Roles of Hardened Husks and Membranes Surrounding Brachypodium hybridum Grains on Germination and Seedling Growth
Abstract
1. Introduction
2. Results
2.1. Effects of Husk Treatments on Germination
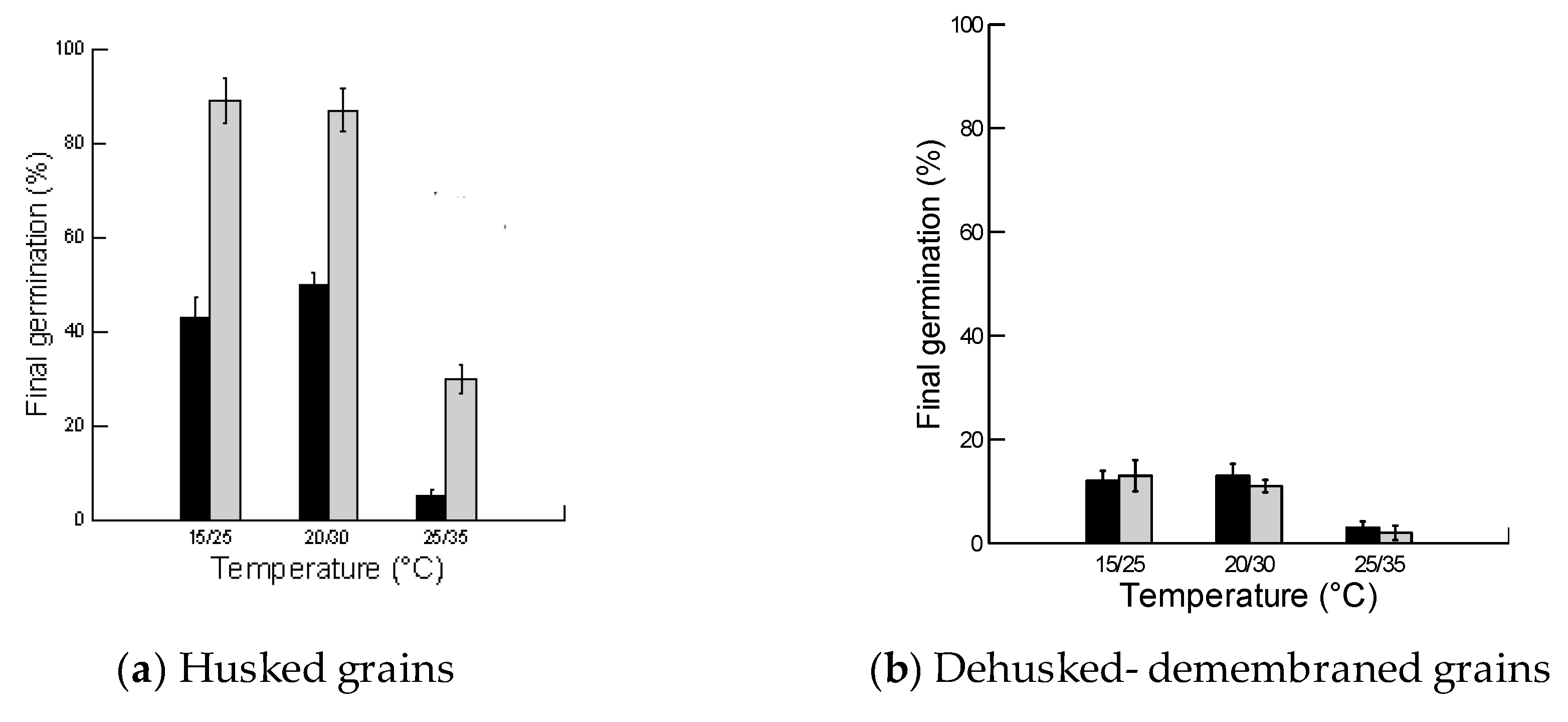
2.2. Effect of Husk, Light and Temperature on Final Germination
2.3. Effect of Husk and Temperature on Germination Rate Index
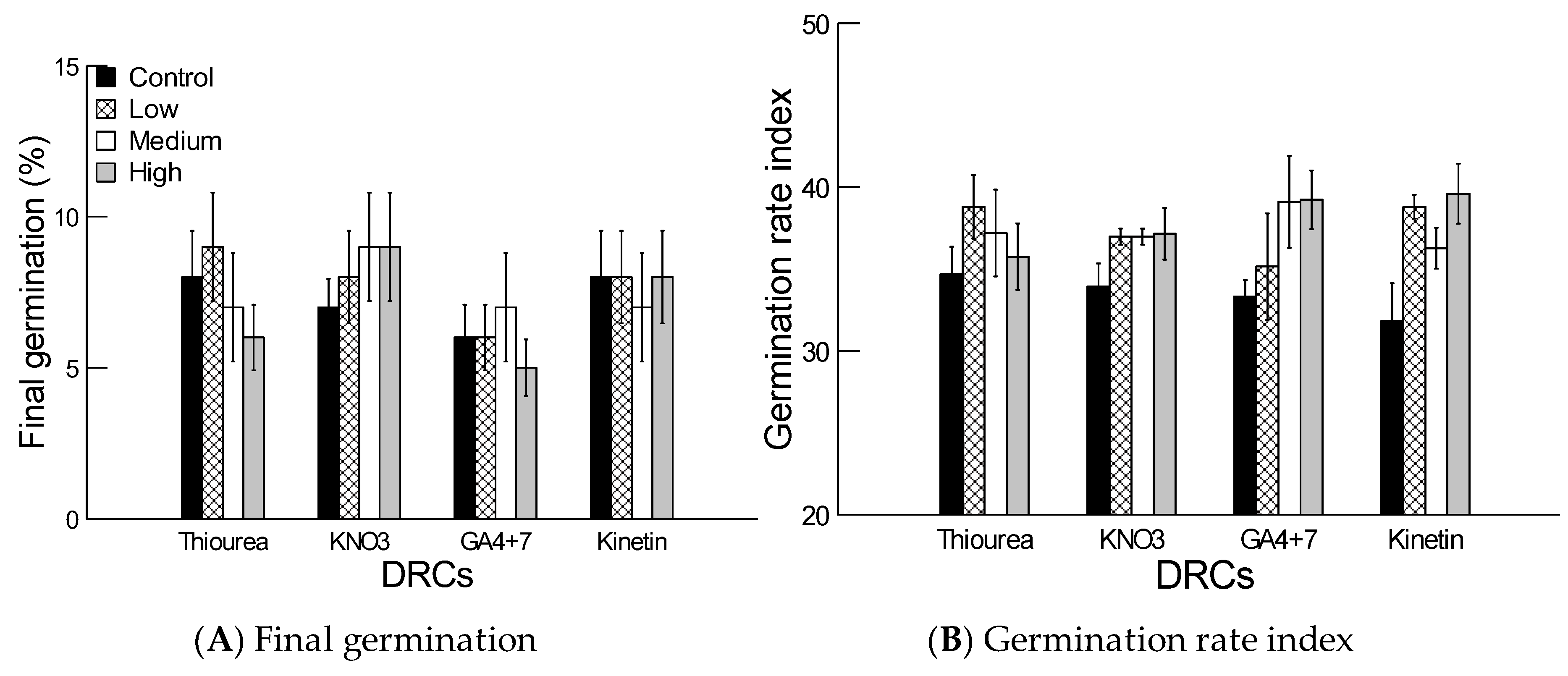
2.4. Effect of Dormancy Regulating Compounds
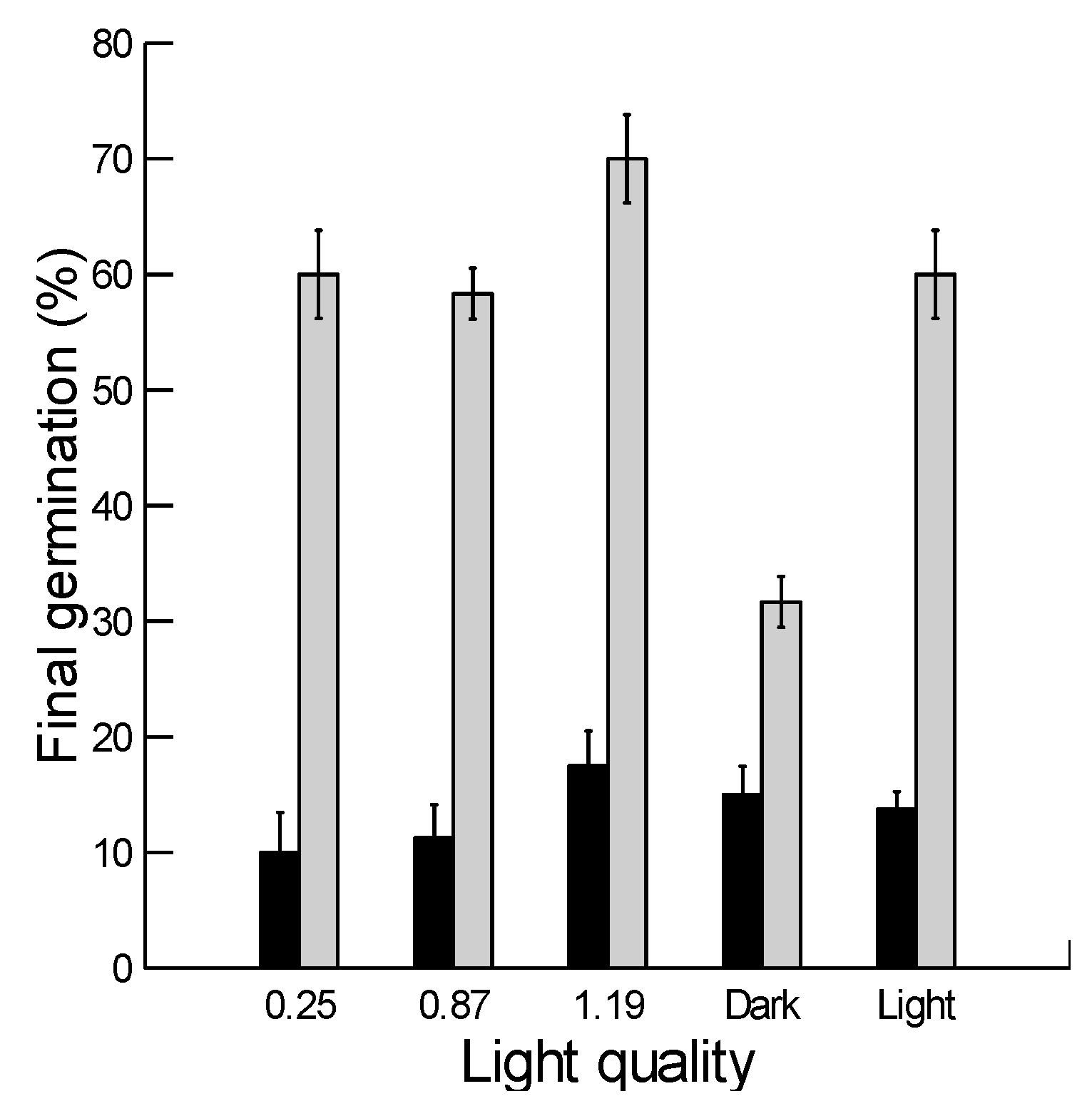
2.5. Effect of Light Quality
2.6. Effects of Husk Treatments on Plant Growth
2.6.1. Seedling Longest Leaf in Petri Dishes
2.6.2. Seedling Growth in Potted Soil
3. Discussion
4. Material and methods
4.1. Seed Collection and Preparation
4.2. Effects of Husk Treatments on Germination and Seedling Growth in Petri Dishes
4.3. Effect of Husk, Light and Temperature on Final Germination
4.4. Effect of Dormancy Regulating Compounds
4.5. Effect of Light Quality
4.6. Effects of Husk Treatments on Seedling Growth in a Pot Experiment
4.7. Data Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rudall, P.J.; Stuppy, W.; Cunniff, J.; Kellogg, E.A.; Briggs, B.G. Evolution of reproductive structures in grasses (Poaceae) inferred by sister-group comparison with their putative closest living relatives, Ecdeiocoleaceae. Am. J. Bot. 2005, 92, 1432–1443. [Google Scholar] [CrossRef]
- Preston, J.C.; Wang, H.; Kursel, L.; Doebley, J.; Kellogg, E.A. The role of teosinte glume architecture (tga1) in coordinated regulation and evolution of grass glumes and inflorescence axes. New Phytol. 2012, 193, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Raviv, B.; Godwin, J.; Granot, G.; Grafi, G. The dead can nurture: Novel Insights into the function of dead organs enclosing embryos. Int. J. Mol. Sci. 2018, 19, 2455. [Google Scholar] [CrossRef] [PubMed]
- Raviv, B.; Aghajanyan, L.; Granot, G.; Makover, V.; Frenkel, O.; Gutterman, Y.; Grafi, G. The dead seed coat functions as a long-term storage for active hydrolytic enzymes. PLoS ONE 2017, 12, e0181102. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.; Raviv, B.; Grafi, G. Dead pericarps of dry fruits function as long-term storage for active hydrolytic enzymes and other substances that affect germination and microbial growth. Plants 2017, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Brown, R. The absorption of water by seeds of Lolium perenne (L.) and certain other Gramineae. Ann. Appl. Biol. 1931, 18, 559–573. [Google Scholar] [CrossRef]
- Bülow-Olsen, A. Germination response to salt in Festuca rubra in a population from a salt marsh. Ecography 1983, 6, 194–198. [Google Scholar] [CrossRef]
- Raviv, B.; Granot, G.; Chalifa-Caspi, V.; Grafi, G. The dead, hardened floral bracts of dispersal units of wild wheat function as storage for active hydrolases and in enhancing seedling vigor. PLoS ONE 2017, 12, e0177537. [Google Scholar] [CrossRef]
- Wurzburger, J.; Leshem, Y. Physiological action of the germination inhibitor in the husk of Aegilops kotschyi Boiss. New Phytol. 1969, 68, 337–341. [Google Scholar] [CrossRef]
- Puglia, G.; Grimaldi, S.; Carta, A.; Pavone, P.; Toorop, P. Pericarp structure of Glebionis coronaria (L.) Cass. ex Spach (Asteraceae) cypselae controls water uptake during germination. Seed Sci. Res. 2015, 25, 255–266. [Google Scholar] [CrossRef]
- Dominguez, C.P.; Rodríguez, M.V.; Batlla, D.; de Salamone, I.E.G.; Mantese, A.I.; Andreani, A.L.; Benech-Arnold, R.L. Sensitivity to hypoxia and microbial activity are instrumental in pericarp-imposed dormancy expression in sunflower (Helianthus annuus L.). Seed Sci. Res. 2019, 29, 85–96. [Google Scholar] [CrossRef]
- Barrero, J.M.; Jacobsen, J.V.; Talbot, M.J.; White, R.G.; Swain, S.M.; Garvin, D.F.; Gubler, F. Grain dormancy and light quality effects on germination in the model grass Brachypodium distachyon. New Phytol. 2012, 193, 376–386. [Google Scholar] [CrossRef] [PubMed]
- El-Keblawy, A. Germination response to light and temperature in eight annual grasses from disturbed and natural habitats of an arid Arabian desert. J. Arid Environ. 2017, 147, 17–24. [Google Scholar] [CrossRef]
- Donohue, K.; Schmitt, J. Maternal environmental effects in plants: Adaptive plasticity? In Maternal Effects as Adaptations; Mousseau, T.A., Fox, C.W., Eds.; Oxford University Press: Oxford, UK, 1998; pp. 137–158. [Google Scholar]
- Galloway, L.F. Parental environmental effects on life history in the herbaceous plant Campanula americana. Ecology 2001, 82, 2781–2789. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Quintas-Soriano, C.; Castro, A.J.; García-Llorente, M.; Cabello, J.; Castro, H. From supply to social demand: A landscape-scale analysis of the water regulation service. Landsc. Ecol. 2014, 29, 1069–1082. [Google Scholar] [CrossRef]
- Roach, D.A.; Wulff, R.D. Maternal effects in plants. Annu. Rev. Ecol. Syst. 1987, 18, 209–235. [Google Scholar] [CrossRef]
- Baskin, C.; Baskin, J. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Elgabra, M.; El-Keblawy, A.; Mosa, K.; Soliman, S. Factors controlling seed dormancy and germination response of Brachypodium hybridum growing in the hot arid mountains of the Arabian Desert. Botany 2019, 97, 371–379. [Google Scholar] [CrossRef]
- El-Keblawy, A. Impacts of dormancy-regulating chemicals on innate and salinity-induced dormancy of four forage grasses native to A rabian deserts. Grass Forage Sci. 2013, 68, 288–296. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Shabana, H.A.; Navarro, T.; Soliman, S. Effect of maturation time on dormancy and germination of Citrullus colocynthis (Cucurbitaceae) seeds from the Arabian hyper-arid deserts. BMC Plant Biol. 2017, 17, 263. [Google Scholar] [CrossRef]
- Khan, M.A.; Shaikh, F.; Zehra, A.; Ahmed, M.Z.; Gul, B.; Ansari, R. Role of chemicals in alleviating salinity and light related seed dormancy in sub-tropical grasses. Flora 2017, 233, 150–155. [Google Scholar] [CrossRef]
- Khan, M.; Ungar, I. Effect of germination promoting compounds on the release of primary and salt-enforced seed dormancy in halophyte Sporobolus arabicus Boiss. Seed Sci. Technol. 2001, 29, 299–306. [Google Scholar]
- El-Keblawy, A.; Al-Ansari, F.; Al-Shamsi, N. Impact of dormancy regulating chemicals on salinity induced dormancy in Lasiurus scindicus and Panicum turgidum: Two desert glycophytic grasses. Plant Growth Regul. 2010, 62, 163–170. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Gairola, S. Dormancy regulating chemicals alleviate innate seed dormancy and promote germination of desert annuals. J. Plant Growth Regul. 2017, 36, 300–311. [Google Scholar] [CrossRef]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Batak, I.; Dević, M.; Gibal, Z.; Grubišić, D.; Poff, K.L.; Konjević, R. The effects of potassium nitrate and NO-donors on phytochrome A-and phytochrome B-specific induced germination of Arabidopsis thaliana seeds. Seed Sci. Res. 2002, 12, 253–259. [Google Scholar] [CrossRef]
- Hartley, W. Studies on the origin, evolution, and distribution of the Gramineae. V. The subfamily Festucoideae. Aust. J. Bot. 1973, 21, 201–234. [Google Scholar] [CrossRef]
- Garvin, D.F.; Gu, Y.Q.; Hasterok, R.; Hazen, S.P.; Jenkins, G.; Mockler, T.C.; Mur, L.A.J.; Vogel, J.P. Development of genetic and genomic research resources for Brachypodium distachyon, a new model system for grass crop research. Crop Sci. 2008, 48, S69–S84. [Google Scholar] [CrossRef]
- Catalán, P.; Müller, J.; Hasterok, R.; Jenkins, G.; Mur, L.A.; Langdon, T.; Betekhtin, A.; Siwinska, D.; Pimentel, M.; López-Alvarez, D. Evolution and taxonomic split of the model grass Brachypodium distachyon. Ann. Bot. 2012, 109, 385–405. [Google Scholar] [CrossRef]
- Wilson, P.; Streich, J.; Borevitz, J. Genomic diversity and climate adaptation in Brachypodium. In Genetics and Genomics of Brachypodium; Springer: Berlin/Heidelberg, Germany, 2015; pp. 107–127. [Google Scholar]
- López-Alvarez, D.; López-Herranz, M.L.; Betekhtin, A.; Catalán, P. A DNA barcoding method to discriminate between the model plant Brachypodium distachyon and its close relatives B. stacei and B. hybridum (Poaceae). PLoS ONE 2012, 7, e51058. [Google Scholar] [CrossRef]
- Wilson, P.B.; Streich, J.C.; Murray, K.D.; Eichten, S.R.; Cheng, R.; Aitken, N.; Spokas, K.; Warthmann, N.; Contributors, A.; Borevitz, J.O. Population structure of the Brachypodium species complex and genome wide association of agronomic traits in response to climate. BioRxiv 2018, 246074. [Google Scholar] [CrossRef]
- Jongbloed, M.; Feulner, G.; Böer, B.; Western, A.R. Environmental Research and Wildlife Development Agency. In The Comprehensive Guide to the Wild Flowers of the United Arab Emirates; Environmental Research and Wildlife Development Agency: Abu Dhabi, UAE, 2003. [Google Scholar]
- Karim, F.; Fawzi, N. Flora of the United Arab Emirates; UAE University Publications: Al-Ain, UAE, 2007. [Google Scholar]
- Draper, J.; Mur, L.A.; Jenkins, G.; Ghosh-Biswas, G.C.; Bablak, P.; Hasterok, R.; Routledge, A.P. Brachypodium distachyon. A new model system for functional genomics in grasses. Plant Physiol. 2001, 127, 1539–1555. [Google Scholar] [CrossRef] [PubMed]
- Koller, D.; Poljakoff-Mayber, A.; Berg, A.; Diskin, T. Germination-regulating mechanisms in Citrullus colocynthis. Am. J. Bot. 1963, 50, 597–603. [Google Scholar] [CrossRef]
- Soler, C.; Casanova, C.; Rojo, A. Desarrollo de cubiertas vegetales a partir de gramíneas seleccionadas, para su explotación en tierras de olivar. Actas Hortic 2004, 41, 97–100. [Google Scholar]
- González Moreno, A.; Casanova Pena, C.; Gascó, A.; Rodríguez Martín, J. Brachypodium hybridum plant cover improves water infiltration in Mediterranean crop soils. J. Plant Chem. Echophysiol. 2016, 1, 1008. [Google Scholar]
- Ruiz-Colmenero, M.; Bienes, R.; Marques, M. Soil and water conservation dilemmas associated with the use of green cover in steep vineyards. Soil Tillage Res. 2011, 117, 211–223. [Google Scholar] [CrossRef]
- Galiè, M.; Casavecchia, S.; Galdenzi, D.; Gasparri, R.; Soriano, P.; Estrelles, E.; Biondi, E. Seed germination behavior of two Brachypodium species with a key role in the improvement of marginal areas. Plant Sociol. 2013, 50, 91–107. [Google Scholar]
- Hunt, M.G.; Rasmussen, S.; Newton, P.C.; Parsons, A.J.; Newman, J.A. Near-term impacts of elevated CO2, nitrogen and fungal endophyte-infection on Lolium perenne L. growth, chemical composition and alkaloid production. Plant Cell Environ. 2005, 28, 1345–1354. [Google Scholar] [CrossRef]
- Luzuriaga, A.; Escudero, A.; Pérez-García, F. Environmental maternal effects on seed morphology and germination in Sinapis arvensis (Cruciferae). Weed Res. 2006, 46, 163–174. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Soliman, S.; Al-Khoury, R.; Ghauri, A.; Al Rammah, H.; Hussain, S.E.; Rashid, S.; Manzoor, Z. Effect of maturation conditions on light and temperature requirements during seed germination of Citrullus colocynthis from the Arabian Desert. Plant Biol. 2019, 21, 292–299. [Google Scholar] [CrossRef]
- Casal, J.J.; Sánchez, R.A. Phytochromes and seed germination. Seed Sci. Res. 1998, 8, 317–329. [Google Scholar] [CrossRef]
- Baron, F.J. Moisture and temperature in relation to seed structure and germination of sugar pine (Pinus lambertiana Dougl.). Am. J. Bot. 1978, 65, 804–810. [Google Scholar] [CrossRef]
- Hoff, R. Dormancy in Pinus monticola seed related to stratification time, seed coat, and genetics. Can. J. For. Res. 1987, 17, 294–298. [Google Scholar] [CrossRef]
- Khan, M.A.; Gul, B. Halophyte seed germination. In Ecophysiology of High Salinity Tolerant Plants; Springer: Berlin/Heidelberg, Germany, 2006; pp. 11–30. [Google Scholar]
- Booth, D.T.; Schuman, G.E. Seedbed ecology of winterfat: Fruits versus threshed seeds. J. Range Manag. 1983, 36, 387–390. [Google Scholar] [CrossRef]
- Francoz, E.; Lepiniec, L.; North, H.M. Seed coats as an alternative molecular factory: Thinking outside the box. Plant Reprod. 2018, 31, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Pill, W.; Necker, A. The effects of seed treatments on germination and establishment of Kentucky bluegrass (Poa pratensis L.). Seed Sci. Technol. 2001, 29, 65–72. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Pre-sowing seed treatment—A shotgun approach to improve germination, plant growth, and crop yield under saline and non-saline conditions. Adv. Agron. 2005, 88, 223–271. [Google Scholar]
- El-Keblawy, A.; Khedr, A.; Khafaga, T. Vegetation composition and diversity of Wadi Helo: A case study in Hajar Mountains. Arid Land Res. Manag. 2016, 10, 1136970. [Google Scholar]
- Khan, M.A.; Ungar, I.A. The effect of salinity and temperature on the germination of polymorphic seeds and growth of Atriplex triangularis Willd. Am. J. Bot. 1984, 71, 481–489. [Google Scholar] [CrossRef]


| Husk Treatments | FG% | GRI | Longest Leaf (cm) |
|---|---|---|---|
| Husked | 70.0 ± 4.3 b | 43.1 ± 1.4 b | 5.28 ± 0.67 b |
| Soaked husked | 91.7 ± 3.2 a | 47.7 ± 0.8 a | 6.00 ± 0.15 a |
| Dehusked-membraned | 64.3 ±2.4 b | 43.8 ± 1.0 b | 2.53 ± 0.19 d |
| Dehusked-membraned + detached husk | 63.4 ± 3.5 b | 41.7 ± 3.9 b | 2.33 ± 0.17 d |
| Soaked dehusked-membraned | 63.8 ± 3.4 b | 44.0 ± 1.7 b | 3.93 ± 0.28 c |
| Dehusked-demembraned | 8.3 ± 1.7 c | 28.5 ± 2.9 c | 1.83 ± 0.44 d |
| Dehusked-demembraned + detached husk | 8.3 ± 1.7 c | 31.3 ± 2.6 c | 1.88 ± 0.36 d |
| Soaked dehusked-demembraned | 10.0 ± 1.9 c | 31.3 ± 2.6 c | 3.55 ± 0.24 c |
| F-value, P (One way ANOVA) | 58.5, p < 0.001 | 7.99, p < 0.001 | 14.22, p < 0.001 |
| Source of Variation | Df | Mean Squares | F-Ratio | p-Value |
|---|---|---|---|---|
| (a) Final germination | ||||
| Husk | 1 | 2.953 | 475.794 | <0.001 |
| Temperature (Temp) | 2 | 0.674 | 108.626 | <0.001 |
| Light | 1 | 0.709 | 114.277 | <0.001 |
| Husk × Temp | 2 | 0.354 | 57.095 | <0.001 |
| Husk × Light | 1 | 0.749 | 120.641 | <0.001 |
| Temp × Light | 2 | 0.050 | 8.125 | <0.01 |
| Husk × Temp*Light | 2 | 0.044 | 7.086 | <0.01 |
| Error | 36 | 0.006 | ||
| (b) Germination rate index | ||||
| Husk | 1 | 2.416 | 88.575 | <0.001 |
| Temperature (Temp) | 2 | 0.770 | 28.209 | <0.001 |
| Husk × Temp | 2 | 0.579 | 21.236 | <0.001 |
| Error | 16 | 0.027 | ||
| Source of Variation. | Df | Mean Squares | F-Ratio | p-Value |
|---|---|---|---|---|
| (a) Final germination | ||||
| DRCs | 3 | 0.002 | 1.452 | ns |
| Concentration (C) | 3 | 0.000 | 0.161 | ns |
| DRCs × C | 9 | 0.000 | 0.398 | ns |
| Error | 48 | 0.001 | ||
| (b) Germination rate index | ||||
| DRCs | 3 | 0.000 | 0.008 | ns |
| Concentration (C) | 3 | 0.054 | 4.189 | <0.01 |
| DRCs × C | 9 | 0.010 | 0.769 | ns |
| Error | 48 | 0.013 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Keblawy, A.; Elgabra, M.; Mosa, K.A.; Fakhry, A.; Soliman, S. Roles of Hardened Husks and Membranes Surrounding Brachypodium hybridum Grains on Germination and Seedling Growth. Plants 2019, 8, 322. https://doi.org/10.3390/plants8090322
El-Keblawy A, Elgabra M, Mosa KA, Fakhry A, Soliman S. Roles of Hardened Husks and Membranes Surrounding Brachypodium hybridum Grains on Germination and Seedling Growth. Plants. 2019; 8(9):322. https://doi.org/10.3390/plants8090322
Chicago/Turabian StyleEl-Keblawy, Ali, Masarra Elgabra, Kareem A. Mosa, Amal Fakhry, and Sameh Soliman. 2019. "Roles of Hardened Husks and Membranes Surrounding Brachypodium hybridum Grains on Germination and Seedling Growth" Plants 8, no. 9: 322. https://doi.org/10.3390/plants8090322
APA StyleEl-Keblawy, A., Elgabra, M., Mosa, K. A., Fakhry, A., & Soliman, S. (2019). Roles of Hardened Husks and Membranes Surrounding Brachypodium hybridum Grains on Germination and Seedling Growth. Plants, 8(9), 322. https://doi.org/10.3390/plants8090322






