Root Development of Bell Pepper (Capsicum annuum L.) as Affected by Water Salinity and Sink Strength
Abstract
1. Introduction
2. Results
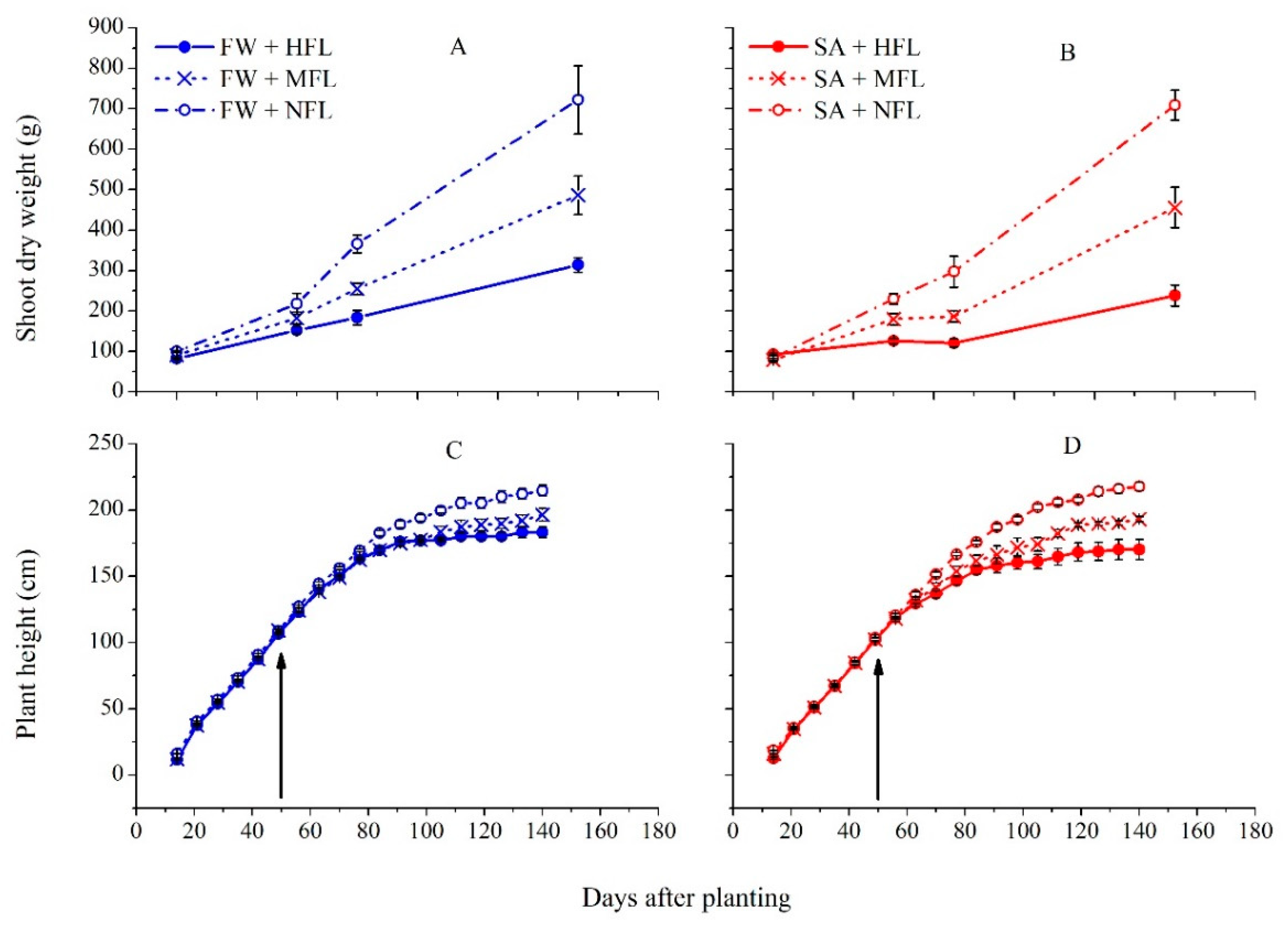
2.1. Plant Growth Dynamic
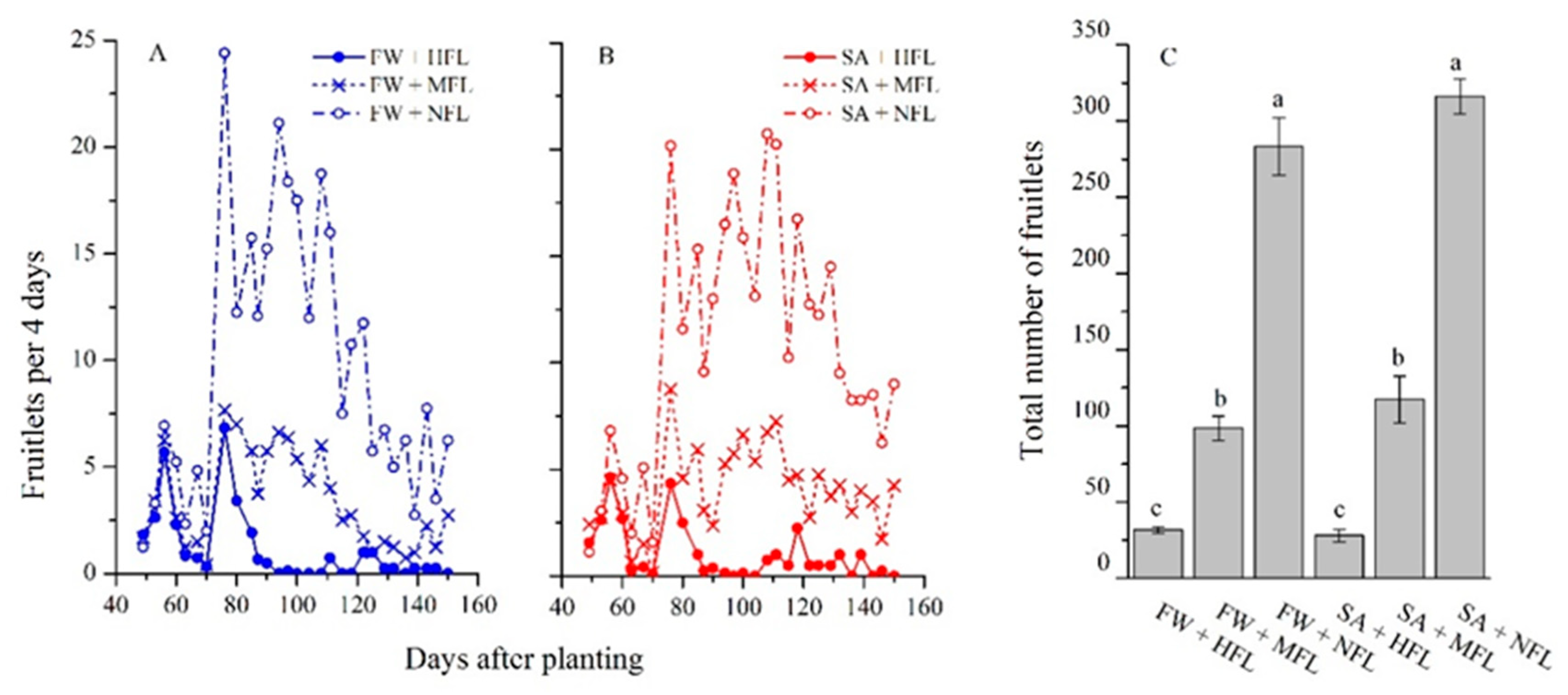
2.2. Fruit Production
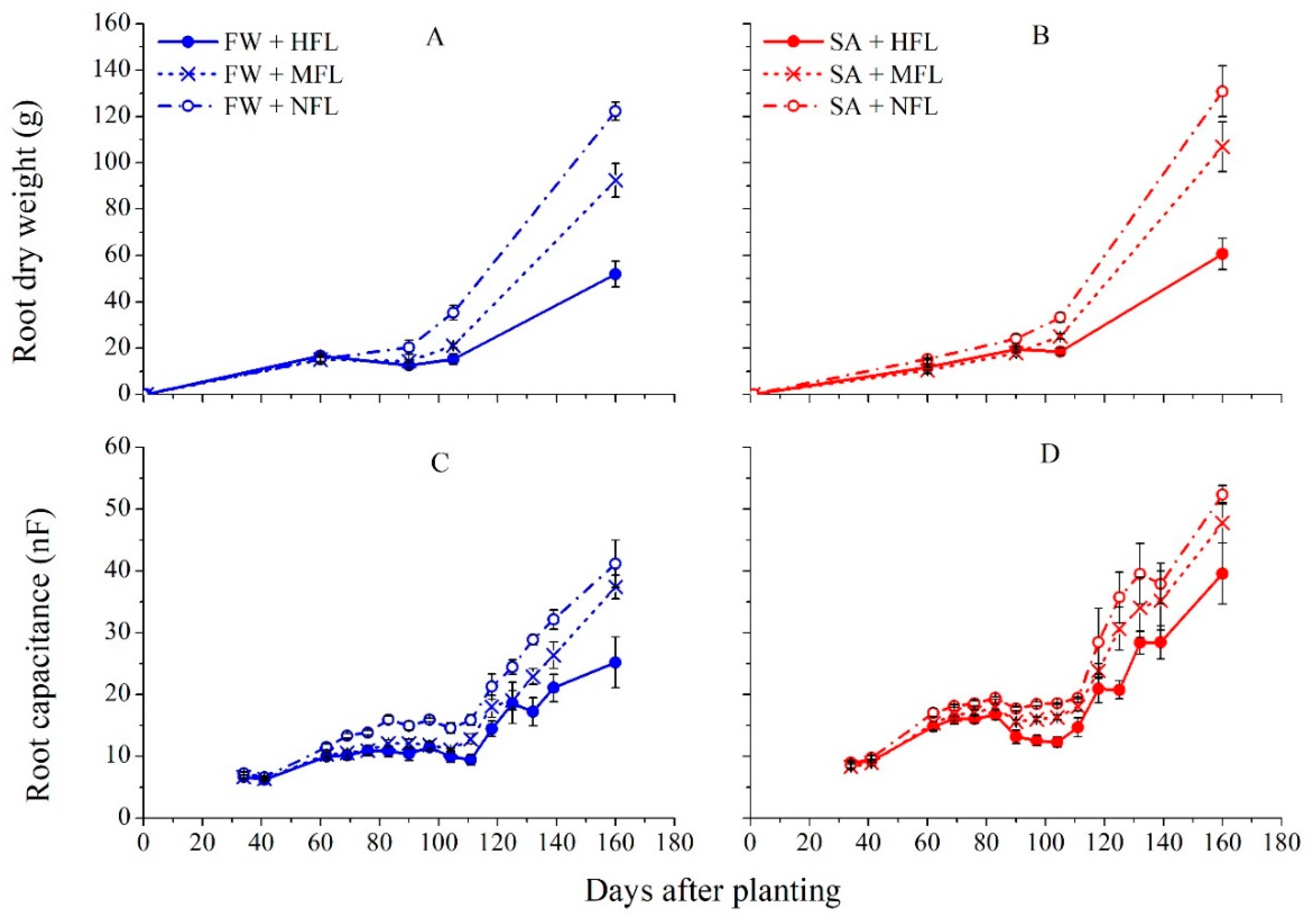
2.3. Root Capacitance
2.4. Root Biomass
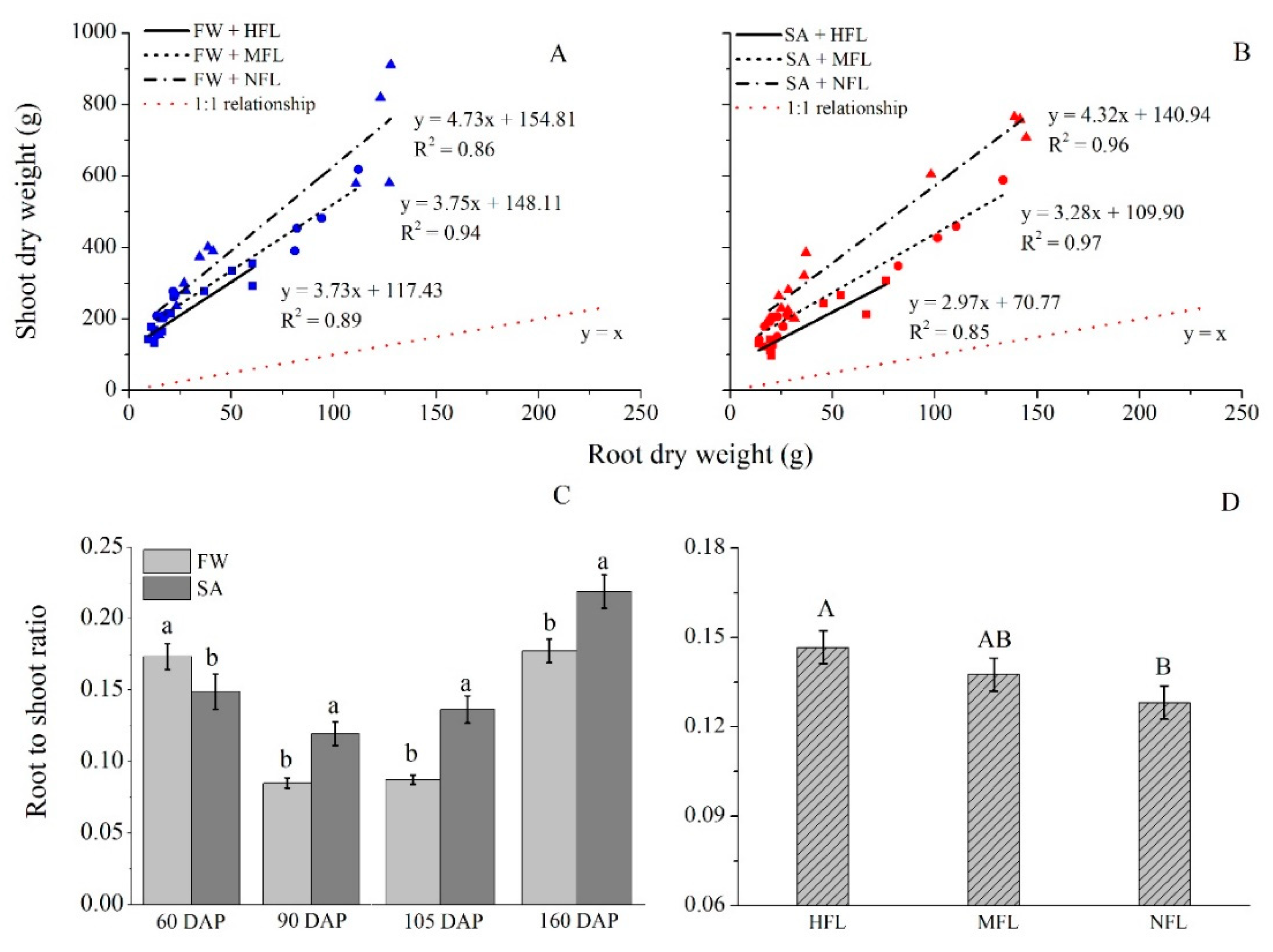
2.5. Root Weight
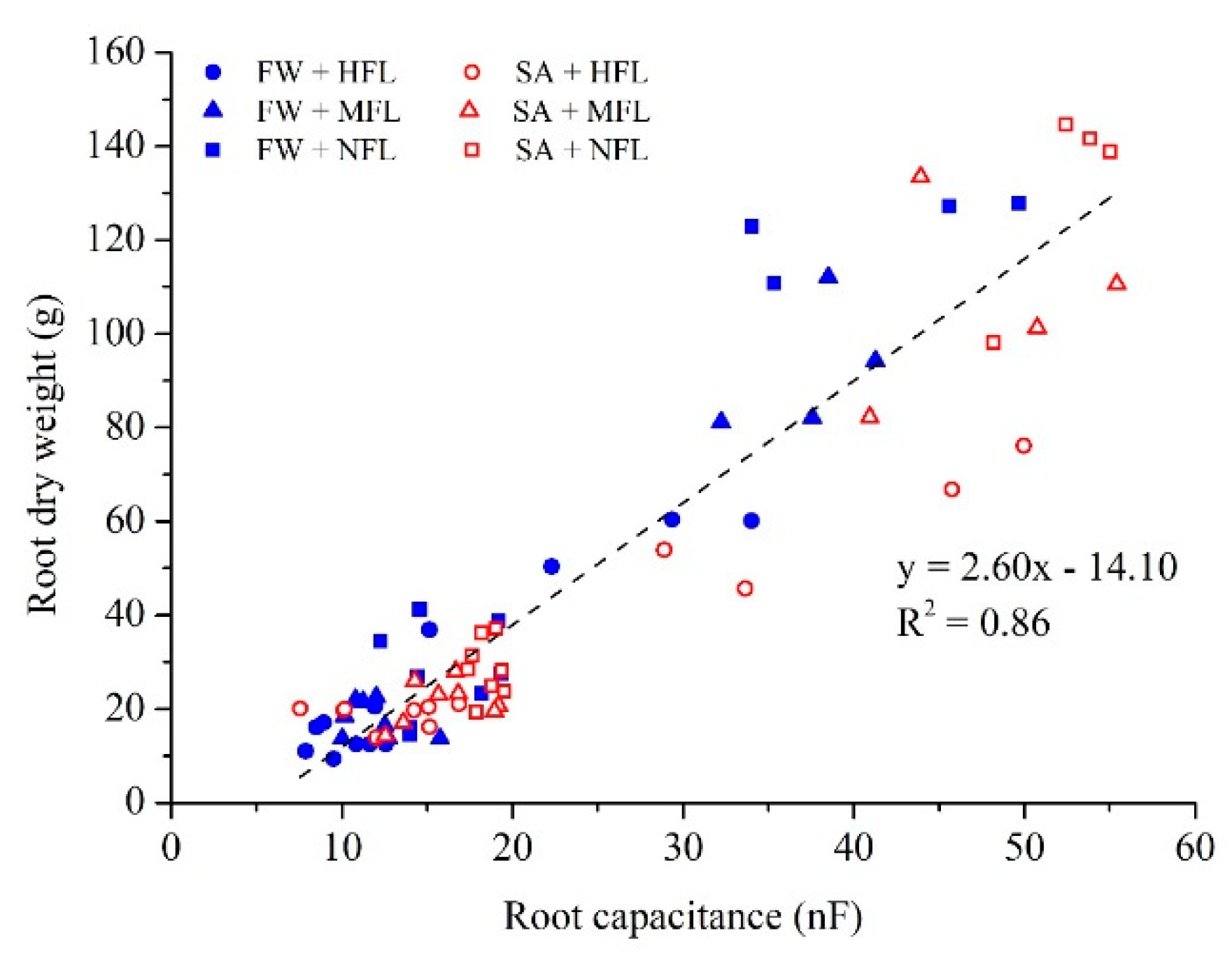
2.6. Growth Dynamic by Root Capacitance
2.7. Root/Shoot Ratio
2.8. Mineral Analysis
3. Discussion
4. Methods
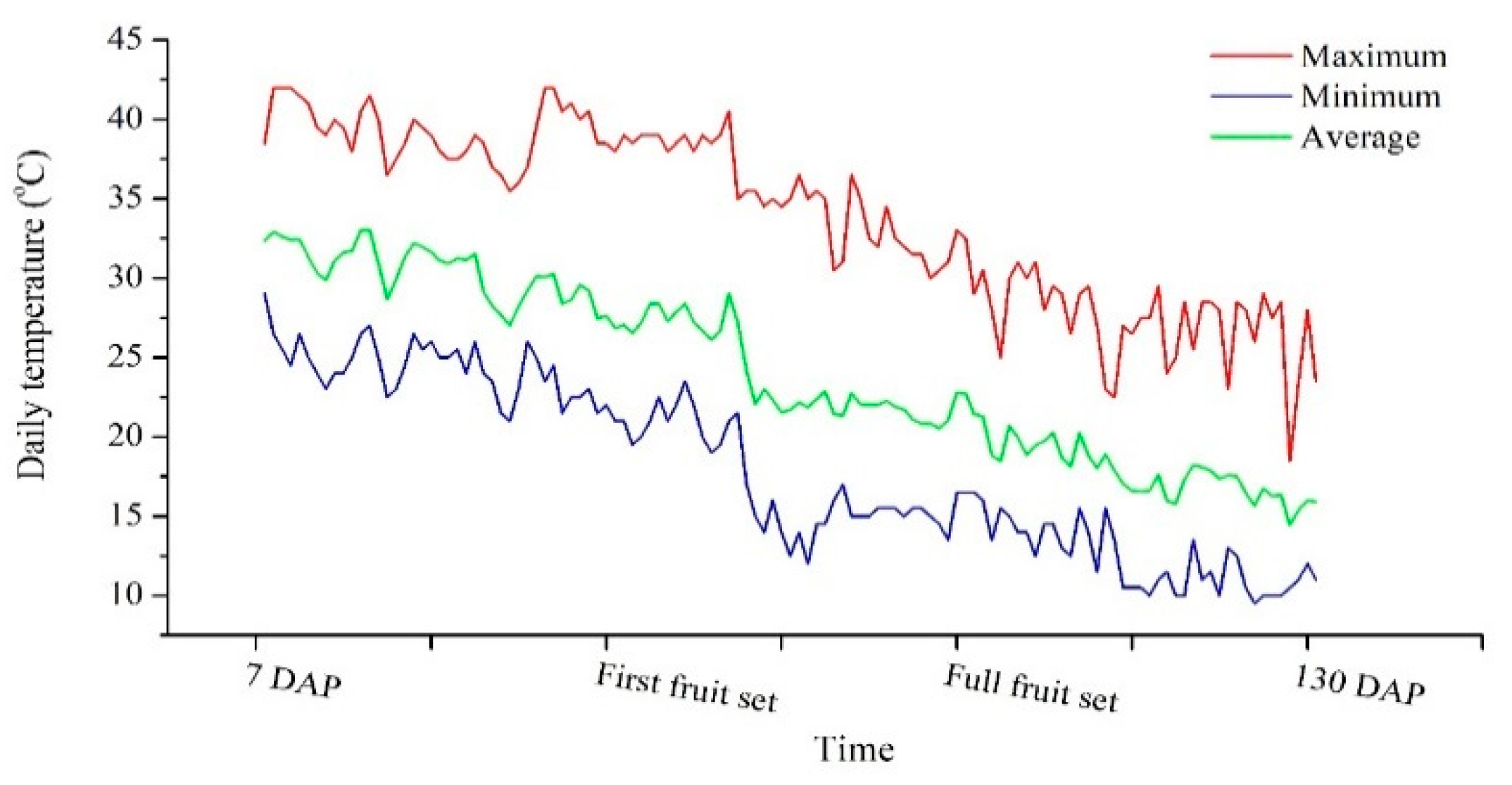
4.1. Experimental Design and Growth Conditions
4.2. Treatments
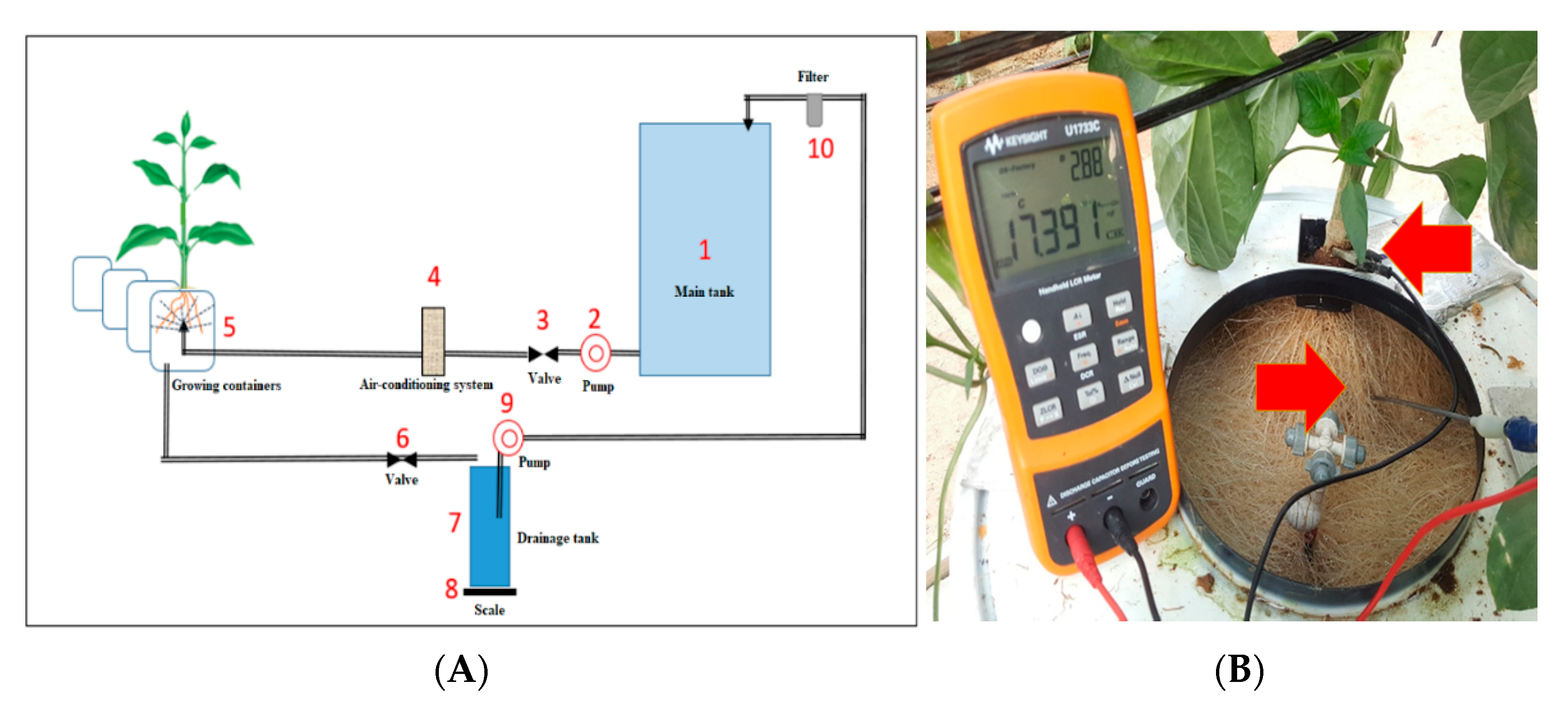
4.3. Aeroponic System
4.4. Measurements
4.4.1. Height
4.4.2. Fruit Set
4.4.3. Root Capacitance
4.4.4. Mineral Analysis
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zhou, L.; Christopher, D.A.; Paull, R.E. Defoliation and fruit removal effects on papaya fruit production, sugar accumulation, and sucrose metabolism. J. Am. Soc. Hortic. Sci. 2000, 125, 644–652. [Google Scholar] [CrossRef]
- Sergeeva, L.I.; Keurentjes, J.J.; Bentsink, L.; Vonk, J.; van der Plas, L.H.; Koornneef, M.; Vreugdenhil, D. Vacuolar invertase regulates elongation of Arabidopsis thaliana roots as revealed by QTL and mutant analysis. Proc. Natl. Acad. Sci. USA 2006, 103, 2994–2999. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Rao, I.M.; Merchant, A. Source-sink relationships in crop plants and their influence on yield development and nutritional quality. Front. Plant Sci. 2018, 9, 1889. [Google Scholar] [CrossRef] [PubMed]
- Lo Bianco, R.; Rieger, M.; Sung, S.J.S. Carbohydrate metabolism of vegetative and reproductive sinks in the late-maturing peach cultivar ‘Encore’. Tree Physiol. 1999, 19, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.G.; Zhu, X.G. Source–sink interaction: A century old concept under the light of modern molecular systems biology. J. Exp. Bot. 2017, 68, 4417–4431. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, K.; Imai, S.; Yakushiji, H.; Koshita, Y. Effects of fruit load on partitioning of 15N and 13C, respiration, and growth of grapevine roots at different fruit stages. Sci. Hortic. 2003, 97, 239–253. [Google Scholar] [CrossRef]
- Feild, T.S.; Arens, N.C. The ecophysiology of early angiosperms. Plant Cell Environ. 2007, 30, 291–309. [Google Scholar] [CrossRef]
- Choi, S.T.; Park, D.S.; Kang, S.M.; Cho, Y.C. Effect of fruit-load on the growth, absorption, and partitioning of inorganic nutrients in young ‘Fuyu’persimmon trees. Sci. Hortic. 2010, 126, 408–412. [Google Scholar] [CrossRef]
- Villena, I.A.; Puente, W.C.; Martínez, L.L.; Muñoz, J.V.; Sánchez, M.d.C.R. Root and aerial growth in early-maturing peach trees under two crop load treatments. Span. J. Agric. Res. 2017, 15, 18. [Google Scholar]
- Lenz, F. Fruit effects on the dry matter-and carbohydrate distribution in apple trees. Acta Hortic. 2009, 835, 21–38. [Google Scholar] [CrossRef]
- Nielsen, T.H.; Veierskov, B. Distribution of dry matter in sweet pepper plants (Capsicum annuum L.) during the juvenile and generative growth phases. Sci. Hortic. 1988, 35, 179–187. [Google Scholar] [CrossRef]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef] [PubMed]
- Shani, U.; Waisel, Y.; Eshel, A.; Xue, S.; Ziv, G. Responses to salinity of grapevine plants with split root systems. New Phytol. 1993, 124, 695–701. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, T. Responses of woody plants to flooding and salinity. Tree Physiol. 1997, 17, 490. [Google Scholar] [CrossRef]
- Ayer, R.; Westcot, D. Water Quality for Agriculture, Irrigation and Drainage; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985; p. 97. [Google Scholar]
- Ben-Gal, A.; Ityel, E.; Dudley, L.; Cohen, S.; Yermiyahu, U.; Presnov, E.; Zigmond, L.; Shani, U. Effect of irrigation water salinity on transpiration and on leaching requirements: A case study for bell peppers. Agric. Water Manag. 2008, 95, 587–597. [Google Scholar] [CrossRef]
- Yasuor, H.; Ben-Gal, A.; Yermiyahu, U.; Beit-Yannai, E.; Cohen, S. Nitrogen management of greenhouse pepper production: Agronomic, nutritional, and environmental implications. HortScience 2013, 48, 1241–1249. [Google Scholar] [CrossRef]
- Ben-Gal, A.; Yermiyahu, U.; Cohen, S. Fertilization and blending alternatives for irrigation with desalinated water. J. Environ. Qual. 2009, 38, 529–536. [Google Scholar] [CrossRef]
- Trachsel, S.; Kaeppler, S.M.; Brown, K.M.; Lynch, J.P. Shovelomics: High throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil 2011, 341, 75–87. [Google Scholar] [CrossRef]
- Rewald, B.; Ephrath, J.E. Minirhizotron Techniques. In Plant Roots: The Hidden Half, 4th ed.; Eshel, A., Beeckman, T., Eds.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Metzner, R.; Eggert, A.; van Dusschoten, D.; Pflugfelder, D.; Gerth, S.; Schurr, U.; Uhlmann, N.; Jahnke, S. Direct comparison of MRI and X-ray CT technologies for 3D imaging of root systems in soil: Potential and challenges for root trait quantification. Plant Methods 2015, 11, 17. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Gao, J.; Syed, T.N.; Chandio, F.A.; Buttar, N.A. Modern plant cultivation technologies in agriculture under controlled environment: A review on aeroponics. J. Plant Interact. 2018, 13, 338–352. [Google Scholar] [CrossRef]
- Dalton, F.N. In-situ root extent measurements by electrical capacitance methods. Plant Soil 1995, 173, 157–165. [Google Scholar] [CrossRef]
- Aulen, M.; Shipley, B. Non-destructive estimation of root mass using electrical capacitance on ten herbaceous species. Plant Soil 2012, 355, 41–49. [Google Scholar] [CrossRef]
- Fallik, E.; Alkalai-Tuvia, S.; Chalupowicz, D.; Zaaroor-Presman, M.; Offenbach, R.; Cohen, S.; Tripler, E. How water quality and quantity affect pepper yield and postharvest quality. Horticulturae 2019, 5, 4. [Google Scholar] [CrossRef]
- Wyngaard, S.R.; Kissinger, M. Materials flow analysis of a desert food production system: The case of bell peppers. J. Clean. Prod. 2019, 227, 512–521. [Google Scholar] [CrossRef]
- Kaner, A.; Tripler, E.; Hadas, E.; Ben-Gal, A. Feasibility of desalination as an alternative to irrigation with water high in salts. Desalination 2017, 416, 122–128. [Google Scholar] [CrossRef]
- Heuvelink, E.; Marcelis, L.; Körner, O. How to reduce yield fluctuations in sweet pepper? Acta Hortic. 2002, 633, 349–355. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Navarro, J.; Garrido, C.; Flores, P.; Martínez, V. The effect of salinity on yield and fruit quality of pepper grown in perlite. Span. J. Agric. Res. 2010, 8, 142–150. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Klapaki, G. Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci. Hortic. 2000, 86, 247–260. [Google Scholar] [CrossRef]
- Aidoo, M.K.; Sherman, T.; Lazarovitch, N.; Fait, A.; Rachmilevitch, S. Physiology and metabolism of grafted bell pepper in response to low root-zone temperature. Func. Plant Biol. 2019, 46, 339–349. [Google Scholar] [CrossRef]
- Marcelis, L.; Heuvelink, E.; Baan Hofman-Eijer, L.; Den Bakker, J.; Xue, L. Flower and fruit abortion in sweet pepper in relation to source and sink strength. J. Exp. Bot. 2004, 55, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Brady, C.J. Assimilate source-sink relationships in Capsicum annuum L. II. Effects of fruiting and defloration on the photosynthetic capacity and senescence of the leaves. Func. Plant Biol. 1977, 4, 771–783. [Google Scholar] [CrossRef]
- Bustan, A.; Erner, Y.; Goldschmidt, E. Interactions between developing citrus fruits and their supportive vascular system. Ann. Bot. 1995, 76, 657–666. [Google Scholar] [CrossRef]
- Bustan, A.; Dag, A.; Yermiyahu, U.; Erel, R.; Presnov, E.; Agam, N.; Kool, D.; Iwema, J.; Zipori, I.; Ben-Gal, A. Fruit load governs transpiration of olive trees. Tree Physiol. 2016, 36, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Silber, A.; Israeli, Y.; Levi, M.; Keinan, A.; Chudi, G.; Golan, A.; Noy, M.; Levkovitch, I.; Narkis, K.; Naor, A. The roles of fruit sink in the regulation of gas exchange and water uptake: A case study for avocado. Agric. Water Manag. 2013, 116, 21–28. [Google Scholar] [CrossRef]
- De Pascale, S.; Ruggiero, C.; Barbieri, G.; Maggio, A. Physiological responses of pepper to salinity and drought. J. Am. Soc. Hortic. Sci. 2003, 128, 48–54. [Google Scholar] [CrossRef]
- Bolu, W.H.; Polle, A. Growth and stress reactions in roots and shoots of a salt-sensitive poplar species (Populus x canescens). Trop. Ecol. 2004, 45, 161–172. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops–what is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- Paranychianakis, N.; Chartzoulakis, K. Irrigation of Mediterranean crops with saline water: From physiology to management practices. Agric. Ecosyst. Environ. 2005, 106, 171–187. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Wakeel, A. Potassium–sodium interactions in soil and plant under saline-sodic conditions. J. Plant Nutr. Soil Sci. 2013, 176, 344–354. [Google Scholar] [CrossRef]
- Polomski, J.; Kuhn, N. Root Research Methods. In Plant Roots: The Hidden Half, 3rd ed.; Waisel, Y., Eshel, A., Kafkafi, U., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2002; pp. 295–321. [Google Scholar]
- Postic, F.; Doussan, C. Benchmarking electrical methods for rapid estimation of root biomass. Plant Methods 2016, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, R.C.; Bengough, A.G.; Jones, H.G.; White, P.J. Can root electrical capacitance be used to predict root mass in soil? Ann. Bot. 2013, 112, 457–464. [Google Scholar] [CrossRef] [PubMed]
| Root to Shoot Ratio | Freshwater | Saline Water | ||
|---|---|---|---|---|
| df | p | df | p | |
| Fruit load | 2 | 0.1761 | 2 | <0.0001 |
| Root biomass | 1 | <0.0001 | 1 | <0.0001 |
| Interaction | 2 | 0.2865 | 2 | 0.0015 |
| DAP | Shoot (%) | Fruit | |||||
|---|---|---|---|---|---|---|---|
| N | P | K | N | P | K | ||
| Saline | 4.10 ± 0.05 | 0.41 ± 0.01 | 6.50 ± 0.12 | 3.48 ± 0.13 | 0.59 ± 0.02 | 4.46 ± 0.24 | |
| 60 | Freshwater | 4.03 ± 0.07 | 0.47 ± 0.01 | 6.30 ± 0.11 | 3.60 ± 0.13 | 0.69 ± 0.04 | 4.90 ± 0.30 |
| significance | n.s | ** | n.s | n.s | n.s | n.s | |
| Saline | 4.00 ± 0.07 | 0.33 ± 0.01 | 5.06 ± 0.08 | 2.59 ± 0.04 | 0.39 ± 0.06 | 3.59 ± 0.10 | |
| 90 | Freshwater | 4.20 ± 0.07 | 0.34 ± 0.01 | 5.80 ± 0.13 | 2.66 ± 0.04 | 0.40 ± 0.05 | 3.52 ± 0.07 |
| significance | * | n.s | *** | n.s | n.s | n.s | |
| Saline | 3.57 ± 0.08 | 0.29 ± 0.01 | 4.68 ± 0.12 | 2.23 ± 0.04 | 0.33 ± 0.01 | 2.85 ± 0.07 | |
| 105 | Freshwater | 3.73 ± 0.08 | 0.31 ± 0.01 | 4.97 ± 0.12 | 2.38 ± 0.09 | 0.35 ± 0.01 | 2.90 ± 0.08 |
| significance | n.s | n.s | n.s | ** | * | n.s | |
| Saline | 3.20 ± 0.05 | 0.30 ± 0.01 | 3.16 ± 0.14 | 3.04 ± 0.15 | 0.37 ± 0.04 | 2.80 ± 0.19 | |
| 160 | Freshwater | 3.25 ± 0.08 | 0.34 ± 0.01 | 3.16 ± 0.14 | 3.36 ± 0.08 | 0.42 ± 0.01 | 3.26 ± 0.14 |
| significance | n.s | * | ** | n.s | n.s | n.s | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erel, R.; T. Le, T.; Eshel, A.; Cohen, S.; Offenbach, R.; Strijker, T.; Shtein, I. Root Development of Bell Pepper (Capsicum annuum L.) as Affected by Water Salinity and Sink Strength. Plants 2020, 9, 35. https://doi.org/10.3390/plants9010035
Erel R, T. Le T, Eshel A, Cohen S, Offenbach R, Strijker T, Shtein I. Root Development of Bell Pepper (Capsicum annuum L.) as Affected by Water Salinity and Sink Strength. Plants. 2020; 9(1):35. https://doi.org/10.3390/plants9010035
Chicago/Turabian StyleErel, Ran, Thuc T. Le, Amram Eshel, Shabtai Cohen, Rivka Offenbach, Tobias Strijker, and Ilana Shtein. 2020. "Root Development of Bell Pepper (Capsicum annuum L.) as Affected by Water Salinity and Sink Strength" Plants 9, no. 1: 35. https://doi.org/10.3390/plants9010035
APA StyleErel, R., T. Le, T., Eshel, A., Cohen, S., Offenbach, R., Strijker, T., & Shtein, I. (2020). Root Development of Bell Pepper (Capsicum annuum L.) as Affected by Water Salinity and Sink Strength. Plants, 9(1), 35. https://doi.org/10.3390/plants9010035








