; Yang, W.; Zhang, M.F.; Zeng, Y.L.; Xu, J.; Deng, X.X.; Cheng, Y.J. GABA pathway rate-limit citrate degradation in postharvest citrus fruit evidence from HB Pumelo (Citrus grandis)× Fairchild (Citrus reticulata) hybrid population. J. Agric. Food Chem. 2017, 65, 1669–1676. [Google Scholar] [CrossRef]
- Chen, Q.S.; Ding, J.; Cai, J.R.; Zhao, J.W. Rapid measurement of total acid content (TAC) in vinegar using near infrared spectroscopy based on efficient variables selection algorithm and nonlinear regression tools. Food Chem. 2012, 135, 590–595. [Google Scholar] [CrossRef]
- Marín-Rodríguez, M.C.; Orchard, J.; Seymour, G.B. Pectate lyases, cell wall degradation and fruit softening. J. Exp. Bot. 2002, 53, 2115–2119. [Google Scholar] [CrossRef]
- Robertson, G.L. The determination of pectic substances in citrus juices and grapes by two spectrophotometric methods. J. Food Biochem. 1981, 5, 139–143. [Google Scholar] [CrossRef]
- Li, H.P. Plant Microscopy Technique; Science Press: Beijing, China, 2009. [Google Scholar]
- Dong, T.; Xia, R.; Wang, M.; Xiao, Z.; Liu, P. Changes in dietary fibre, polygalacturonase, cellulase of navel orange (Citrus sinensis (L.) Osbeck ‘Cara Cara’) fruit under different storage conditions. Sci. Hortic. 2008, 116, 414–420. [Google Scholar] [CrossRef]
- Ledergerber, C.; Dessimoz, C. Base-calling for next-generation sequencing platforms. Brief. Bioinform. 2011, 12, 489–497. [Google Scholar] [CrossRef]
- Li, R.; Yu, C.; Li, Y.; Lam, T.-W.; Yiu, S.-M.; Kristiansen, K.; Wang, J. SOAP2: An improved ultrafast tool for short read alignment. Bioinformatics 2009, 25, 1966–1967. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods. 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Audic, S.; Claverie, J.M. The significance of digital gene expression profiles. Genome Res. 1997, 7, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, S.; Alcalde, F.G.; Dopazo, J.; Ferrer, A.; Conesa, A. Differential expression in RNA-seq: A matter of depth. Genome Res. 2011, 21, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Fang, L.; Zheng, H.K.; Zhang, Y.; Chen, J.; Wang, J.; Li, S.T.; Li, R.Q.; Bolund, L.; Wang, J. WEGO: A web tool for plotting GO annotations. Nucleic Acids Res. 2006, 34, 293–297. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lohani, S.; Trivedi, P.K.; Nath, P. Changes in activities of cell wall hydrolases during ethylene-induced ripening in banana: Effect of 1-MCP, ABA and IAA. Postharvest Biol. Technol. 2004, 31, 119–126. [Google Scholar] [CrossRef]

| Gene ID. | E-Value | RPKM Value | Log2 Fold Change | Functional Description | |
|---|---|---|---|---|---|
| CK | GR | ||||
| Cs1g04910 | 3.75 × 10−77 | 0.35 | 0.90 | 1.35 | Cinnamyl-alcohol dehydrogenase |
| Cs1g12840 | 1.36 × 10−49 | 0.80 | 1.75 | 1.13 | Polygalacturonase |
| Cs1g15830 | 6.71 × 10−27 | 14.35 | 1.72 | −3.06 | Auxin-responsive protein IAA26-like |
| Cs1g16550 | 1.26 × 10−176 | 101.57 | 218.40 | 1.10 | Pectin methylesterase |
| Cs1g17210 | 2.69 × 10−20 | 34.70 | 4.05 | −3.10 | Protein TIFY 10A |
| Cs1g19920 | 0 | 60.62 | 6.48 | −3.23 | Cell wall protein PRY3 |
| Cs1g21210 | 1.26 × 10−114 | 3.44 | 0.25 | −3.81 | 1-Aminocyclopropane-1-carboxylate synthase, aminotransferase |
| Cs2g07660 | 3.25 × 10−110 | 63.99 | 3.19 | −4.32 | Pectin methylesterase |
| Cs2g31410 | 0 | 11.99 | 0.11 | −6.74 | Alcohol acyl transferase |
| Cs3g22560 | 0 | 3.26 | 9.00 | 1.47 | Citrus sucrose transporter 1 |
| Cs4g13870 | 4.29 × 10−128 | 562.74 | 50.38 | −3.48 | 1-Aminocyclopropane-1-carboxylate oxidase |
| Cs5g01960 | 2.28 × 10−95 | 1.20 | 6.04 | 2.33 | Cellulose synthase-like protein G3-like |
| Cs5g12420 | 9.56 × 10−160 | 64.54 | 2.42 | −4.74 | Epidermis-specific secreted glycoprotein EP1-like protein |
| Cs5g20320 | 5.70 × 10−90 | 0.64 | 0.02 | −4.70 | Basic cellulase |
| Cs5g22170 | 5.01 × 10−130 | 4.62 | 19.80 | 2.10 | 1-Associated receptor kinase 1 precursor |
| Cs5g31400 | 1.82 × 10−100 | 22.66 | 10.79 | −1.07 | Myc-like proanthocyanidin regulatory protein |
| Cs6g21320 | 3.94 × 10−143 | 0.16 | 2.90 | 4.13 | Amino acid transporter |
| Cs6g21420 | 6.71 × 10−51 | 8.02 | 58.95 | 2.88 | Calcium-binding protein |
| Cs7g01980 | 1.56 × 10−147 | 5.12 | 12.78 | 1.32 | Polygalacturonase-inhibiting protein |
| Cs7g08620 | 0 | 61.27 | 204.54 | 1.74 | Probable polygalacturonase non-catalytic subunit JP630-like |
| Cs7g08770 | 2.27 × 10−92 | 1.65 | 6.47 | 1.97 | Galactinol synthase |
| Cs7g18970 | 7.68 × 10−180 | 1121.82 | 542.19 | −1.05 | Polygalacturonase-inhibiting protein |
| Cs7g31410 | 2.45 × 10−111 | 28.17 | 97.83 | 1.80 | plasma membrane intrinsic protein |
| Cs8g04610 | 0 | 17.72 | 0.49 | −5.17 | GH3 family protein |
| Cs8g06820 | 0 | 0.54 | 3.50 | 2.70 | Mannitol transporter |
| Cs8g16290 | 0 | 0.48 | 2.12 | 2.13 | Phenylalanine ammonia-lyase |
| Cs9g06490 | 1.53 × 10−113 | 5.29 | 40.58 | 2.94 | Carboxylesterase |
| orange1.1t00214 | 3.45 × 10−149 | 2.40 | 0.00 | −7.91 | Pectin methylesterase |
| orange1.1t02040 | 5.65 × 10−58 | 0.04 | 0.11 | 1.45 | Peroxidase |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.-M.; Wang, C.; He, L.-G.; Wang, Z.-J.; Tong, Z.; Song, F.; Tu, J.-F.; Qiu, W.-M.; Liu, J.-H.; Jiang, Y.-C.; et al. Transcriptome Analysis Unravels Metabolic and Molecular Pathways Related to Fruit Sac Granulation in a Late-Ripening Navel Orange (Citrus sinensis Osbeck). Plants 2020, 9, 95. https://doi.org/10.3390/plants9010095
Wu L-M, Wang C, He L-G, Wang Z-J, Tong Z, Song F, Tu J-F, Qiu W-M, Liu J-H, Jiang Y-C, et al. Transcriptome Analysis Unravels Metabolic and Molecular Pathways Related to Fruit Sac Granulation in a Late-Ripening Navel Orange (Citrus sinensis Osbeck). Plants. 2020; 9(1):95. https://doi.org/10.3390/plants9010095
Chicago/Turabian StyleWu, Li-Ming, Ce Wang, Li-Gang He, Zhi-Jing Wang, Zhu Tong, Fang Song, Jun-Fan Tu, Wen-Ming Qiu, Ji-Hong Liu, Ying-Chun Jiang, and et al. 2020. "Transcriptome Analysis Unravels Metabolic and Molecular Pathways Related to Fruit Sac Granulation in a Late-Ripening Navel Orange (Citrus sinensis Osbeck)" Plants 9, no. 1: 95. https://doi.org/10.3390/plants9010095
APA StyleWu, L.-M., Wang, C., He, L.-G., Wang, Z.-J., Tong, Z., Song, F., Tu, J.-F., Qiu, W.-M., Liu, J.-H., Jiang, Y.-C., & Peng, S.-A. (2020). Transcriptome Analysis Unravels Metabolic and Molecular Pathways Related to Fruit Sac Granulation in a Late-Ripening Navel Orange (Citrus sinensis Osbeck). Plants, 9(1), 95. https://doi.org/10.3390/plants9010095






