Abstract
In vitro regeneration is a pre-requisite for developing transgenic plants through tissue culture-based genetic engineering approaches. Huge variations among different genotypes of the genus Brassica necessitate the identification of a set of regeneration conditions for a genotype, which can be reliably used in transformation experiments. In this study, we evaluated the morphogenesis potential of four commercial cultivars (Faisal canola, Punjab canola, Aari canola, Nifa Gold) and one model, Westar, from four different explants namely cotyledons, hypocotyls, petioles and roots on three different Brassica regeneration protocols, BRP-I, -II and -III. The regeneration efficiency was observed in the range of 6–73%, 4–79.3%, 0–50.6%, and 0–42.6% from cotyledons, petioles, hypocotyls and roots, respectively, whereas, the regeneration response in terms of average shoots per explant was found to be 0.76–10.9, 0.2–3.2, 0–3.4 and 0–2.7 from these explants. Of the commercial varieties tested, almost all varieties showed poorer regeneration than Westar except Aari canola. In comparison to Westar, its regeneration frequency from cotyledons was up to 7.5-fold higher on BRP-I, while it produced up to 21.9-fold more shoots per explant. Our data show that the explant has strong influence on the regeneration response, ranging from 24% to 92%. While the growth of commercial cultivars was least affected by the regeneration conditions provided, the effect on Westar was twice that of the commercial cultivars. After determining the optimal explant type and regeneration conditions, we also determined the minimum kanamycin concentration levels required to selectively inhibit the growth of untransformed cells for these cultivars. Regenerated shoots of Aari canola could be successfully grown to maturity within 16–18 weeks, with no altered phenotype noted and normal seed yields obtained. Therefore, the commercial variety, Aari canola, could be a good candidate for future genetic transformation studies.
1. Introduction
Brassica, from the family Brassicaceae, is an economically important genus. It includes several species that are often used as oilseed crops, vegetables, fodder crops as well as condiments. Brassica oilseed varieties producing oil low in anti-nutritive aliphatic glucosinolates and erucic acid as well as rich in unsaturated fatty acids are generally termed as ‘canola’. Conventionally, the term ‘canola’ was more often used for B. napus but now some canola quality varieties of B. rapa and B. juncea are also available [1,2,3,4]. Being rich in omega-6 and omega-3 fatty acids and low saturate fats, canola oil is considered as a heart-healthy oil. Due to its high quantity of proteins, its meal for poultry and livestock is considered as good as soybean [5,6].
With the increasing world population, the demand for vegetable oil is also increasing. According to the United States Department of Agriculture, the largest importers of vegetable oil in 2018 were the European Union, the US, China and India. Pakistan also gets over 80% of its edible oil requirements from imports [7]. Since the increase in cultivable land is not possible in the face of an increasing population, the viable option to meet the challenges of edible oil requirements, as well as industrial applications, is to develop stress-resilient high-yielding Brassica genotypes. The development of stress tolerant Brassica is possible by transferring genes from the plant species that are adapted to harsh environmental conditions. These species present a rich reservoir of the traits that enable them to grow under stressful conditions. However, transferring these traits to salt or drought sensitive crops is only possible by genetic transformation, as they cannot be cross bred through conventional breeding approaches. GM (genetic manipulation) tools developed in the 1980s allow the transferring of traits from a wide taxon for engineering novel traits into field crops. Herbicide tolerance, insect resistance, β-carotene synthesis (golden rice) and vitamin-enrichment (multivitamin corn) are few examples among the list of engineered traits through GM technology.
The introduction of transgenes into plants to engineer useful novel traits may seem trivial now [8], but it has its own challenges and limitations [9,10,11,12]. For example, a plant species must be responsive to in vitro regeneration protocols, and a robust regeneration system is one of the key pre-requisites for successful genetic transformation. Several indigenous Brassica varieties developed locally have canola characteristics. Being stress-sensitive, these varieties are unable to grow on marginal lands. To develop stress-resilient transgenic versions, it is necessary to determine the morphogenesis potential of these varieties. Although transformation of Brassica species has been reported in several studies [13,14,15,16,17,18,19,20,21,22], several Brassica genotypes remain recalcitrant to genetic transformation [2,19,23]. Several factors including susceptibility to Agrobacterium infection, choice of explant and tissue culture conditions mainly responsible for these variations have been identified [13,19,22]. These factors vary from genotype to genotype, indicating a strong genetic control on in vitro regeneration and transformation of Brassica genotypes [22,24,25,26]. Therefore, it is important to find out responsive genotypes, as well as a type of explant, which show reliable regeneration efficiency to be used in future transformation experiments.
Traditionally, model cultivars such as Westar have been used in transformation and regeneration experiments for the introduction of transgenes for desired traits [19,27]. While these model cultivars are valuable for studying gene function, there is a need to work directly on elite cultivars for the development of climate-resilient crops. The undesired agronomic characters presented by the model plants require an exhaustive process of crossing and back-crossing to transfer engineered traits into field varieties [10]. The challenge of transferring traits from model plants is greatly increased if the number of genes to introduce increases. Therefore, the transformation of commercial cultivars with desired agronomic performance adapted to the prevailing climatic conditions is highly desirable, making the process of developing transgenic plants quicker and more efficient. However, their transformation is often hampered with genotypic recalcitrance, which makes it necessary to test them for in vitro regeneration prior to genetic transformation experiments.
In this study, we evaluated the regeneration potential of different commercial varieties of Brassica grown in Pakistan using different explants and growth conditions for establishing transgenic technology in commercial varieties of Brassica. One of the commercial varieties, Aari canola, was found to be highly responsive to the given conditions. We also determined kanamycin concentrations to be used in future transformation experiments for the recovery of transformants. The in vitro Aari canola plants grown to maturity showed a normal plant morphology, with normal seed setting and without any observable phenotypic variations. The information generated in this study will be useful for developing stress-resilient Brassica varieties by directly transforming the commercial cultivars.
2. Results
2.1. Shoot Regeneration from Cotyledons
All the explants, including cotyledons, normally regenerated multiple shoots and occasionally a single shoot per explant. Regenerates formed from the cut end of the 2 mm petiole attached to the cotyledon. It was essential when explants were isolated that no traces of meristem region were left behind during the explant cutting to avoid the emergence of shoots from meristematic tissue (however, it is acknowledged that for those not familiar with the technique this can be difficult at first, but shoots resulting from meristematic tissue will be visible as shoots emerging within a few days of explant isolation and should be discarded). Typically, the explants would swell in the first week of incubation, and shoot primordia will start appearing in the second week. The regeneration efficiency was calculated as following:
The highest regeneration efficiency was observed for Aari canola on all the three protocols with 70.6% on BRP-I, 69.3% on BRP-II, and 73.3% on BRP-III (Figure 1a,e,i). Faisal canola showed the lowest regeneration efficiency on BRP-I (6.0%) and it was Punjab canola which showed the lowest regeneration on BRP-II and BRP-III with 13.3% and 10.0% efficiency, respectively. The regeneration efficiency of Aari canola was found to be 7.5-fold higher than the model, Westar, on BRP-I, 3.8-fold higher on BRP-II and 2.5-fold higher on BRP-III.
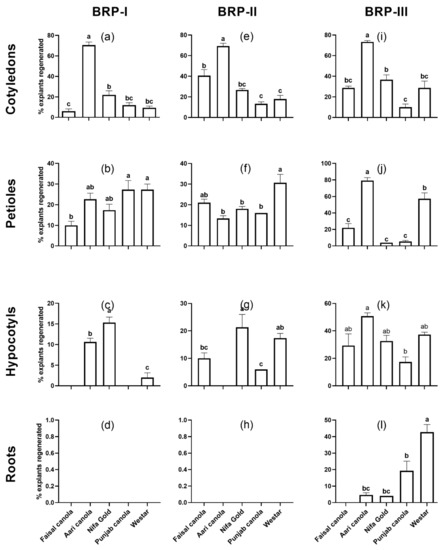
Figure 1.
Regeneration efficiency of five Brassica cultivars from different explants on three different regeneration protocols, BRP-I (a–d), BRP-II (e–h) and BRP-III (i–l) from cotyledons (a,e,i), petioles (b,f,j), hypocotyls (c,g,k) and roots (d,h,l). Data points represent the mean ± SE of three replicates.
In terms of total shoots regenerated, the highest shoot formation was observed in Aari canola on all the three protocols with 548 shoots on BRP-I (average 10.96 shoots/explant), 436.3 on BRP-II (8.72 shoots/explant) and 366.3 on BRP-III (7.3 shoots/explant) from a total of 50 explants (Figure 2a,e,i). The least regeneration responsive was Westar (25 shoots/50 explants; 0.50/explant) on BRP-I, and Punjab canola on BRP-II (38.3 shoots/50 explants; 0.76 shoots/explant) and BRP-III (25 shoots/50 explants; 0.50/explant). In terms of average number of shoots per explant, it was in the range of 0.50–10.9, 0.76–8.7 and 0.5–7.3 from cotyledons on BRP-I BRP-II and BRP-III, respectively. Overall, the regeneration response of Aari canola, in terms of total shoots formation, was 21.9-fold higher than Westar on BRP-I, 7.0-fold higher on BRP-II and 2.3-fold higher on BRP-III.
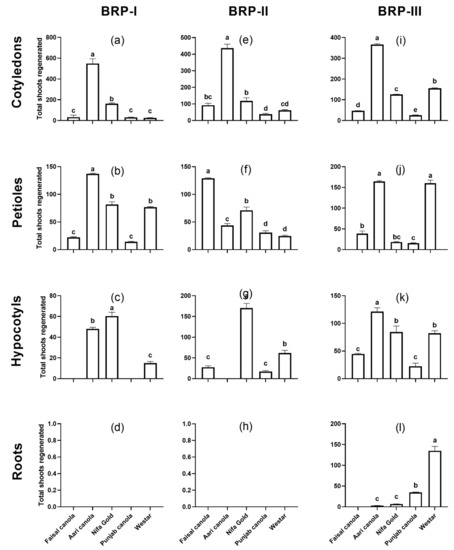
Figure 2.
Regeneration of total number of shoots from different explants of five Brassica cultivars on three different regeneration protocols, BRP-I (a–d), BRP-II (e–h) and BRP-III (i–l) from cotyledons (a,e,i), petioles (b,f,j), hypocotyls (c,g,k) and roots (d,h,l). Data points represent the mean ± SE of three replicates.
2.2. Shoot Regeneration from Detached Petioles
Petiole explants regenerated shoots along the middle rib after 3 weeks on SIM. The highest regeneration efficiency from detached petioles was observed for Aari canola on BRP-III (79.3%), Westar on BRP-I and BRP-II with a regeneration of 27.3% and 30.6%, respectively (Figure 1b,f,j). The lowest regeneration efficiency was observed for Faisal canola on BRP-I (10.0%), followed by Aari canola on BRP-II (13.3%), and Nifa Gold on BRP-III (4.0%). The regeneration efficiency of Aari canola was 1.2-fold and 2.3-fold lower than Westar on BRP-I and BRP-II, respectively, while 1.3-fold higher on BRP-III.
For the total number of shoots, Aari canola produced highest number of shoots on BRP-I and BRP-II with 137 and 164.33 shoots from 50 explants, respectively, while Faisal canola produced the highest number of shoots (129 shoots) on BRP-II (Figure 2b,f,j). Punjab canola produced least number of shoots on BRP-I (14.3 shoots/50 explants) and BRP-III (15.6 shoots) while it was Westar, which showed the lowest response on BRP-II with 24.6 shoots from 50 explants. The average number of shoots per explant was in the range of 0.2–2.7, 0.4–2.5 and 0.3–3.2 on BRP-I, BRP-II and BRP-III, respectively. The number of shoots produced by Aari canola was 1.7-fold, 5.2-fold and 1.0-fold higher than Westar on BRP-I, BRP-II and BRP-III respectively.
Overall, the regeneration conditions provided in BRP-I and BRP-III seemed more conducive for generating shoots from detached petioles for Aari canola and Westar. The regeneration conditions in BRP-II appeared favorable for Faisal canola.
2.3. Shoot Regeneration from Hypocotyls
Regeneration from hypocotyl segments usually started after 3 weeks on SIM although callus formation started in the second week. The upper end of the hypocotyl cut 2 mm below the epicotyl region showed more swelling and calli formation, ultimately producing higher number of shoots as compared to the other end of the explant. Shoot regeneration was rarely observed from the middle rib portion.
The highest regeneration efficiency from hypocotyls was observed in Aari canola on BRP-III (50.6%) followed by Westar and Nifa Gold with 37.3% and 32.6%, respectively (Figure 1k). The highest regeneration efficiency on BRP-I and BRP-II was of Nifa Gold with 15.3% and 21.3%, respectively, as compared to the other cultivars, suggesting the suitability of these protocols for Nifa Gold for obtaining regeneration from hypocotyls sections. Faisal canola, Punjab canola and Westar were least responsive on BRP-I, while, Aari canola did not respond to BRP-II at all. When the efficiency of Aari canola was compared with Westar, it was 5.3-fold higher on BRP-I, 17.3-fold lower on BRP-II and 1.3-fold higher on BRP-III.
When the total number of shoots regenerated were counted, it was Nifa Gold that produced highest number of shoots on BRP-I (60.3 shoots from 50 explants) and BRP-II (170 shoots/50 explants) while Aari canola produced highest shoots on BRP-III (121 shoots/50 explants) (Figure 2c,g,k). The hypocotyl segments of Aari canola did not respond to BRP-II at all like Faisal canola and Punjab canola on BRP-I. In contrast, Westar showed regeneration on all three protocols, with highest shoot count on BRP-III (82 shoots/50 explants), followed by BRP-II with 61.6 shoots and BRP-I with 15 shoots. The average number of shoots generated from hypocotyls were in the range of 0–1.2, 0–3.4, and 0.4–2.4 per explant on BRP-I, BRP-II and BRP-III, respectively.
Overall, the regeneration conditions of BRP-III appeared more responsive to hypocotyls of three cultivars, Aari canola, Nifa Gold and Westar producing more than one shoot per explant. BRP-I and BRP-II protocols were found suitable for regeneration from hypocotyls of Nifa Gold only. The conditions in BRP-I were not suitable for obtaining plants from hypocotyls of any cultivars other than Nifa Gold. Likewise, the conditions in BRP-II did not appear suitable for hypocotyls of Aari canola, Faisal canola and Punjab canola too. However, hypocotyl explants of Nifa Gold and Aari canola generated good number of shoots on BRP-II and BRP-III, respectively, though they were far less efficient and slower than the cotyledonary explants.
2.4. Shoot Regeneration from Roots
Roots explants turned green and showed extraordinary elongation on SIM followed by little swelling and callus formation in the previous 2–3 weeks but could not form any shoots till the fourth week on the medium. The conditions in BRP-I and BRP-II were not suitable for regeneration from roots for any cultivar. Regeneration was observed only on BRP-III, with Westar showing the highest regeneration efficiency (42.66%) followed by Punjab canola with 19.3% regeneration efficiency (Figure 1d,h,l). The total number of shoots from these two cultivars was 135 and 26 shoots from 50 explants for Westar and Punjab canola, respectively (Figure 2d,h,l). A significantly small number of shoot formations was observed in Aari canola and Nifa Gold, whereas, Faisal canola could not regenerate any shoots at all.
Figure 3 shows the regeneration of Aari canola from four different explants on different protocols.
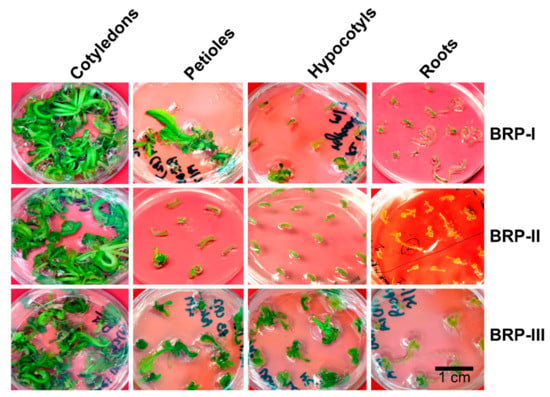
Figure 3.
Regeneration of Aari canola on different regeneration protocols from four different explants cotyledons, excised petioles, hypocotyls and roots. Only representative figures of the three replicates are shown. Pictures were taken after 30 days. Abbreviations: BRP, Brassica regeneration protocol.
2.5. Effect of Explant, Regeneration Conditions, and Their Interaction on In Vitro Regeneration
The data obtained was analyzed for the significance and the degree of effect of the explant type, the growth regimes, as well as their interaction on the regeneration of all the cultivars. A standard analysis of variance was applied to analyze the regeneration data. Table 1 shows the summary of the analyses. The explant had a highly significant effect on regeneration (p ≤ 0.0001). The effect of regeneration conditions was less significant in Westar compared to other cultivars (p ≤ 0.001), whereas, it was non-significant in Nifa Gold. Only in one cultivar, Punjab canola, it was highly significant (p ≤ 0.0001). The effect of ‘explant × regeneration conditions’ was statistically non-significant only in one cultivar, Nifa Gold (p ≥ 0.05), whereas it was less significant for Westar compared to the other three. The effect of replication was non-significant on all cultivars except Punjab canola.

Table 1.
Mean square and significance levels from analysis of variance of data from the regeneration of five Brassica cultivars.
In terms of percentage contribution of these effects to the regeneration efficiency, the effect of explant type was highest from all the other factors except for Westar (Table 2). It was highest in Aari canola (91.87%) while lowest in Westar (24.13%). The effect of regeneration conditions was much more pronounced on Westar regeneration (57.21%) compared to that of the commercial varieties. The regeneration conditions were less likely to influence the regeneration of Aari canola and Nifa Gold unlike of Faisal canola and Westar. The effect of ‘explant × regeneration conditions’ was variable among different cultivars. It was highest for Faisal canola (27.12%) and Punjab canola (31.44%) while lowest for Aari canola (4.95%). The contribution of the replicate effect was negligible. The lower residual error values of these cultivars except Nifa Gold indicate a high degree of uniformity in their regeneration response.

Table 2.
Percent contribution of explant type, growth conditions, and their interaction on the regeneration frequencies of five Brassica cultivars.
2.6. Determining Kanamycin Sensitivity Levels
From the regeneration experiments, cotyledons were found to exhibit highest regeneration potential on all three protocols. Likewise, the regeneration protocol, BRP-I, was observed to be more conducive for multiple shoot regeneration compared to the other two protocols used in this study. Therefore, in this experiment, only cotyledons were used to determine sensitivity of each cultivar to kanamycin on BRP-I at 0, 10, 15, 20, 25 and 30 mg/L of kanamycin. In total, 30 explants (10 explants per plate) were divided into three subgroups. The sensitivity level of each cultivar was determined where explants did not produce any viable shoots.
Different cultivars showed a mixed response to kanamycin dosage. The minimum concentration at which complete inhibition was observed was different for each cultivar. For example, it was 20 mg/L for Westar and Nifa Gold, 25 mg/L for Punjab and Aari canola and 30 mg/L for Faisal canola (Figure 4).
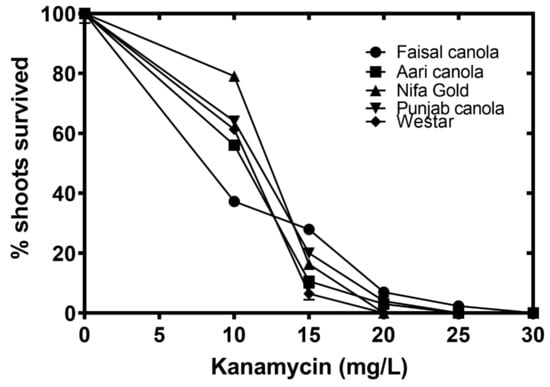
Figure 4.
Determining optimum concentration of kanamycin. Cotyledons of 4 day old seedlings of Faisal canola (circles), Aari canola (squares), Nifa Gold (triangles), Punjab canola (inverted triangles) and Westar (diamonds) were cultured on different concentrations of kanamycin. Data points represent the means ± SE of three replicates.
2.7. Completion of Regeneration Cycle for Aari canola
The Aari canola shoots were matured for flowering and seed setting, as described in the Section 4.5 of the Methods. The regeneration cycle with timescale for recovering fully mature plants is shown in Figure 5. It was observed that the complete recovery cycle from cotyledons would take ~18 weeks, around two week earlier than Brassica oleracea, which has been reported to take ~20 weeks on the same protocol and same explant [23].
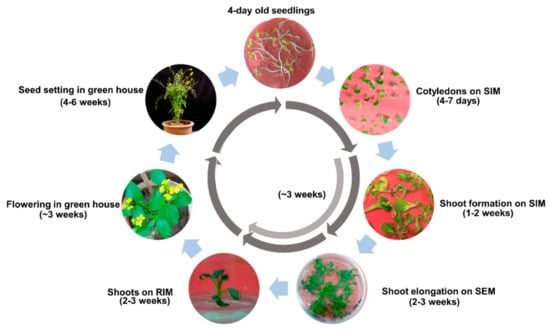
Figure 5.
Complete regeneration cycle of Aari canola on BRP-I. Different phases of plant regeneration along with the timeline are shown. The regeneration cycle from cotyledons to seed collection takes ~18 weeks. It can be shortened by another two weeks by skipping the SEM step and increasing the SIM incubation for three weeks (light arrow). Abbreviations: SIM, shoot initiation medium; SEM, shoot elongation medium; RIM, root induction medium.
2.8. Rooting Efficiency
Almost all isolated shoots were capable of developing roots successfully regardless of the explant type they originated from (data not shown).
3. Discussion
The present study was carried out to evaluate the regeneration potential of commercial cultivars of Brassica belonging to Brassica juncea and Brassica napus. The regeneration and transformation protocols for Brassica are mainly limited to model cultivar Westar and attempts have been made to extend these developments to transform the locally adapted Brassica cultivars in many parts of the world [28,29,30,31,32]. Although the success of genetic transformation depends on several factors including susceptibility of the genotype to Agrobacterium infection, explant type as well as age, and in vitro regeneration efficiency are the key factors. Transformation of the elite varieties is manly hindered because of the high degree of recalcitrance of these varieties to regeneration protocols [23]. Therefore, the first pre-requisite to embark on transformation experiment of elite cultivars is to evaluate their regeneration potential to available regeneration protocols.
Regeneration in Brassica has been reported from several tissues and organs including leaves, stem sections, petioles, roots, hypocotyls, cotyledons, immature zygotic embryos, protoplasts and cell suspension cultures [16,27,33,34,35,36,37,38,39,40,41]. Out of these, cotyledons, hypocotyls and roots have been frequently used for genetic transformation [2,16,19,37,42,43]. Therefore, only the commonly used explants were tested in this study. Explant age and type also significantly influences regeneration potential. Ono, Takahata and Kaizuma [42] compared regeneration from the explants isolated from seedlings of different ages. The study reported that explants from 4 day old seedlings gave higher regeneration response compared to the those isolated from 5 and 6 day old seedlings. In another study, when the explant age was increased from 4 to 10 days, the regeneration frequency was significantly decreased [44]. Therefore, in this study, only 4 day old seedlings were used for explant preparation.
Cotyledonary explants showed the highest regeneration efficiency on all the tested protocols followed by petioles and hypocotyls, respectively (Figure 1). Overall, the roots were found to be least responsive among all the types of explants used (Figure 1d,h,l). This observation was in line with earlier studies. Zhang and Bhalla [34] tested the regeneration potential of seven Australian commercial cultivars of Brassica napus using cotyledons, hypocotyls, and roots. In six cultivars, the regeneration response from roots was comparatively low and slow compared to cotyledons and hypocotyls.
We observed that the regeneration protocol BRP-I was the most conducive for shoot formation from cotyledons (up to 10.9 average shoots per explant) while BRP-II was best for petioles (up to 3.2 average shoots per explant) and BRP-III promoted shoot formation both in hypocotyls (up to 3.4 average shoots per explant) and root explants (up to 2.7 shoots per explant) (Figure 2). We applied these protocols without introducing any major modification. The regeneration of these cultivars, including that of Aari canola, could be further improved upon by optimizing the plant regeneration conditions. It has been observed that varying the media components affects the shoot formation efficiencies significantly [33,35,36,37,42,43,45,46,47]. The fact that huge variations have been observed among different explants, a generalized recommendation cannot be made. For example, all the three protocols can be efficiently used to recover transformants of Aari canola from cotyledons, while the regeneration efficiency of other explants was greatly reduced in these protocols (Figure 1). The poor regeneration of elite varieties except Aari canola indicates the recalcitrant nature of these cultivars to regeneration as reported in the literature. The regeneration response of Westar to BRP-III from all the explants confirms the fact that it was developed for Westar (Figure 1 and Figure 2). Nevertheless, this study provides a snapshot of the in vitro regeneration potential of these cultivars on three different regeneration protocols.
In vitro regeneration in the genus Brassica is highly genotype specific and huge variations have been reported in the regeneration potential of different genotypes. In the present study, all the tested genotypes exhibited variable regeneration response in a manner that appears highly genotype specific. For example, huge variations were observed in regeneration from different explants ranging from 6% to 73%, 4% to 79.3%, 0% to 50.6% and 0% to 42.6% from cotyledons, petioles, hypocotyls and roots, respectively (Figure 1). Similar variations have been reported in Brassica. Hachey, et al. [48] screened six cultivars of Brassica campestris and observed 0–70% regeneration in these genotypes. Ono, Takahata and Kaizuma [42] investigated the regeneration potential of 100 genotypes of Brassica napus by in vitro regeneration and reported huge variations in the regeneration response, which ranged from no regeneration at all to 97%. Zhang and Bhalla [34] reported huge variations (0–96.7%) in seven Australian cultivars of Brassica napus. Similar genotype-dependent variations among different Brassica species have been reported in several studies [23,33,35,36,37,38,39,40,43,45,46,47,49].
The regeneration response appeared specific to the type of cultivar and media components used. For example, Aari canola showed exceptional regeneration on all the tested regeneration protocols. The model variety, Westar, showed a good regeneration on BRP-III but lagged far behind Aari canola. Both Faisal and Punjab canola were least responsive to BRP-I and BRP-III. The results presented here show that explant type had a significant effect on the in vitro regeneration response of the tested cultivars (Table 1 and Table 2). The effect that regeneration conditions provided was less significant compared to the explant type and often non-significant like that of the replicate. Khehra and Mathias [27] studied the effect of genotype along with explant type on shoot regeneration frequency in four Brassica napus varieties, one spring (Westar) and three winter (Ariana, Cobra and Libravo) by culturing cotyledons, hypocotyls and stem sections on different growth mediums. The study showed that both the genotype and explant type had significant effect on shoot regeneration frequency.
Determination of the threshold values of the selective agent, kanamycin in this study, is a preliminary requirement before starting transformation experiments that will allow the selective growth of transformants while killing the non-transformants. Different cultivars showed a complete inhibition at different kanamycin concentrations (Figure 4) perhaps due to the genotypic variations. The findings of this study are in close agreement with [50] who reported that the optimum kanamycin concentration for mustard (Brassica juncea Coss.) was 30 mg/L.
A notable observation of this study is the exceptional regeneration response of an elite variety Aari canola to different regeneration conditions particularly from cotyledons. The regeneration efficiency of Aari canola from these explants was found to be up to 7.5-fold higher than the model cultivar Westar (Figure 1a,e,i), whereas, when compared in terms of total number of shoots obtained per explant, it was up to 21.9-fold higher than Westar (Figure 2a,e,i). Shoots of Aari canola were successfully grown to maturity using the procedure outlined in BRP-I [23]. The complete regeneration of mature Aari canola plants took approximately 18 weeks (Figure 5), which is 2 weeks shorter than the Brassica oleracea for which this protocol was originally designed [23]. The efficient regeneration of Aari canola both in terms of regeneration efficiency and as well as total number of shoots obtained, and relatively quicker recovery of mature plants make it a good candidate for genetic transformation. One of the reasons of faster recovery of mature plants grown from in vitro regenerated shoots may be since Aari canola is a short-duration variety. However, such a correlation between the cropping type, short-day or long-day, with the in vitro regeneration potential has not been observed in Brassica [42]. Aari canola is a recently approved, high-yielding commercial canola cultivar with many good agronomic attributes, and is currently grown in many parts of the country [51]. Transformation of an elite variety has several advantages over the transformation in a model genotype. Transformations are usually made in a few selected genotypes of a species, which can be easily manipulated by genetic means. Often, these genotypes have poor agronomic performance and therefore, the engineered traits must be transferred into commercial yet cross-compatible varieties through crossing. After crossing, it takes several more years breeding work, labor and cost to recover the recurrent parent genome, and to minimize the ‘linkage drag’. The associated labor and costs with the back-crossing programs increase several times if the number of traits to be transferred increases [10,52]. Aari canola belongs to Brassica juncea (Table 1), which is the most widely cultivated genotype in the Southeast Asian countries like India and Pakistan [30]. The use of Aari canola (commercial variety) in transformation experiments will help expedite the improvement of canola through genetic transformation against biotic and abiotic stresses as well as facilitate the functional genomic studies.
In conclusion, we have used simple regeneration protocols, with slight modifications, to successfully regenerate shoots from different explants of commercial Brassica varieties. Our work has identified commercial cultivar, Aari canola, which is highly responsive to regeneration conditions tested. The quicker recovery of in vitro plants and extra-ordinary regeneration potential make this variety an ideal candidate for future transformation-based studies. The regeneration conditions and the kanamycin levels identified in this work will be useful for improving canola through different genetic engineering approaches against various biotic and abiotic stresses as well as functional genomic studies.
4. Materials and Methods
4.1. Plant Material and Growth Conditions
Five different cultivars (four local and one model) belonging to Brassica napus and B. juncea were obtained from different institutes of Pakistan (Table 3).

Table 3.
List of cultivars with their sources used in this study.
4.2. Sterilization and Sowing
Seeds were stratified at 4 °C for 48 h before sowing to ensure uniform germination. Seeds were surface sterilized by immersing in 100% ethanol for 2 min followed by immersing in commercial bleach (sodium hypochlorite solution) containing 4–6% available chlorine plus 2–3 drops of 10% tween-20 for 10 min. The seeds were then rinsed four times with sterile distilled water in a laminar flow and placed on sterile filter paper to dry and germinated on seed germination media (MS salts, 3% sucrose, 1 mg/L pyridoxine, 1 mg/L nicotinic acid, 10 mg/L thiamine-HCl, 100 mg/L myo-inositol, 4 g/L phytagel, pH 5.7–5.8) at a density of 20 seeds per 90 × 30 mm petri dish in a growth room maintained at 23 °C at 16 h light/8 h dark cycles under a light intensity of 50 µmol m−2 s−1 provided by cool fluorescent bulbs.
4.3. Explant Isolation and Shoot Induction
Four different explants intact cotyledons with approximately 2 mm of petiole, hypocotyls (approximately 3–4 mm), petioles (approximately 2 mm, with the ‘leaf of the cotyledon removed’, and roots (3–4 mm) sections were subjected to in vitro regeneration conditions. Four day old seedlings were used for explant isolation. A pair of long, sterile forceps were used to remove the seedlings from the germination media and placed in sterile petri dishes. Explants were cut and separated using a sharp scalpel blade and transferred to either shoot induction media (SIM) or callus induction media (CIM) (see Table 4 for composition). Cotyledons were excised with a sharp scalpel blade with 2 mm petiole avoiding any part of meristem. Explants were placed on the medium in such a way that the petiole was embedded in media and the cotyledonary lamella was clear of the media. 10 explants were established on each plate and transferred to a growth room at 23 °C under scattered light. A total of 50 explants of all varieties were used for each experiment, and the experiments were repeated three times. Cultures were transferred to fresh medium every two weeks. The explants were subjected to regeneration under three different regeneration protocols termed as Brassica Regeneration Protocols, BRP-I [23], BRP-II [53] and BRP-III [19]. A number of protocols for the in vitro regenerations of different Brassica genotypes have been reported in the literature, which are highly specialized for a certain genotype and are often complicated [1,3,16,28,29,32,33,34,35,36,37,40,42,43,44,45,46,48,54,55,56,57,58,59,60]. Further, different explant types have been shown to respond differently to different hormonal combinations [44], which is further complicated by the fact that regeneration in Brassica is highly variable among different genotypes [42]. These protocols were chosen because of the simplicity and fewer steps involved, which ultimately will enable an easier transfer of the protocols to other laboratories, as well as the nature of work in our lab.

Table 4.
Composition of different mediums used in the study.
The final data was recorded in two ways: (a) total number of explants showing regeneration and (b) the total number of shoots regenerated from all the number of explants. The first type of data did not consider the multiple number of shoots per explant, therefore, we chose to record data for the total number of shoots to account for the fact that many explants regenerated multiple shoots.
4.4. Kanamycin Selection Levels
Kanamycin was added in the shoot induction medium (SIM) (see Table 4 for composition) at a concentration of 0, 10, 15, 20, 25 and 30 mg/L. The cotyledons were obtained, excised and placed on SIM as described above for regeneration using BRP-I. Culture conditions and sub-culturing duration were also the same. Cultures were monitored regularly, and the time taken for shoot induction at different levels of kanamycin noted, together with the total number of shoots per explant after two-week intervals.
4.5. Growing In Vitro Regenerated Shoots to Maturity
The selected in vitro regenerated shoots of Aari canola were grown to maturity for flowering and seed setting as described in Hundleby and Irwin [23] with slight modifications to determine the timescale for the complete recovery of mature plants. Briefly, shoots were isolated and transferred to 100 mL jars containing 25 mL root induction medium (RIM; ½ MS salts, 1 mL vitamin B5 stock, 1% (w/v) glucose, 4 g/L phytagel and 2 mg/L IBA). The sub-culturing was repeated in the case of multiple shoots arising from a single explant until single-stemmed shoots were obtained. The shoots were maintained at 40 µmol m−2 sec−1 16 h/8 h day/night cycle at 23 °C until enough root mass was obtained. The rooted shoots were transferred to sterile peat pots before transferring them to a greenhouse. Plants were transferred to pots filled with compost-soil mixture. The pots were covered with white polythene bags to help plants adjust to the greenhouse conditions. The plants were grown at 18/12 °C day/night temperature and 16/8 h day/night photoperiod under ambient light conditions (~200 µmol m−2 sec−1). Plants were fed weekly with 2:1:1 NPK solution. Once the plants were established under greenhouse conditions, the white bags were gradually removed. After seed setting and pod formation, the watering schedule was reduced to help pods dry on the plant. Pods were harvested when pods were 70–80% brown in color to avoid seed loss from pod shattering. The collected pods were dried for 2–3 days to eliminate moisture, and then stored.
4.6. Rooting Efficiency
The rooting efficiency of regenerated shoots from each protocol was determined by taking five (05) random shoots from each replicate by transferring them to RIM (see Table 4 for composition). Two of the rooted shoots were transferred to peat moss and grown under greenhouse conditions for seed setting as described in Section 4.5.
4.7. Statistical Analysis
The statistical significance of the data obtained was validated using Chi-square test at p = 0.05 with 95% confidence interval (CI) and a two-way ANOVA at α = 0.05 by using the statistical software SPSS v18.
Author Contributions
N.A. and P.H. conceived and designed the study. N.F. and M.A.N. conducted experiments. N.F., N.A. and Z.M. analyzed data. N.F. and M.A.N. wrote the manuscript. N.A., P.H. and Z.M. reviewed the manuscript. I.A. provided seeds of the varieties used, as well as technical advice. All authors read and approved the manuscript.
Funding
“This research was funded by HIGHER EDUCATION COMMISSION, grant number 3402” and “The APC was funded by the John Innes Center, UK”.
Acknowledgments
N.A. is thankful to the Higher Education Commission (HEC), Pakistan, for supporting research in his lab, and the Biotechnology and Biological Sciences Research Council (BBSRC) via grant BB/P013511/1 to the John Innes Centre for the funding of P.H.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Naz, S.; Siddiquiland, M.F.; Raza, S. Effect of different growth regulators on in vitro propagation of Brassica napus L. Pak. J. Bot. 2018, 50, 1871–1876. [Google Scholar]
- Guoliang, L.; Lixin, Y.; Fei, L.; Zhang, S.; Zhang, H.; Wei, Q.; Zhiyuan, F.; Jian, W.; Xiaowu, W.; Zhang, S. Research progress on Agrobacterium tumefaciens-based transgenic technology in Brassica rapa. Hortic. Plant J. 2018, 4, 126–132. [Google Scholar]
- Gerszberg, A. Tissue culture and genetic transformation of cabbage (Brassica oleracea var. capitata): An overview. Planta 2018, 248, 1037–1048. [Google Scholar] [CrossRef]
- Rani, T.; Yadav, R.C.; Yadav, N.R.; Rani, A.; Singh, D. Genetic transformation in oilseed Brassicas–A review. Indian J. Agric. Sci. 2013, 83, 367–373. [Google Scholar]
- O’Brien, D. Canola: Good protein source for dairy cattle. Agric. Res. 2016, 64, 1–3. [Google Scholar]
- Wickramasuriya, S.S.; Yi, Y.-J.; Yoo, J.; Kang, N.K.; Heo, J.M. A review of canola meal as an alternative feed ingredient for ducks. J. Anim. Sci. Technol. 2015, 57, 29. [Google Scholar] [CrossRef]
- Government of Pakistan, E.A.W. Pakistan Economic Survey 2016–17–Agriculture; Division, F., Ed.; Ministry of Finance: Islamabad, Pakistan, 2017.
- Christou, P. Plant genetic engineering and agricultural biotechnology 1983–2013. Trends Biotechnol. 2013, 31, 125–127. [Google Scholar] [CrossRef]
- Ahmad, N.; Mukhtar, Z. Genetic manipulations in crops: Challenges and opportunities. Genomics 2017, 109, 494–505. [Google Scholar] [CrossRef]
- Baltes, N.J.; Gil-Humanes, J.; Voytas, D.F. Genome engineering and agriculture: Opportunities and challenges. In Progress in Molecular Biology and Translational Science; Weeks, D.P., Yang, B., Eds.; Academic Press, Elsevier: Cambridge, Ma, USA, 2017; Volume 149, pp. 1–26. [Google Scholar]
- Paul, M.J.; Nuccio, M.L.; Basu, S.S. Are GM crops for yield and resilience possible? Trends Plant Sci. 2017, 23, 10–16. [Google Scholar] [CrossRef]
- Tyczewska, A.; Woźniak, E.; Gracz, J.; Kuczyński, J.; Twardowski, T. Towards food security: Current state and future prospects of agrobiotechnology. Trends Biotechnol. 2018, 36, 1219–1229. [Google Scholar] [CrossRef]
- Augustine, R.; Bisht, N.C. Targeted silencing of genes in polyploids: Lessons learned from Brassica juncea-glucosinolate system. Plant Cell Rep. 2018. [Google Scholar] [CrossRef]
- Nour-Eldin, H.H.; Madsen, S.R.; Engelen, S.; Jørgensen, M.E.; Olsen, C.E.; Andersen, J.S.; Seynnaeve, D.; Verhoye, T.; Fulawka, R.; Denolf, P. Reduction of antinutritional glucosinolates in Brassica oilseeds by mutation of genes encoding transporters. Nat. Biotechnol. 2017, 35, 377–382. [Google Scholar] [CrossRef]
- Liang, Y.; Xiong, Z.; Zheng, J.; Xu, D.; Zhu, Z.; Xiang, J.; Gan, J.; Raboanatahiry, N.; Yin, Y.; Li, M. Genome-wide identification, structural analysis and new insights into late embryogenesis abundant (LEA) gene family formation pattern in Brassica napus. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Maheshwari, P.; Selvaraj, G.; Kovalchuk, I. Optimization of Brassica napus (canola) explant regeneration for genetic transformation. Nat. Biotechnol. 2011, 29, 144–155. [Google Scholar] [CrossRef]
- Cheng, L.; Li, H.-P.; Qu, B.; Huang, T.; Tu, J.-X.; Fu, T.-D.; Liao, Y.-C. Chloroplast transformation of rapeseed (Brassica napus) by particle bombardment of cotyledons. Plant Cell Rep. 2010, 29, 371–381. [Google Scholar] [CrossRef]
- Liu, C.W.; Lin, C.C.; Yiu, J.C.; Chen, J.J.; Tseng, M.J. Expression of a Bacillus thuringiensis toxin (Cry1Ab) gene in cabbage (Brassica oleracea L. var. capitata L.) chloroplasts confers high insecticidal efficacy against Plutella xylostella. Theor. Appl. Genet. 2008, 117, 75–88. [Google Scholar] [CrossRef]
- Bhalla, P.L.; Singh, M.B. Agrobacterium-mediated transformation of Brassica napus and Brassica oleracea. Nat. Protoc. 2008, 3, 181–189. [Google Scholar] [CrossRef]
- Liu, C.W.; Lin, C.C.; Chen, J.J.; Tseng, M.J. Stable chloroplast transformation in cabbage (Brassica oleracea L. var. capitata L.) by particle bombardment. Plant Cell Rep. 2007, 26, 1733–1744. [Google Scholar] [CrossRef]
- Skarjinskaia, M.; Svab, Z.; Maliga, P. Plastid transformation in Lesquerella fendleri, an oilseed Brassicacea. Transgenic Res. 2003, 12, 115–122. [Google Scholar] [CrossRef]
- Sparrow, P.; Dale, P.; Irwin, J. The use of phenotypic markers to identify Brassica oleracea genotypes for routine high-throughput Agrobacterium-mediated transformation. Plant Cell Rep. 2004, 23, 64–70. [Google Scholar] [CrossRef]
- Hundleby, P.A.; Irwin, J.A. Brassica oleracea and B. napus. Methods Mol. Biol. 2015, 1223, 287–297. [Google Scholar] [CrossRef]
- Sparrow, P.; Snape, J.; Dale, P.; Irwin, J. The rapid identification of B. napus genotypes, for high-throughput transformation, using phenotypic tissue culture markers. Acta Hortic. 2006, 706, 239–246. [Google Scholar] [CrossRef]
- Sparrow, P.; Townsend, T.; Arthur, A.; Dale, P.; Irwin, J. Genetic analysis of Agrobacterium tumefaciens susceptibility in Brassica oleracea. Theor. Appl. Genet. 2004, 108, 644–650. [Google Scholar] [CrossRef]
- Sparrow, P.; Townsend, T.; Morgan, C.; Dale, P.; Arthur, A.; Irwin, J. Genetic analysis of in vitro shoot regeneration from cotyledonary petioles of Brassica oleracea. Theor. Appl. Genet. 2004, 108, 1249–1255. [Google Scholar] [CrossRef]
- Khehra, G.; Mathias, R. The interaction of genotype, explant and media on the regeneration of shoots from complex explants of Brassica napus L. J. Exp. Bot. 1992, 43, 1413–1418. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Arumugam, N.; Nandakumar, P.B.A.; Pradhan, A.K.; Gupta, V.; Pental, D. Agrobacterium-mediated genetic transformation of oilseed Brassica campestris: Transformation frequency is strongly influenced by the mode of shoot regeneration. Plant Cell Rep. 1992, 11, 506–513. [Google Scholar] [CrossRef]
- Zhang, Y.; Singh, M.B.; Swoboda, I.; Bhalla, P.L. Agrobacterium-mediated transformation and generation of male sterile lines of Australian canola. Aust. J. Agric. Res. 2005, 56, 353–361. [Google Scholar] [CrossRef]
- Das, B.; Goswami, L.; Ray, S.; Ghosh, S.; Bhattacharyya, S.; Das, S.; Majumder, A.L. Agrobacterium-mediated transformation of Brassica juncea with a cyanobacterial (Synechocystis PCC6803) delta-6 desaturase gene leads to production of gamma-linolenic acid. Plant Cell Tissue Organ Cult. 2006, 86, 219–231. [Google Scholar] [CrossRef]
- Dutta, I.; Saha, P.; Das, S. Efficient Agrobacterium-mediated genetic transformation of oilseed mustard [Brassica juncea (L.) Czern.] using leaf piece explants. In Vitro Cell. Dev. Biol.-Plant 2008, 44, 401–411. [Google Scholar] [CrossRef]
- Baskar, V.; Gangadhar, B.H.; Park, S.W.; Nile, S.H. A simple and efficient Agrobacterium tumefaciens-mediated plant transformation of Brassica rapa ssp. pekinensis. 3 Biotech 2016, 6, 88. [Google Scholar] [CrossRef]
- Daud, N.; Hasbullah, N.; Azis, N.; Rasad, F.; Amin, M.; Lassim, M. In vitro regeneration of Brassica oleracea var. capitata trough stems, roots, leaves and petioles cultures. In Proceedings of the International Conference on Agricultural, Ecological and Medical Sciences (AEMS-2015), Phuket, Thailand, 7–8 April 2015; pp. 7–8. [Google Scholar]
- Zhang, Y.; Bhalla, P.L. In vitro shoot regeneration from commercial cultivars of Australian canola (Brassica napus L.). Aust. J. Agric. Res. 2004, 55, 753–756. [Google Scholar] [CrossRef]
- Darçın, E.S.; Kolsarıcı, Ö.; Yıldız, M. Establishment of efficient regeneration protocol for three rapeseed cultivars. Biotechnol. Biotechnol. Equip. 2014, 28, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Srivastava, D. High frequency organogenesis in hypocotyl, cotyledon, leaf and petiole explants of broccoli (Brassica oleracea L. var. italica), an important vegetable crop. Physiol. Mol. Biol. Plants 2015, 21, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, G.; Kumar, P.; Srivastava, D. High frequency regeneration of plants from cotyledon and hypocotyl cultures in Brassica oleracea cv. Pride of India. Biotechnol. Rep. 2017, 15, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, B.; Shariatpanahi, M.E. Proline and chitosan enhanced efficiency of microspore embryogenesis induction and plantlet regeneration in Brassica napus L. Plant Cell Tissue Organ Cult. 2015, 123, 57–65. [Google Scholar] [CrossRef]
- Chikkala, V.R.; Nugent, G.D.; Dix, P.J.; Stevenson, T.W. Regeneration from leaf explants and protoplasts of Brassica oleracea var. botrytis (cauliflower). Sci. Hortic. 2009, 119, 330–334. [Google Scholar] [CrossRef]
- Khan, M.; Robin, A.A.H.K.; Nazim-Ud-Dowla, M.; Talukder, S.; Hassan, L. In vitro regeneration potentiality of Brassica genotypes in differential growth regulators. Bangladesh J. Agric. Res. 2010, 35, 189–199. [Google Scholar] [CrossRef]
- Cao, J.; Earle, E. Transgene expression in broccoli (Brassicaoleracea var. italica) clones propagated in vitro via leaf explants. Plant Cell Rep. 2003, 21, 789–796. [Google Scholar]
- Ono, Y.; Takahata, Y.; Kaizuma, N. Effect of genotype on shoot regeneration from cotyledonary explants of rapeseed (Brassica napus L.). Plant Cell Rep. 1994, 14, 13–17. [Google Scholar] [CrossRef]
- Jonoubi, P.; Mousavi, A.; Majd, A.; Salmanian, A.; Javaran, M.J.; Daneshian, J. Efficient regeneration of Brassica napus L. hypocotyls and genetic transformation by Agrobacterium tumefaciens. Biol. Plant. 2005, 49, 175–180. [Google Scholar] [CrossRef]
- Tang, G.; Zhou, W.; Li, H.; Mao, B.; He, Z.; Yoneyama, K. Medium, explant and genotype factors influencing shoot regeneration in oilseed Brassica spp. J. Agron. Crop Sci. 2003, 189, 351–358. [Google Scholar] [CrossRef]
- Ċosiċ, T.; Motyka, V.; Raspor, M.; Savić, J.; Cingel, A.; Vinterhalter, B.; Vinterhalter, D.; Trávníčková, A.; Dobrev, P.I.; Bohanec, B. In vitro shoot organogenesis and comparative analysis of endogenous phytohormones in kohlrabi (Brassica oleracea var. gongylodes): Effects of genotype, explant type and applied cytokinins. Plant Cell Tissue Organ Cult. 2015, 121, 741–760. [Google Scholar] [CrossRef]
- Gerszberg, A.; Hnatuszko-Konka, K.; Kowalczyk, T. In vitro regeneration of eight cultivars of Brassica oleracea var. capitata. In Vitro Cell. Dev. Biol.-Plant 2015, 51, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Uliaie, E.D.; Farsi, M.; Ghreyazie, B.; Imani, J. Effects of genotype and AgNO3 on shoot regeneration in winter cultivars of rapeseed (Brassica napus). Pak. J. Biol. Sci. 2008, 11, 2040–2043. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hachey, J.E.; Sharma, K.K.; Moloney, M.M. Efficient shoot regeneration of Brassica campestris using cotyledon explants cultured in vitro. Plant Cell Rep. 1991, 9, 549–554. [Google Scholar] [CrossRef]
- Bano, R.; Khan, M.H.; Khan, R.S.; Rashid, H.; Swati, Z.A. Development of an efficient regeneration protocol for three genotypes of Brassica juncea. Pak. J. Bot. 2010, 42, 963–969. [Google Scholar]
- Zhao, S.; Lei, J.; Chen, G.; Cao, B. Application of kanamycin in transgenic mustard (Brassica juncea Coss.). Yi Chuan Hered. 2008, 30, 501–507. [Google Scholar] [CrossRef]
- Mahmood, T.; Hussain, M.; Mustafa, H.; Hasan, E.; Aftab, M. Aari canola: Pakistan’s first ever canola quality and short duration mustard (Brassica juncea L.) cultivar resilient to climate change. Int. J. Biol. Pharm. Al. Sci 2017, 6, 777–787. [Google Scholar]
- Ribaut, J.-M.; Hoisington, D. Marker-assisted selection: New tools and strategies. Trends Plant Sci. 1998, 3, 236–239. [Google Scholar] [CrossRef]
- Lu, X.-M.; Yin, W.-B.; Hu, Z.-M. Chloroplast transformation. In Plant Cell Culture Protocols; Springer: Berlin, Germany, 2006; pp. 285–303. [Google Scholar]
- Díaz, A.H. Regeneration and plastid transformation approaches in Arabidopsis thaliana and Rapid-Cycling Brassica rapa; Ludwig Maximilian University of Munich: Munich, Germany, 2011. [Google Scholar]
- Cogbill, S.; Faulcon, T.; Jones, G.; McDaniel, M.; Harmon, G.; Blackmon, R.; Young, M. Adventitious shoot regeneration from cotyledonary explants of rapid-cycling fast plants of Brassica rapa L. Plant Cell Tiss. Org. Cult. 2010, 101, 127–133. [Google Scholar] [CrossRef]
- Khan, M.R.; Rashid, H.; Ansar, M.; Chaudry, Z. High frequency shoot regeneration and Agrobacterium-mediated DNA transfer in Canola (Brassica napus). Plant Cell Tiss. Org. Cult. 2003, 75, 223–231. [Google Scholar] [CrossRef]
- Khan, M.R.; Rashid, H.; Quraishi, A. High frequency shoot regeneration from hypocotyl of canola (Brassica napus L.) cv. Dunkled. Plant Tissue Cult 2002, 12, 131–138. [Google Scholar]
- Zhang, Y.; Bhalla, P. Shoot regeneration potential from seedling explants of Australian cultivars of oil seed rape (Brassica napus L.). In Proceedings of the New Horizons for an Old Crop, 10th International Rapeseed Congress, Canberra, Australia, 26–29 September 1999. [Google Scholar]
- Damgaard, O.; Rasmussen, O. Direct regeneration of transformed shoots in Brassica napus from hypocotyl infections with Agrobacterium rhizogenes. Plant Mol. Biol. 1991, 17, 1–8. [Google Scholar] [CrossRef]
- Barsby, T.L.; Yarrow, S.A.; Shepard, J.F. A rapid and efficient alternative procedure for the regeneration of plants from hypocotyl protoplasts of Brassica napus. Plant Cell Rep. 1986, 5, 101–103. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).