Stomatal and Photosynthetic Traits Are Associated with Investigating Sodium Chloride Tolerance of Brassica napus L. Cultivars
Abstract
1. Introduction
2. Results
2.1. Seedling Growth and Biomass Yield
2.2. Chlorophyll Content (SPAD)
2.3. K+, Total Soluble Sugar (TSS) and Proline Concentrations (ProC)
2.4. Relative Water Content (RWC) and Electrolyte Leakage (EL)
2.5. Enzymatic Antioxidants
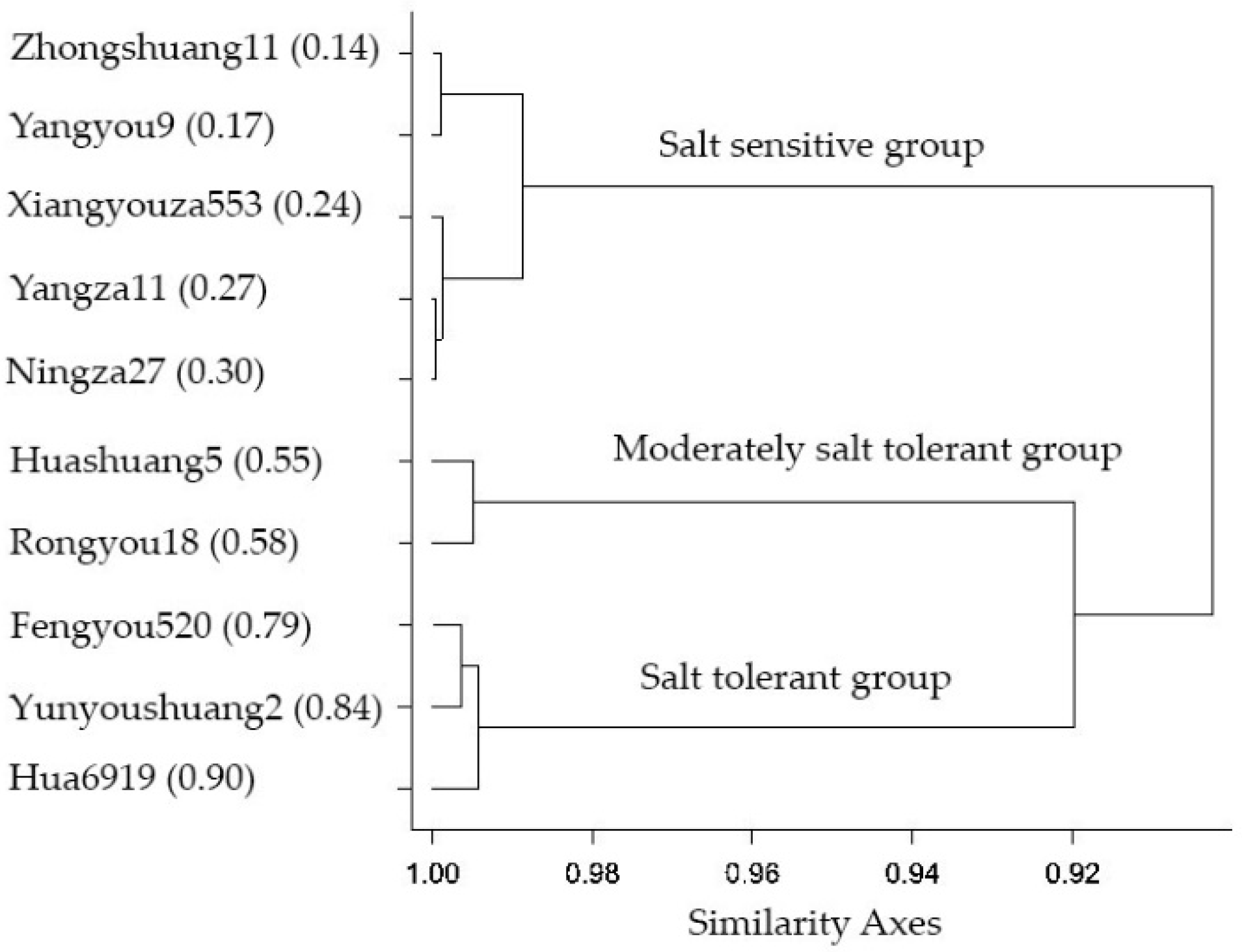
2.6. Ranking and Grouping of Rapeseed Cultivars for Salt Tolerance Evaluation
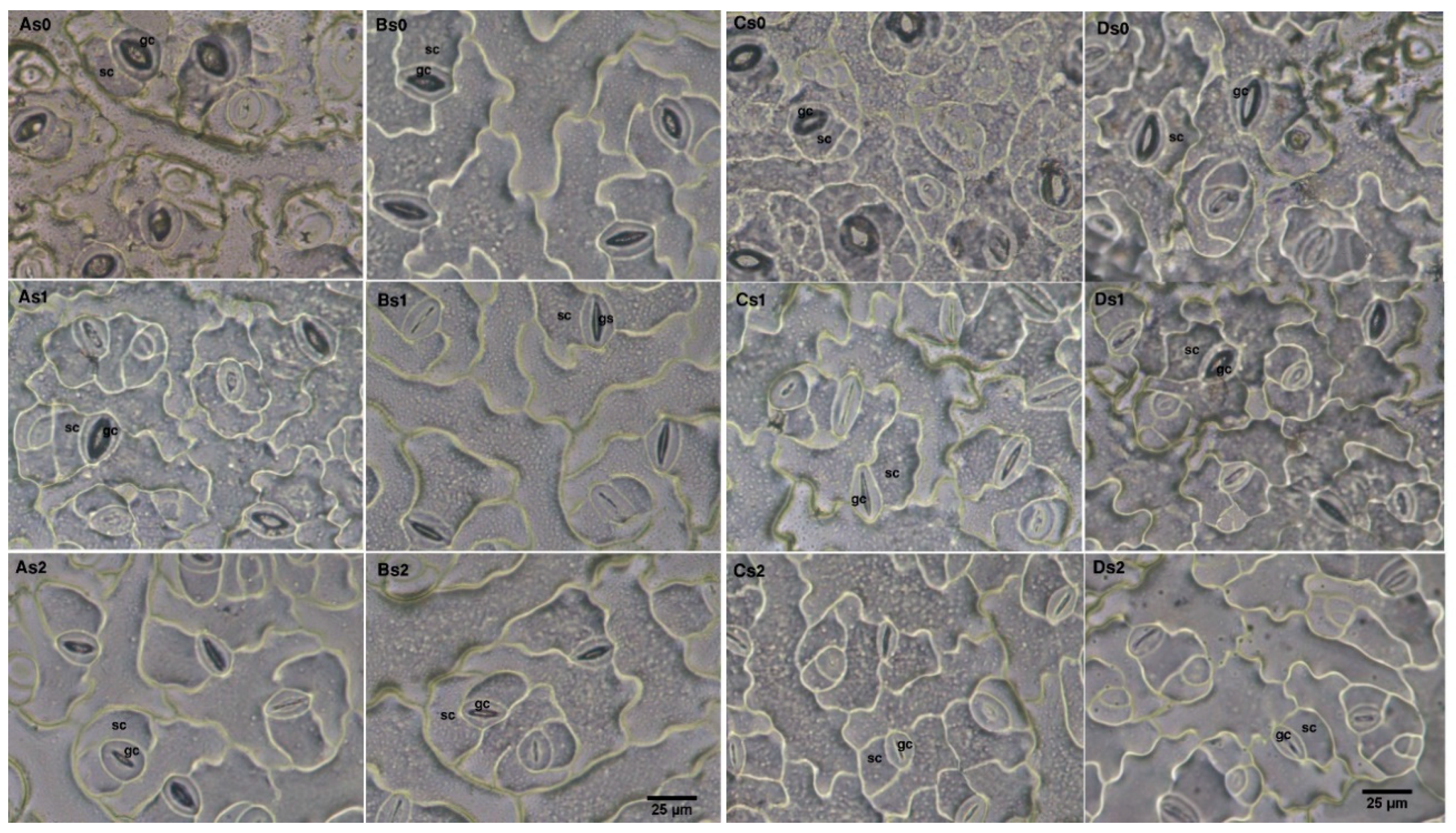
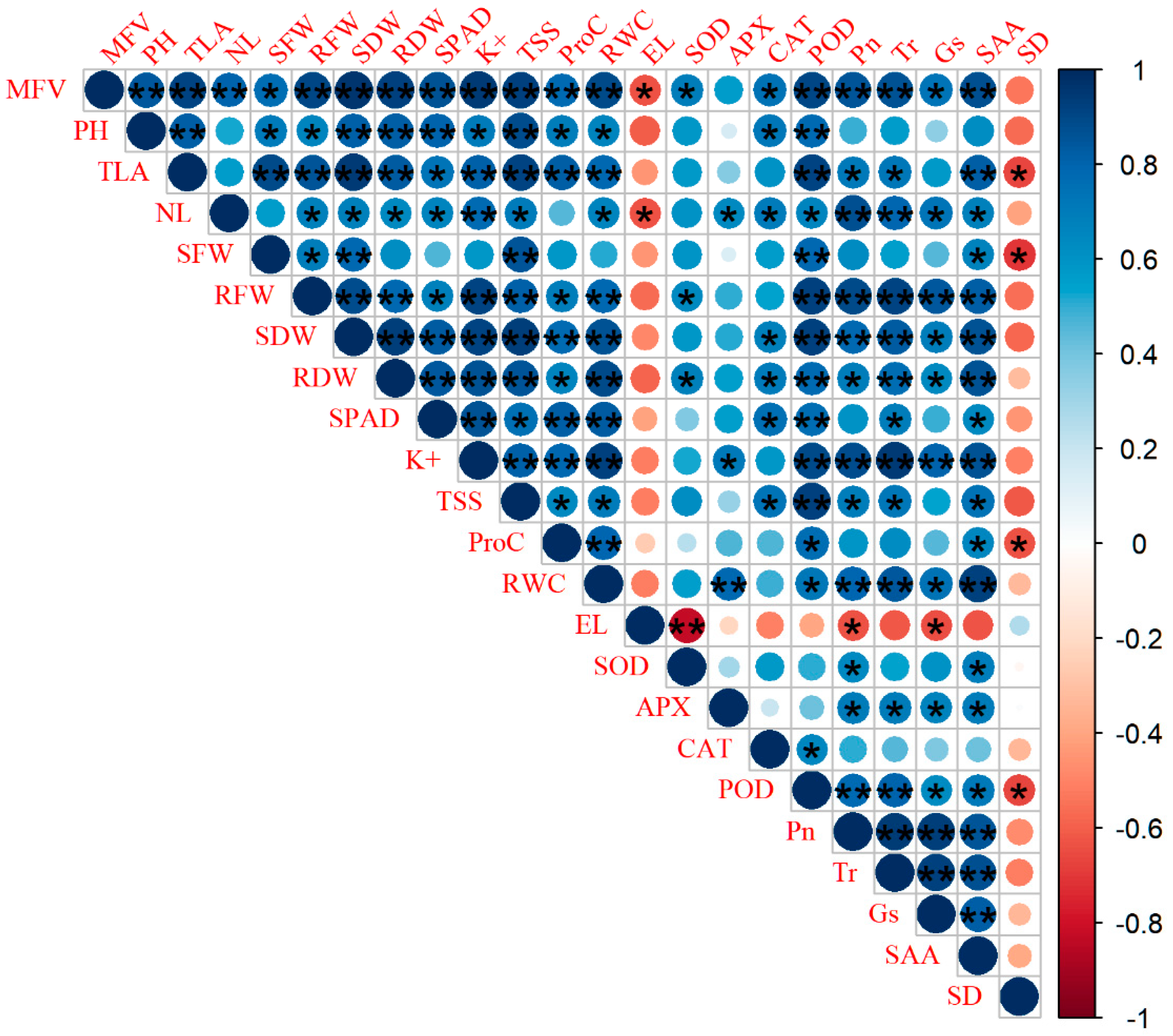
2.7. Assessment Role of Photosynthetic Gas Exchange Parameters and Stomata Anatomical Traits for Salinity Tolerance of Rapeseed Cultivars
3. Discussion
4. Materials and Methods
4.1. Materials, Conditions of Growth, and Experimental Treatments
4.2. Assessment of Growth and Biomass Yield
4.3. Assessment of Physiological and Biochemical Traits
4.3.1. Chlorophyll Content (SPAD)
4.3.2. Determination of K+, Proline, and Total Soluble Sugars Concentrations
4.3.3. Determination of Water Relations and Electrolyte Leakage
4.3.4. Assessment of Antioxidant Enzymes Activity
4.4. Salt Tolerance Evaluation
4.5. Measurement of Photosynthetic Gas Exchange Parameters
4.6. Assessment of Stomata Anatomical Parameters
4.7. Analysis of Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Machado, R.; Serralheiro, R. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.; Du, X.; Tang, H.; Shen, C.; Wu, J. Salicylic acid alleviates the adverse effects of salt stress in Torreya grandis cv. merrillii seedlings by activating photosynthesis and enhancing antioxidant systems. PLoS ONE 2014, 9, e109492. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; James, R.A.; Xu, B.; Athman, A.; Conn, S.J.; Jordans, C.; Byrt, C.S.; Hare, R.A.; Tyerman, S.D.; Tester, M. Wheat grain yield on saline soils is improved by an ancestral Na+ transporter gene. Nat. Biotechnol. 2012, 30, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Agami, R.A. Alleviating the adverse effects of NaCl stress in maize seedlings by pretreating seeds with salicylic acid and 24-epibrassinolide. South Afr. J. Bot. 2013, 88, 171–177. [Google Scholar] [CrossRef]
- Isayenkov, S.V. Physiological and molecular aspects of salt stress in plants. Cytol. Genet. 2012, 46, 302–318. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Miyamoto, S.; Oster, M.F.; Rostle, C.T.; Lenn, E.G. Salt tolerance of oilseed crops during establishment. J. Arid Land Stud. 2012, 22, 147–151. [Google Scholar]
- Dolatabadi, N.; Toorchi, M.; Valizadeh, M.; Bandehagh, A.; Ir, M.A. Effect of salinity stress on some physiological traits of spring rapeseed genotypes at seedling stage. J. Biodivers. Environ. Sci. 2016, 9, 2220–6663. [Google Scholar]
- Kholghi, M.; Toorchi, M.; Bandeh, A.L.I.; Shakiba, M.R. An evaluation of canola genotypes under salinity stress at vegetative stage via morphological and physiological traits. Pak. J. Bot 2018, 50, 447–455. [Google Scholar]
- Keshavarzi, M.H.B. Effect of salt stress on germination and early seedling growth of savory (Satureja hortensis). Aust. J. Basic Appl. Sci. 2011, 5, 3274–3279. [Google Scholar]
- Corpas, F.J.; Gómez, M.; Hernández, J.A.; Del Río, L.A. Metabolism of Activated Oxygen in Peroxisomes from two Pisum sativum L. Cultivars with Different Sensitivity to Sodium Chloride. J. Plant Physiol. 1993, 141, 160–165. [Google Scholar] [CrossRef]
- Hernández, J.A.; Corpas, F.J.; Gómez, M.; del Rio, L.A.; Sevilla, F. Salt-induced oxidative stress mediated by activated oxygen species in pea leaf mitochondria. Physiol. Plant. 1993, 89, 103–110. [Google Scholar] [CrossRef]
- Hernández, J.A.; Olmos, E.; Corpas, F.J.; Sevilla, F.; Del Rio, L.A. Salt-induced oxidative stress in chloroplasts of pea plants. Plant Sci. 1995, 105, 151–167. [Google Scholar] [CrossRef]
- Agami, R.A.; Ghramh, H.A.; Hasheem, M. Seed inoculation with Azospirillum lipoferum alleviates the adverse effects of drought stress on wheat plants. J. Appl. Bot. Food Qual. 2017, 90, 165–173. [Google Scholar]
- Paul, D.; Lade, H. Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: A review. Agron. Sustain. Dev. 2014, 34, 737–752. [Google Scholar] [CrossRef]
- Shokri-Gharelo, R.; Noparvar, P.M. Molecular response of canola to salt stress: Insights on tolerance mechanisms. PeerJ 2018, 6, e4822. [Google Scholar] [CrossRef]
- Kusvuran, S.; Kiran, S.; Ellialtioglu, S.S. Antioxidant Enzyme Activities and Abiotic Stress Tolerance Relationship in Vegetable Crops. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; Shanker, A., Shanker, C., Eds.; InTech: London, UK, 2016; pp. 481–506. [Google Scholar]
- Purty, R.S.; Kumar, G.; Singla-Pareek, S.L.; Pareek, A. Towards salinity tolerance in Brassica: An overview. Physiol. Mol. Biol. Plants 2008, 14, 39–49. [Google Scholar] [CrossRef]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Abdelaziz, M.N.; Xuan, T.D.; Mekawy, A.M.M.; Wang, H.; Khanh, T.D. Relationship of salinity tolerance to Na+ exclusion, proline accumulation, and antioxidant enzyme activity in rice seedlings. Agriculture 2018, 8, 166. [Google Scholar] [CrossRef]
- Hoque, M.A.; Banu, M.N.A.; Okuma, E.; Amako, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Exogenous proline and glycinebetaine increase NaCl-induced ascorbate–glutathione cycle enzyme activities, and proline improves salt tolerance more than glycinebetaine in tobacco Bright Yellow-2 suspension-cultured cells. J. Plant Physiol. 2007, 164, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Hussain Wani, S.; Brajendra Singh, N.; Haribhushan, A.; Iqbal Mir, J. Compatible solute engineering in plants for abiotic stress tolerance-role of glycine betaine. Curr. Genom. 2013, 14, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Sevengor, S.; Yasar, F.; Kusvuran, S.; Ellialtioglu, S. The effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidative enzymes of pumpkin seedling. Afr. J. Agric. Res. 2011, 6, 4920–4924. [Google Scholar]
- Banu, M.N.A.; Hoque, M.A.; Watanabe-Sugimoto, M.; Matsuoka, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J. Plant Physiol. 2009, 166, 146–156. [Google Scholar]
- Nounjan, N.; Charoensawan, V.; Chansongkrow, P.; Siangliw, J.L.; Toojinda, T.; Chadchawan, S.; Theerakulpisut, P. High performance of photosynthesis and osmotic adjustment are associated with salt tolerance ability in rice carrying drought tolerance QTL: Physiological and co-expression network analysis. Front. Plant Sci. 2018, 9, 1135. [Google Scholar] [CrossRef]
- Allel, D.; Ben-Amar, A.; Abdelly, C. Leaf photosynthesis, chlorophyll fluorescence and ion content of barley (Hordeum vulgare) in response to salinity. J. Plant Nutr. 2018, 41, 497–508. [Google Scholar] [CrossRef]
- FAO Oil Crops, Oils and Meals. Available online: http://www.fao.org/fileadmin/templates/est/COMM_MARKETS_MONITORING/Oilcrops/Documents/Food_outlook_oilseeds/FO_Oilcrops.pdf (accessed on 27 November 2019).
- Singh, J.; Singh, V.; Sharma, P.C. Elucidating the role of osmotic, ionic and major salt responsive transcript components towards salinity tolerance in contrasting chickpea (Cicer arietinum L.) genotypes. Physiol. Mol. Biol. Plants 2018, 24, 441–453. [Google Scholar] [CrossRef]
- Wu, H.; Guo, J.; Wang, C.; Li, K.; Zhang, X.; Yang, Z.; Li, M.; Wang, B. An effective screening method and a reliable screening trait for salt tolerance of Brassica napus at the germination stage. Front. Plant Sci. 2019, 10, 530. [Google Scholar] [CrossRef]
- Long, W.H.; Pu, H.M.; Zhang, J.F.; Qi, C.K.; Zhang, X.K. Screening of Brassica napus for salinity tolerance at germination stage. Chin. J. Oil Crop Sci. 2013, 35, 271–275. [Google Scholar]
- Liu, Y.; Li, P.; Xu, G.C.; Xiao, L.; Ren, Z.P.; Li, Z. Bin Growth, morphological, and physiological responses to drought stress in Bothriochloa ischaemum. Front. Plant Sci. 2017, 8, 230. [Google Scholar] [PubMed]
- Chen, X.; Min, D.; Yasir, T.A.; Hu, Y.-G. Evaluation of 14 morphological, yield-related and physiological traits as indicators of drought tolerance in Chinese winter bread wheat revealed by analysis of the membership function value of drought tolerance (MFVD). Field Crop. Res. 2012, 137, 195–201. [Google Scholar] [CrossRef]
- Wassie, M.; Zhang, W.; Zhang, Q.; Ji, K.; Chen, L. Effect of Heat Stress on Growth and Physiological Traits of Alfalfa (Medicago sativa L.) and a Comprehensive Evaluation for Heat Tolerance. Agronomy 2019, 9, 597. [Google Scholar] [CrossRef]
- Ma, T.; Zeng, W.; Li, Q.; Wu, J.; Huang, J. Effects of water, salt and nitrogen stress on sunflower (Helianthus annuus L.) at different growth stages. J. Soil Sci. Plant Nutr. 2016, 16, 1024–1037. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol. Trace Elem. Res. 2011, 143, 1758–1776. [Google Scholar] [CrossRef]
- Agami, R.A. Pre-soaking in indole-3-acetic acid or spermidine enhances copper tolerance in wheat seedlings. S. Afr. J. Bot. 2016, 104, 167–174. [Google Scholar] [CrossRef]
- Ashraf, M.; McNeilly, T. Salinity tolerance in Brassica oilseeds. CRC Crit. Rev. Plant Sci. 2004, 23, 157–174. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. BioMed Res. Int. 2014, 2014, 757219. [Google Scholar] [CrossRef]
- Tatar, Ö.; Brueck, H.; Gevrek, M.N.; Asch, F. Physiological responses of two turkish rice (oryza sativa l.) varieties to salinity. Turk. J. Agric. For. 2010, 34, 451–459. [Google Scholar]
- Chunthaburee, S.; Dongsansuk, A.; Sanitchon, J.; Pattanagul, W.; Theerakulpisut, P. Physiological and biochemical parameters for evaluation and clustering of rice cultivars differing in salt tolerance at seedling stage. Saudi J. Biol. Sci. 2016, 23, 467–477. [Google Scholar] [CrossRef]
- Xu, X.; Xu, H.; Wang, Y.; Wang, X.; Qiu, Y.; Xu, B. The effect of salt stress on the chlorophyll level of the main sand-binding plants in the shelterbelt along the Tarim Desert Highway. Chin. Sci. Bull. 2008, 53, 109–111. [Google Scholar] [CrossRef][Green Version]
- Ma, N.; Hu, C.; Wan, L.; Hu, Q.; Xiong, J.; Zhang, C. Strigolactones improve plant growth, photosynthesis, and alleviate oxidative stress under salinity in rapeseed (Brassica napus L.) by regulating gene expression. Front. Plant Sci. 2017, 8, 1671. [Google Scholar] [CrossRef] [PubMed]
- Stepien, P.; Johnson, G.N. Contrasting Responses of Photosynthesis to Salt Stress in the Glycophyte Arabidopsis and the Halophyte Thellungiella: Role of the Plastid Terminal Oxidase as an Alternative Electron Sink. Plant Physiol. 2009, 149, 1154–1165. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Diaz-Vivancos, P.; Álvarez, S.; Fernández-García, N.; Sanchez-Blanco, M.J.; Hernández, J.A. Physiological and biochemical mechanisms of the ornamental Eugenia myrtifolia L. plants for coping with NaCl stress and recovery. Planta 2015, 242, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Maathuis, F.J.M. Cellular and tissue distribution of potassium: Physiological relevance, mechanisms and regulation. J. Plant Physiol. 2014, 171, 708–714. [Google Scholar] [CrossRef]
- Sun, Y.; Kong, X.; Li, C.; Liu, Y.; Ding, Z. Potassium retention under salt stress is associated with natural variation in salinity tolerance among Arabidopsis accessions. PLoS ONE 2015, 10, e0124032. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, M.; Shabala, L.; Zhou, M.; Shabala, S. K+ retention in leaf mesophyll, an overlooked component of salinity tolerance mechanism: A case study for barley. J. Integr. Plant Biol. 2015, 57, 171–185. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savoure, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef]
- Abdelhamid, M.T.; Rady, M.M.; Osman, A.S.; Abdalla, M.A. Exogenous application of proline alleviates salt-induced oxidative stress in Phaseolus vulgaris L. plants. J. Hortic. Sci. Biotechnol. 2013, 88, 439–446. [Google Scholar] [CrossRef]
- Xue, X.; Liu, A.; Hua, X. Proline accumulation and transcriptional regulation of proline biothesynthesis and degradation in Brassica napus. BMB Rep. 2009, 42, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Saadia, M.; Jamil, A.; Akram, N.A.; Ashraf, M. A study of proline metabolism in canola (Brassica napus L.) seedlings under salt stress. Molecules 2012, 17, 5803–5815. [Google Scholar] [CrossRef] [PubMed]
- El Bassiouny, H.M.S.; Abdallah, M.M.S.; Rady, M.M.; Gaballah, M.S.; El-Sebai, T.N. Role of blue-green algae, glutathione and salicylic acid on the oxidative defense systems of wheat plant grown in saline soil. Int. J. PharmTech Res. 2015, 8, 18–31. [Google Scholar]
- Slabbert, M.M.; Krüger, G.H.J. Antioxidant enzyme activity, proline accumulation, leaf area and cell membrane stability in water stressed Amaranthus leaves. South Afr. J. Bot. 2014, 95, 123–128. [Google Scholar] [CrossRef]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef]
- Rezaei, H.; Khosh Kholgh Sima, N.A.; Malakouti, M.J.; Pessarakli, M. Salt tolerance of canola in relation to accumulation and xylem transportation of cations. J. Plant Nutr. 2006, 29, 1903–1917. [Google Scholar] [CrossRef]
- Wangsawang, T.; Chuamnakthong, S.; Kohnishi, E.; Sripichitt, P.; Sreewongchai, T.; Ueda, A. A salinity-tolerant japonica cultivar has Na+ exclusion mechanism at leaf sheaths through the function of a Na+ transporter Os HKT 1; 4 under salinity stress. J. Agron. Crop Sci. 2018, 204, 274–284. [Google Scholar] [CrossRef]
- Hniličková, H.; Hnilička, F.; Orsák, M.; Hejnák, V. Effect of salt stress on growth, electrolyte leakage, Na+ and K+ content in selected plant species. Plant Soil Environ. 2018, 65, 90–96. [Google Scholar] [CrossRef]
- Hoque, M.A.; Banu, M.N.A.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycinebetaine enhance antioxidant defense and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. J. Plant Physiol. 2008, 165, 813–824. [Google Scholar] [CrossRef]
- El-Shabrawi, H.; Kumar, B.; Kaul, T.; Reddy, M.K.; Singla-Pareek, S.L.; Sopory, S.K. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 2010, 245, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Biochar mitigates salinity stress in potato. J. Agron. Crop Sci. 2015, 201, 368–378. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups; Springer: Berlin/Heidelberg, Germany, 2003; ISBN 3540435166. [Google Scholar]
- Waqas, M.; Yaning, C.; Iqbal, H.; Shareef, M.; Rehman, H.; Yang, Y. Paclobutrazol improves salt tolerance in quinoa: Beyond the stomatal and biochemical interventions. J. Agron. Crop Sci. 2017, 203, 315–322. [Google Scholar] [CrossRef]
- Curtis, P.S.; Lauchli, A. The effect of moderate salt stress on leaf anatomy in Hibiscus cannabinus (kenaf) and its relation to leaf area. Am. J. Bot. 1987, 74, 538–542. [Google Scholar] [CrossRef]
- Zörb, C.; Mühling, K.H.; Kutschera, U.; Geilfus, C.-M. Salinity stiffens the epidermal cell walls of salt-stressed maize leaves: Is the epidermis growth-restricting? PLoS ONE 2015, 10, e0118406. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Schmidhalter, U. Effect of salinity on the composition, number and size of epidermal cells along the mature blade of wheat leaves. J. Integr. Plant Biol. 2007, 49, 1016–1023. [Google Scholar] [CrossRef]
- Acosta-Motos, J.; Ortuño, M.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.; Hernandez, J. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Wan, H.; Li, Q.; Gao, Y.; Wen, J.; Ma, C.; Tu, J.; Fu, T.; Shen, J. Application of high efficiency and low cost method for subculturing rapeseed microspore regenerated plants based on hydroponics. Chin. J. Oil Crop Sci. 2016, 38, 588–591. [Google Scholar]
- Wu, F.; Wu, L.; Xu, F. Chlorophyll meter to predict nitrogen sidedress requirements for short-season cotton (Gossypium hirsutum L.). Field Crop. Res. 1998, 56, 309–314. [Google Scholar]
- Munns, R.; Wallace, P.A.; Teakle, N.L.; Colmer, T.D. Measuring soluble ion concentrations (Na+, K+, Cl−) in salt-treated plants. In Plant Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2010; pp. 371–382. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Maness, N. Extraction and analysis of soluble carbohydrates. In Plant Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2010; pp. 341–370. [Google Scholar]
- Morant-Manceau, A.; Pradier, E.; Tremblin, G. Osmotic adjustment, gas exchanges and chlorophyll fluorescence of a hexaploid triticale and its parental species under salt stress. J. Plant Physiol. 2004, 161, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Korkmaz, Y.; Demirkıran, A.R. Enhancing chilling stress tolerance of pepper seedlings by exogenous application of 5-aminolevulinic acid. Environ. Exp. Bot. 2010, 67, 495–501. [Google Scholar] [CrossRef]
- Nazar, R.; Umar, S.; Khan, N.A. Exogenous salicylic acid improves photosynthesis and growth through increase in ascorbate-glutathione metabolism and S assimilation in mustard under salt stress. Plant Signal. Behav. 2015, 10, e1003751. [Google Scholar] [CrossRef] [PubMed]
- Hamill, S.D.; Smith, M.K.; Dodd, W.A. In vitro induction of banana autotetraploids by colchicine treatment of micropropagated diploids. Aust. J. Bot. 1992, 40, 887–896. [Google Scholar] [CrossRef]
- Barbieri, G.; Vallone, S.; Orsini, F.; Paradiso, R.; De Pascale, S.; Negre-zakharov, F.; Maggio, A. Stomatal density and metabolic determinants mediate salt stress adaptation and water use efficiency in basil (Ocimum basilicum L.). J. Plant Physiol. 2012, 169, 1737–1746. [Google Scholar] [CrossRef]
- VSN International GENSTAT for Windows 17th Edition (VSN Int. Hemel Hempstead, UK). 2015. Available online: https://GENSTAT.kb.vsni.co.uk (accessed on 27 November 2019).

| Cultivars | PH | TLA | NL | SFW | RFW | SDW | RDW |
|---|---|---|---|---|---|---|---|
| Zhongshuang11 | 71.7 | 39.1 | 77.8 | 41.5 | 48.9 | 45.7 | 46.2 |
| Yangyou9 | 69.2 | 45.6 | 70.5 | 47.2 | 57.1 | 48.2 | 44.3 |
| Hua6919 | 82.9 | 57.4 | 88.2 | 52.7 | 66.8 | 74.3 | 65.7 |
| Xiangyouza553 | 73.8 | 48.8 | 71.4 | 49.1 | 49.1 | 52.3 | 49.7 |
| Yangza11 | 80.2 | 44.5 | 70.3 | 42.6 | 53.1 | 49.1 | 50.4 |
| Fengyou520 | 88.5 | 60.6 | 77.1 | 54.2 | 62.6 | 72.3 | 61.7 |
| Huashuang5 | 76.7 | 53.7 | 78.2 | 48.3 | 62.1 | 64.2 | 52.8 |
| Ningza27 | 71.2 | 43.8 | 71.5 | 37.6 | 54.7 | 52.2 | 53.0 |
| Rongyou18 | 77.5 | 49.5 | 86.2 | 47.4 | 57.6 | 60.1 | 53.7 |
| Yunyoushuang2 | 83.5 | 56.9 | 89.1 | 53.2 | 65.2 | 65.5 | 57.6 |
| Cultivars | SPAD | K+ | TSS | ProC | RWC | EL | SOD | CAT | APX | POD |
|---|---|---|---|---|---|---|---|---|---|---|
| Zhongshuang11 | 100 | 16.2 | 134 | 147 | 76.5 | 190.6 | 149 | 159 | 103 | 135 |
| Yangyou9 | 85 | 15.9 | 130 | 159 | 77.0 | 173.1 | 180 | 141 | 94 | 144 |
| Hua6919 | 119 | 25.6 | 158 | 177 | 88.2 | 121.0 | 245 | 177 | 121 | 182 |
| Xiangyouza553 | 96 | 15.8 | 134 | 172 | 81.0 | 184.8 | 165 | 137 | 105 | 128 |
| Yangza11 | 104 | 17.5 | 134 | 169 | 80.2 | 139.1 | 176 | 143 | 94 | 133 |
| Fengyou520 | 129 | 22.9 | 159 | 202 | 84.7 | 170.1 | 192 | 195 | 100 | 193 |
| Huashuang5 | 115 | 23.9 | 145 | 197 | 84.6 | 189.2 | 130 | 138 | 116 | 179 |
| Ningza27 | 111 | 19.9 | 126 | 179 | 84.3 | 187.5 | 164 | 144 | 120 | 136 |
| Rongyou18 | 120 | 21.5 | 139 | 189 | 83.9 | 144.4 | 174 | 183 | 112 | 154 |
| Yunyoushuang2 | 121 | 24.9 | 153 | 199 | 86.7 | 132.4 | 232 | 172 | 120 | 186 |
| Cultivars | Pn | Tr | Gs | SAA | SD |
|---|---|---|---|---|---|
| Zhongshuang11 | 43.6 | 43.6 | 32.2 | 26.0 | 129.2 |
| Yangyou9 | 53.1 | 48.2 | 47.0 | 36.5 | 123.6 |
| Hua6919 | 75.6 | 74.7 | 67.3 | 77.4 | 123.0 |
| Xiangyouza553 | 45.0 | 42.9 | 33.8 | 48.8 | 124.8 |
| Yangza11 | 44.5 | 49.4 | 38.3 | 40.8 | 122.8 |
| Fengyou520 | 54.4 | 55.2 | 41.2 | 52.2 | 116.4 |
| Huashuang5 | 63.8 | 66.8 | 52.4 | 55.8 | 112.1 |
| Ningza27 | 50.6 | 51.6 | 45.9 | 46.7 | 135.8 |
| Rongyou18 | 64.8 | 60.6 | 54.7 | 50.9 | 117.2 |
| Yunyoushuang2 | 74.3 | 66.6 | 55.6 | 64.9 | 119.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, I.A.A.; Shalby, N.; Bai, C.; Qin, M.; Agami, R.A.; Jie, K.; Wang, B.; Zhou, G. Stomatal and Photosynthetic Traits Are Associated with Investigating Sodium Chloride Tolerance of Brassica napus L. Cultivars. Plants 2020, 9, 62. https://doi.org/10.3390/plants9010062
Mohamed IAA, Shalby N, Bai C, Qin M, Agami RA, Jie K, Wang B, Zhou G. Stomatal and Photosynthetic Traits Are Associated with Investigating Sodium Chloride Tolerance of Brassica napus L. Cultivars. Plants. 2020; 9(1):62. https://doi.org/10.3390/plants9010062
Chicago/Turabian StyleMohamed, Ibrahim A. A., Nesma Shalby, Chenyang Bai, Meng Qin, Ramadan A. Agami, Kuai Jie, Bo Wang, and Guangsheng Zhou. 2020. "Stomatal and Photosynthetic Traits Are Associated with Investigating Sodium Chloride Tolerance of Brassica napus L. Cultivars" Plants 9, no. 1: 62. https://doi.org/10.3390/plants9010062
APA StyleMohamed, I. A. A., Shalby, N., Bai, C., Qin, M., Agami, R. A., Jie, K., Wang, B., & Zhou, G. (2020). Stomatal and Photosynthetic Traits Are Associated with Investigating Sodium Chloride Tolerance of Brassica napus L. Cultivars. Plants, 9(1), 62. https://doi.org/10.3390/plants9010062







