Enhanced Somatic Embryo Induction of a Tree Peony, Paeonia ostii ‘Fengdan’, by a Combination of 6-benzylaminopurine (BA) and 1-naphthylacetic Acid (NAA)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Disinfection
2.2. The Effects of the Basal Medium and Different Plant Growth Regulator (PGR) Combinations on the Induction of Somatic Embryos
2.3. The Effects of the Concentration Ratio of Cytokinin and Auxin on the Induction of Somatic Embryos
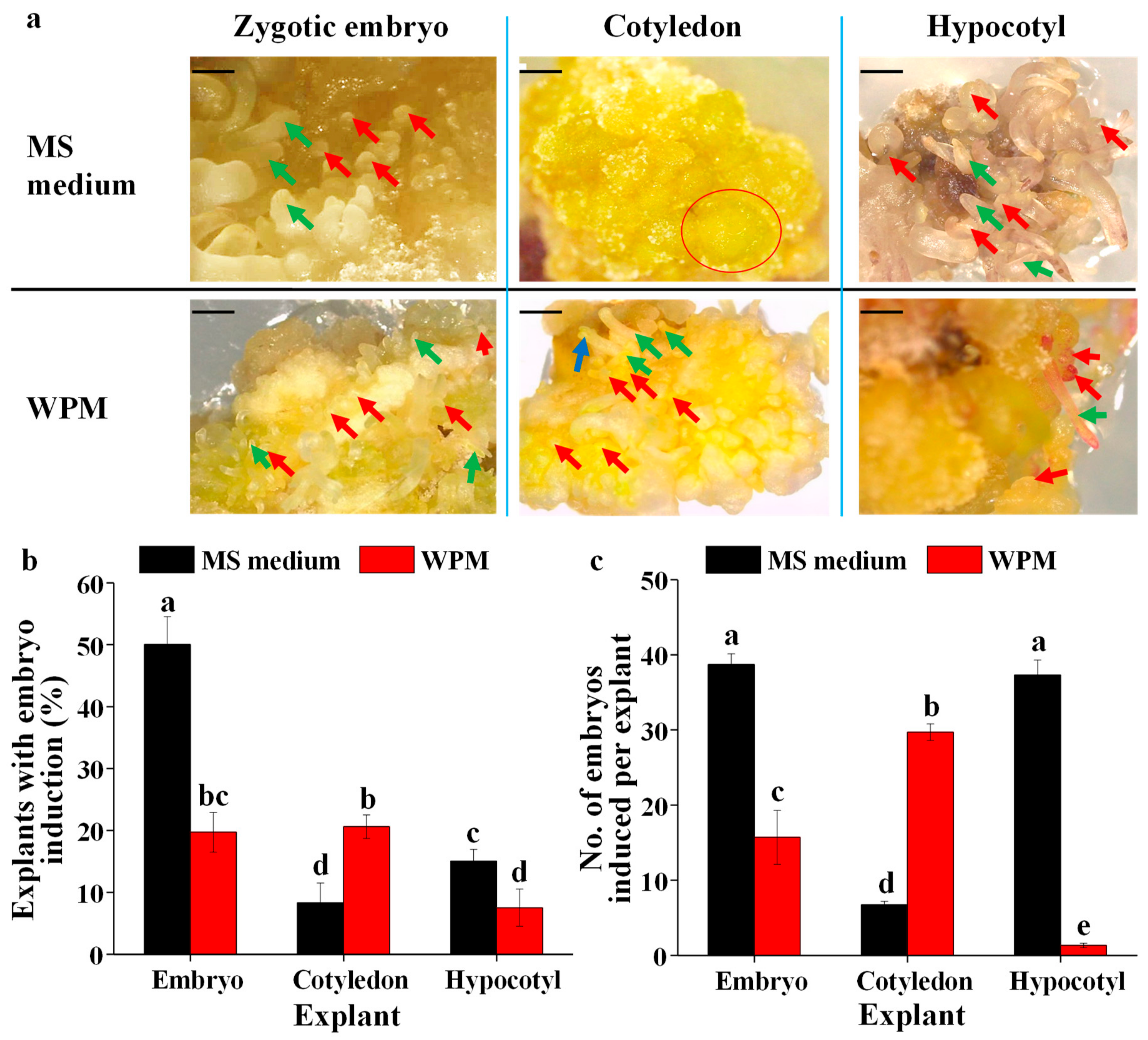
2.4. The Effects of the Basal Medium and Explant Type on the Induction of Somatic Embryos
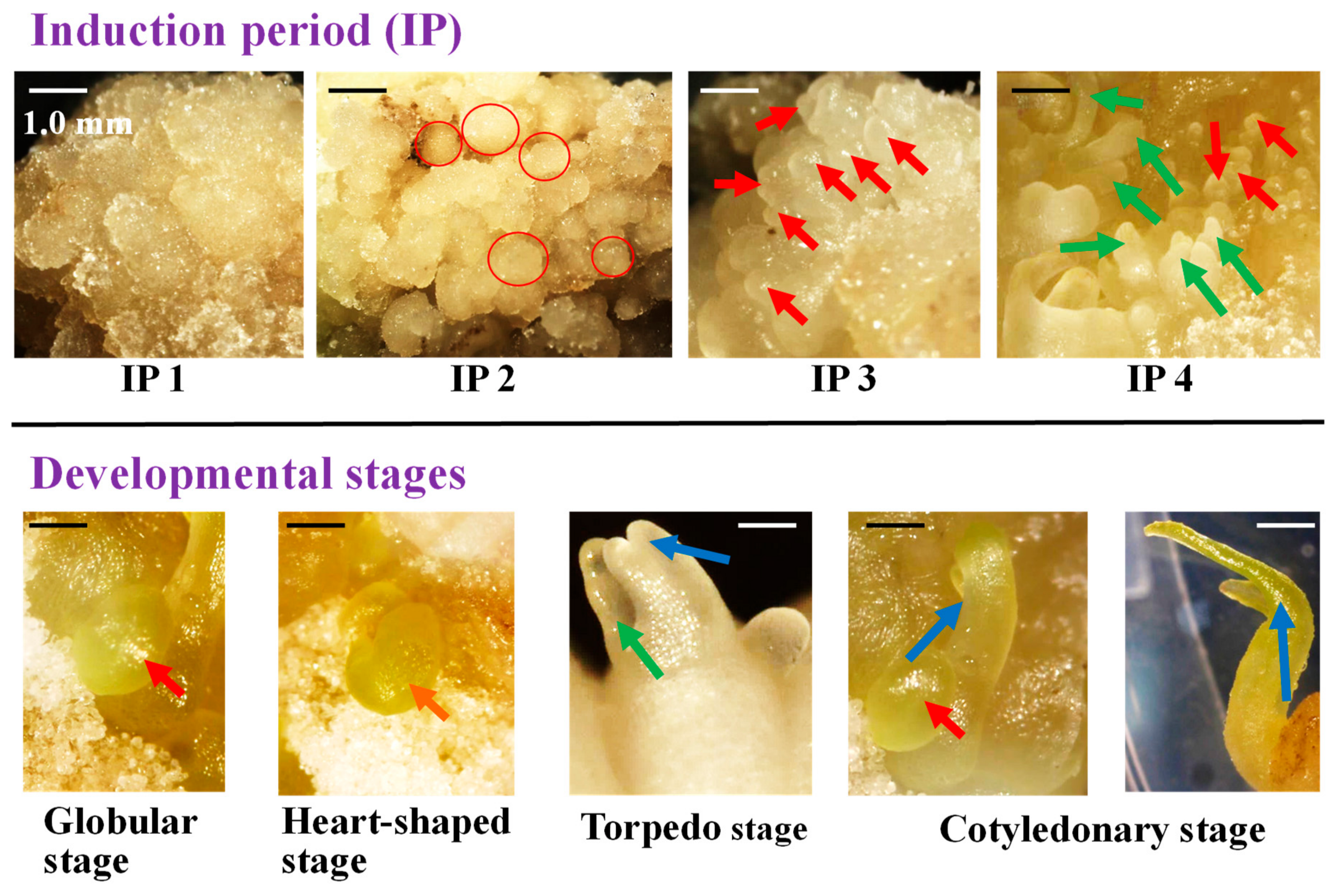
2.5. Morphological Observation of the Induction and Development of Somatic Embryos from the Zygotic Embryo
2.6. Statistical Analysis
3. Results
3.1. Callus and Somatic Embryo Induction as Affected by Basal Medium, PGR Combination, and the Concentration Ratio of Cytokinin and Auxin
3.2. Callus and Somatic Embryo Induction as Affected by the Basal Medium and Explant Type
3.3. The Development of Somatic Embryos
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, H.F.; Li, X.F.; Wu, K.; Wang, M.K.; Liu, P.; Wang, X.S.; Deng, R.X. Antioxidant activities and chemical constituents of flavonoids from the flower of Paeonia ostii. Molecules 2017, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.H.; Niu, L.X.; Zhang, Y.L.; Jin, M.; Ji, D.; Zhang, X.X. Pollen sources influence the traits of seed and seed oil in Paeonia ostii ‘Feng Dan’. HortScience 2017, 52, 700–705. [Google Scholar] [CrossRef]
- Gao, L.L.; Li, Y.Q.; Wang, Z.S.; Sun, G.J.; Qi, X.M.; Mo, H.Z. Physicochemical characteristics and functionality of tree peony (Paeonia suffruticosa Andr.) seed protein. Food Chem. 2018, 240, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.S.; Cheng, F.Y.; Zhong, Y.; Wang, X.; Li, L.Z.M.; Zhang, Y.X.; Qiu, J.M. Efficient protocols for the micropropagation of tree peony (Paeonia suffruticosa ‘Jin Pao Hong’, P. suffruticosa ‘Wu Long Peng Sheng’, and P. x lemoinei ‘High Noon’) and application of arbuscular mycorrhizal fungi to improve plantlet establishment. Sci. Hortic. 2016, 201, 10–17. [Google Scholar] [CrossRef]
- Ji, A.; Geng, X.; Zhang, Y.; Wu, G. Advances in somatic embryogenesis research of horticultural plants. Am. J. Plant Sci. 2011, 2, 727. [Google Scholar] [CrossRef][Green Version]
- Gaj, M.D. Direct somatic embryogenesis as a rapid and efficient system for in vitro regeneration of Arabidopsis thaliana. Plant Cell Tissue Organ Cult. 2001, 64, 39–46. [Google Scholar] [CrossRef]
- Mahdavi-Darvari, F.; Noor, N.M.; Ismanizan, I. Epigenetic regulation and gene markers as signals of early somatic embryogenesis. Plant Cell Tissue Organ Cult. 2015, 120, 407–422. [Google Scholar] [CrossRef]
- Hussein, S.; Ibrahim, R.; Ling Pick Kiong, A. Somatic embryogenesis: An alternative method for in vitro micropropagation. Iran. J. Biotechnol. 2006, 4, 156–161. [Google Scholar]
- Kumaravel, M.; Uma, S.; Backiyarani, S.; Saraswathi, M.S.; Vaganan, M.M.; Muthusamy, M.; Sajith, K.P. Differential proteome analysis during early somatic embryogenesis in Musa spp. Aaa cv. Grand naine. Plant Cell Rep. 2017, 36, 163–178. [Google Scholar] [CrossRef]
- Tang, H.; Ren, Z.; Krczal, G. Somatic embryogenesis and organogenesis from immature embryo cotyledons of three sour cherry cultivars (Prunus cerasus L.). Sci. Hortic. 2000, 83, 109–126. [Google Scholar] [CrossRef]
- Pateña, L.F.; Carlos-Refuerzo, L.R.; Barba, R.C. Somatic embryogenesis and plantlet regeneration in mango (Mangifera indica L.). In Vitro Cell. Dev. Biol. Plant 2002, 38, 173–177. [Google Scholar] [CrossRef]
- Marsoni, M.; Bracale, M.; Espen, L.; Prinsi, B.; Negri, A.S.; Vannini, C. Proteomic analysis of somatic embryogenesis in Vitis vinifera. Plant Cell Rep. 2008, 27, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Guan, R.; Zhu, S.; Deng, X. Proteomic analysis of somatic embryogenesis in valencia sweet orange (Citrus sinensis Osbeck). Plant Cell Rep. 2009, 28, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, A.; Grabowska, A.; Pietraszewska-Bogiel, A.; Tagashira, N.; Zuzga, S.; Wóycicki, R.; Przybecki, Z.; Malepszy, S.; Filipecki, M. Identification of genes up-regulated during somatic embryogenesis of cucumber. Plant Physiol. Biochem. 2012, 50, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Teixeira da Silva, J.A.; Shen, M.; Yu, X. Tissue culture and micropropagation of tree peony (Paeonia suffruticosa Andr.). J. Crop Sci. Biotechnol. 2012, 15, 159–168. [Google Scholar] [CrossRef]
- Han, C.J.; Wang, Q.; Zhang, H.B.; Dong, H.Z. Seed development and nutrient accumulation as affected by light shading in oilseed peony (Paeonia ostii ‘Feng Dan’). Sci. Hortic. 2019, 251, 25–31. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Use of Microculture for Production and Improvement of Rhododendron spp; American Society for Horticultural Science: Alexandria, VA, USA, 1980; pp. 416–417. [Google Scholar]
- Jana, S.; Sivanesan, I.; Lim, M.Y.; Jeong, B.R. In vitro zygotic embryo germination and somatic embryogenesis through cotyledonary explants of Paeonia lactiflora Pall. Flower Res. J. 2013, 21, 17–22. [Google Scholar] [CrossRef]
- Shiota, H.; Satoh, R.; Watabe, K.I.; Harada, H.; Kamada, H. C-ABI3, the carrot homologue of the Arabidopsis ABI3, is expressed during both zygotic and somatic embryogenesis and functions in the regulation of embryo-specific ABA-inducible genes. Plant Cell Physiol. 1998, 39, 1184–1193. [Google Scholar] [CrossRef]
- Birnbaum, K.D.; Alvarado, A.S. Slicing across kingdoms: Regeneration in plants and animals. Cell 2008, 132, 697–710. [Google Scholar] [CrossRef]
- Fehér, A.; Pasternak, T.P.; Dudits, D. Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult. 2003, 74, 201–228. [Google Scholar] [CrossRef]
- Rose, R.J.; Nolan, K.E. Invited review: Genetic regulation of somatic embryogenesis with particular reference to Arabidopsis thaliana and Medicago truncatula. In Vitro Cell. Dev. Biol. Plant 2006, 42, 473–481. [Google Scholar] [CrossRef]
- Boltenkov, E.V.; Kuritskaya, E.V.; Vrzhosek, E.V. Histological analysis of somatic embryogenesis in Itoh peony. Curr. Sci. 2016, 111, 395–398. [Google Scholar] [CrossRef]
- Mantiri, F.R.; Kurdyukov, S.; Lohar, D.P.; Sharopova, N.; Saeed, N.A.; Wang, X.-D.; VandenBosch, K.A.; Rose, R.J. The transcription factor MtSERF1 of the ERF subfamily identified by transcriptional profiling is required for somatic embryogenesis induced by auxin plus cytokinin in Medicago truncatula. Plant Physiol. 2008, 146, 1622–1636. [Google Scholar] [CrossRef]
- Jenik, P.D.; Gillmor, C.S.; Lukowitz, W. Embryonic patterning in Arabidopsis thaliana. Annu. Rev. Cell Dev. Biol. 2007, 23, 207–236. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Zhao, X.Y.; Liu, Y.B.; Zhang, C.L.; O’Neill, S.D.; Zhang, X.S. Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J. 2009, 59, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Wójcikowska, B.; Jaskóła, K.; Gąsiorek, P.; Meus, M.; Nowak, K.; Gaj, M.D. LEAFY COTYLEDON2 (LEC2) promotes embryogenic induction in somatic tissues of Arabidopsis, via YUCCA-mediated auxin biosynthesis. Planta 2013, 238, 425–440. [Google Scholar] [CrossRef]
- Wójcikowska, B.; Gaj, M.D. LEAFY COTYLEDON2-mediated control of the endogenous hormone content: Implications for the induction of somatic embryogenesis in Arabidopsis. Plant Cell Tissue Organ Cult. 2015, 121, 255–258. [Google Scholar] [CrossRef][Green Version]
- Guohua, M. Effects of cytokinins and auxins on cassava shoot organogenesis and somatic embryogenesis from somatic embryo explants. Plant Cell Tissue Organ Cult. 1998, 54, 1–7. [Google Scholar] [CrossRef]
- Friml, J.; Vieten, A.; Sauer, M.; Weijers, D.; Schwarz, H.; Hamann, T.; Offringa, R.; Jürgens, G. Efflux-dependent auxin gradients establish the apical–basal axis of Arabidopsis. Nature 2003, 426, 147–153. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Z.; Chua, N.H. Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell 1993, 5, 621–630. [Google Scholar] [CrossRef]
- Kim, H.M.; Shin, J.H.; Sohn, J.K. Cryopreservation of somatic embryos of the herbaceous peony (Paeonia lactiflora Pall.) by air drying. Cryobiology 2006, 53, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Rajam, M.V. Genotype, explant and position effects on organogenesis and somatic embryogenesis in eggplant (Solanum melongena L.). J. Exp. Bot. 1995, 46, 135–141. [Google Scholar] [CrossRef]
- Trolinder, N.L.; Goodin, J.R. Somatic embryogenesis in cotton (Gossypium) i. Effects of source of explant and hormone regime. Plant Cell Tissue Organ Cult. 1988, 12, 31–42. [Google Scholar] [CrossRef]
- Chen, J.T.; Chang, W.C. Effects of auxins and cytokinins on direct somatic embryogenesison leaf explants of Oncidium ‘Gower Ramsey’. Plant Growth Regul. 2001, 34, 229–232. [Google Scholar] [CrossRef]
- Rout, G.R.; Debata, B.K.; Das, P. Somatic embryogenesis in callus cultures of Rosa hybrida L. Cv. Landora. Plant Cell Tissue Organ Cult. 1991, 27, 65–69. [Google Scholar] [CrossRef]
- Litz, R.E.; Conover, R.A. In vitro somatic embryogenesis and plant regeneration from Carica papaya L. ovular callus. Plant Sci. Lett. 1982, 26, 153–158. [Google Scholar] [CrossRef]
- Singh, R.; Kashyap, S.P.; Kumari, N.; Singh, M. Regeneration of soapnut tree through somatic embryogenesis and assessment of genetic fidelity through ISSR and RAPD markers. Physiol. Mol. Biol. Plants 2016, 22, 381–389. [Google Scholar] [CrossRef]
- Satish, L.; Rency, A.S.; Rathinapriya, P.; Ceasar, S.A.; Pandian, S.; Rameshkumar, R.; Rao, T.B.; Balachandran, S.M.; Ramesh, M. Influence of plant growth regulators and spermidine on somatic embryogenesis and plant regeneration in four indian genotypes of finger millet (Eleusine coracana (L.) Gaertn). Plant Cell Tissue Organ Cult. 2016, 124, 15–31. [Google Scholar] [CrossRef]
- Ali, M.; Mujib, A.; Tonk, D.; Zafar, N. Plant regeneration through somatic embryogenesis and genome size analysis of Coriandrum sativum L. Protoplasma 2017, 254, 343–352. [Google Scholar] [CrossRef]
- Paul, S.; Dam, A.; Bhattacharyya, A.; Bandyopadhyay, T.K. An efficient regeneration system via direct and indirect somatic embryogenesis for the medicinal tree Murraya koenigii. Plant Cell Tissue Organ Cult. 2011, 105, 271–283. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, Y.; Liu, Z.; Zhang, F.; Xiang, F.; Xia, G. High-frequency embryogenesis and regeneration of plants with high content of gentiopicroside from the Chinese medicinal plant Gentiana straminea Maxim. In Vitro Cell. Dev. Biol. Plant. 2009, 45, 730–739. [Google Scholar] [CrossRef]
- Fu, C.; Lei, C.; Gan, L.; Li, M.; Yang, Y.; Yu, L. Optimization of embryogenic-callus induction and embryogenesis of Glycyrrhiza glabra. Afr. J. Biotechnol. 2010, 9, 5823–5829. [Google Scholar]
- Tenning, P.; Wremerth Weich, E.; Kjärsgaard, U.B.; Lelu, M.A.; Nihlgård, M. Somatic embryogenesis from zygotic embryos of sugar beet (Beta vulgaris L.). Plant Sci. 1992, 81, 103–109. [Google Scholar] [CrossRef]
- Kumar, V.; Moyo, M.; Van Staden, J. Somatic embryogenesis in Hypoxis hemerocallidea: An important african medicinal plant. S. Afr. J. Bot. 2017, 108, 331–336. [Google Scholar] [CrossRef]
- Vinoth, A.; Ravindhran, R. Efficient plant regeneration of watermelon (Citrullus lanatus Thunb.) via somatic embryogenesis and assessment of genetic fidelity using ISSR markers. In Vitro Cell. Biol. Plant 2016, 52, 107–115. [Google Scholar] [CrossRef]
- Sivanesan, I.; Lim, M.Y.; Jeong, B.R. Somatic embryogenesis and plant regeneration from leaf and petiole explants of Campanula punctata Lam. Var. Rubriflora makino. Plant Cell Tissue Organ Cult. 2011, 107, 365–369. [Google Scholar] [CrossRef]
- Lee, C.Y.; Kim, Y.K.; Kim, Y.S.; Suh, S.Y.; Lee, S.Y.; Park, S.U. Somatic embryogenesis and plant regeneration in Cnidium officinale Makino. J. Med. Plants Res. 2009, 3, 96–100. [Google Scholar]
- Vengadesan, G.; Pijut, P.M. Somatic embryogenesis and plant regeneration of northern red oak (Quercus rubra L.). Plant Cell Tissue Organ Cult. 2009, 97, 141–149. [Google Scholar] [CrossRef]


| Explant | Medium | PGR Combination (mg·L−1) | Explant with Callus Induction (%) |
|---|---|---|---|
| Embryo | MS medium | 3.0 BA + 1.0 NAA | 0.86 ± 0.07az |
| Embryo | WPM | 3.0 BA + 1.0 NAA | 0.85 ± 0.05a |
| Cotyledon | MS medium | 3.0 BA + 1.0 NAA | 0.53 ± 0.10b |
| Cotyledon | WPM | 3.0 BA + 1.0 NAA | 0.56 ± 0.07b |
| Hypocotyl | MS medium | 3.0 BA + 1.0 NAA | 0.80 ± 0.06a |
| Hypocotyl | WPM | 3.0 BA + 1.0 NAA | 0.82 ± 0.09a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Liu, Y.; Jeong, B.R. Enhanced Somatic Embryo Induction of a Tree Peony, Paeonia ostii ‘Fengdan’, by a Combination of 6-benzylaminopurine (BA) and 1-naphthylacetic Acid (NAA). Plants 2020, 9, 3. https://doi.org/10.3390/plants9010003
Ren X, Liu Y, Jeong BR. Enhanced Somatic Embryo Induction of a Tree Peony, Paeonia ostii ‘Fengdan’, by a Combination of 6-benzylaminopurine (BA) and 1-naphthylacetic Acid (NAA). Plants. 2020; 9(1):3. https://doi.org/10.3390/plants9010003
Chicago/Turabian StyleRen, Xiuxia, Ya Liu, and Byoung Ryong Jeong. 2020. "Enhanced Somatic Embryo Induction of a Tree Peony, Paeonia ostii ‘Fengdan’, by a Combination of 6-benzylaminopurine (BA) and 1-naphthylacetic Acid (NAA)" Plants 9, no. 1: 3. https://doi.org/10.3390/plants9010003
APA StyleRen, X., Liu, Y., & Jeong, B. R. (2020). Enhanced Somatic Embryo Induction of a Tree Peony, Paeonia ostii ‘Fengdan’, by a Combination of 6-benzylaminopurine (BA) and 1-naphthylacetic Acid (NAA). Plants, 9(1), 3. https://doi.org/10.3390/plants9010003






