The Interplay among Polyamines and Nitrogen in Plant Stress Responses
Abstract
1. Introduction
2. Major Genes Involved in Abiotic and Biotic Stress Responses
3. Stress-Related Nitrogen Flow and Polyamines
4. N/PA Biotechnological Approaches for Enhanced Tolerance to Abiotic and Biotic Stress
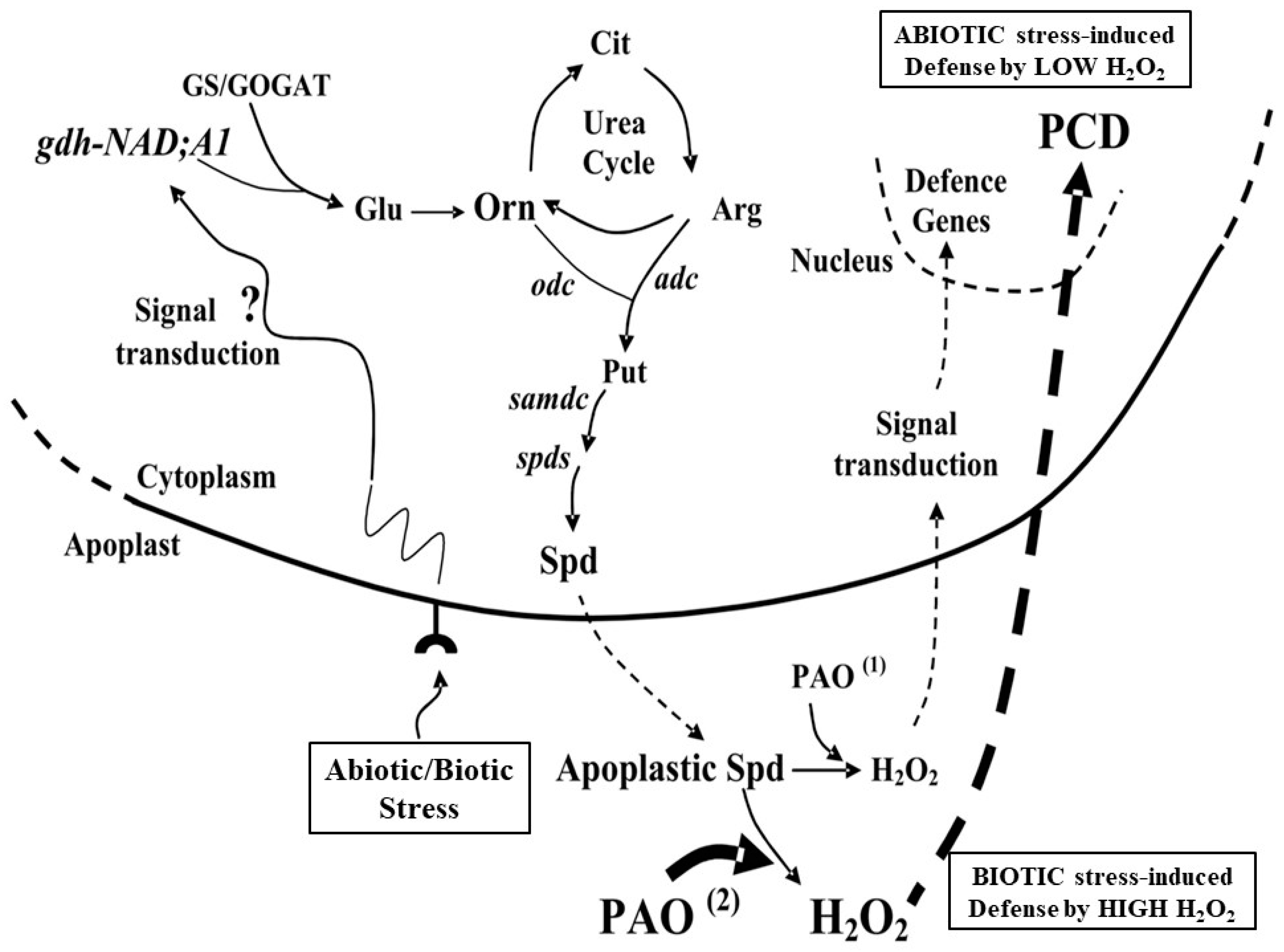
5. Conclusion and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ioannidis, N.E.; Malliarakis, D.; Torne, J.M.; Santos, M.; Kotzabasis, K. The over-expression of the plastidial transglutaminase from maize in arabidopsis increases the activation threshold of photoprotection. Front. Plant Sci. 2016, 7, 635. [Google Scholar] [CrossRef] [PubMed]
- Moschou, P.N.; Paschalidis, K.A.; Roubelakis-Angelakis, K.A. Plant polyamine catabolism: The state of the art. Plant Signal. Behav. 2008c, 3, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Tavladoraki, P.; Cona, A.; Angelini, R. Copper-containing amine oxidases and fad-dependent polyamine oxidases are key players in plant tissue differentiation and organ development. Front. Plant Sci. 2016, 7, 824. [Google Scholar] [CrossRef] [PubMed]
- Minocha, R.; Majumdar, R.; Minocha, S.C. Polyamines and abiotic stress in plants: A complex relationship. Front. Plant Sci. 2014, 5, 175. [Google Scholar] [CrossRef] [PubMed]
- Tiburcio, A.F.; Alcazar, R. Potential applications of polyamines in agriculture and plant biotechnology. Methods Mol. Biol. 2018, 1694, 489–508. [Google Scholar] [PubMed]
- Handa, A.K.; Fatima, T.; Mattoo, A.K. Polyamines: Bio-molecules with diverse functions in plant and human health and disease. Front. Chem. 2018, 6, 10. [Google Scholar] [CrossRef]
- Fortes, A.M.; Agudelo-Romero, P. Polyamine metabolism in climacteric and non-climacteric fruit ripening. Methods Mol. Biol. 2018, 1694, 433–447. [Google Scholar]
- Wang, Y.; Ye, X.; Yang, K.; Shi, Z.; Wang, N.; Yang, L.; Chen, J. Characterization, expression, and functional analysis of polyamine oxidases for their role in selenium-induced hydrogen peroxide production in brassica rapa. J. Sci. Food Agric. 2019, 99, 4082–4093. [Google Scholar] [CrossRef]
- Podlesakova, K.; Ugena, L.; Spichal, L.; Dolezal, K.; De Diego, N. Phytohormones and polyamines regulate plant stress responses by altering gaba pathway. N. Biotechnol. 2019, 48, 53–65. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. The functional role of polyamines in eukaryotic cells. Int. J. Biochem. Cell Biol. 2019, 107, 104–115. [Google Scholar] [CrossRef]
- Bordenave, C.D.; Granados Mendoza, C.; Jimenez Bremont, J.F.; Garriz, A.; Rodriguez, A.A. Defining novel plant polyamine oxidase subfamilies through molecular modeling and sequence analysis. BMC Evol. Biol. 2019, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, E.; Salinas, M.; Manzano-Agugliaro, F. Worldwide research on plant defense against biotic stresses as improvement for sustainable agriculture. Sustainability 2018, 10, 391. [Google Scholar] [CrossRef]
- Miao, H.; Sun, P.; Liu, Q.; Liu, J.; Xu, B.; Jin, Z. The agpase family proteins in banana: Genome-wide identification, phylogeny, and expression analyses reveal their involvement in the development, ripening, and abiotic/biotic stress responses. Int. J. Mol. Sci. 2017, 18, 1581. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.D.; Shao, Q.S.; Yin, L.H.; Younis, A.; Zheng, B.S. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Liu, T.; Yang, S.C.; Jin, X.Q.; Qu, F.; Huang, N.; Hu, X.H. Polyamines are involved in gaba-regulated salinity-alkalinity stress tolerance in muskmelon. Environ. Exp. Bot. 2019, 164, 181–189. [Google Scholar] [CrossRef]
- Takahashi, T. Thermospermine: An evolutionarily ancient but functionally new compound in plants. Methods Mol. Biol. 2018, 1694, 51–59. [Google Scholar] [PubMed]
- Takahashi, T.; Kakehi, J. Polyamines: Ubiquitous polycations with unique roles in growth and stress responses. Ann. Bot. 2010, 105, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Takano, A.; Kakehi, J.; Takahashi, T. Thermospermine is not a minor polyamine in the plant kingdom. Plant Cell Physiol. 2012, 53, 606–616. [Google Scholar] [CrossRef]
- Tsaniklidis, G.; Delis, C.; Nikoloudakis, N.; Katinakis, P.; Aivalakis, G. Low temperature storage affects the ascorbic acid metabolism of cherry tomato fruits. Plant Physiol. Biochem. 2014, 84, 149–157. [Google Scholar] [CrossRef]
- Tsaniklidis, G.; Kotsiras, A.; Tsafouros, A.; Roussos, P.A.; Aivalakis, G.; Katinakis, P.; Delis, C. Spatial and temporal distribution of genes involved in polyamine metabolism during tomato fruit development. Plant Physiol. Biochem. 2016, 100, 27–36. [Google Scholar] [CrossRef]
- Paschalidis, K.A.; Aziz, A.; Geny, L.; Primikirios, N.I.; Roubelakis-Angelakis, K.A. Polyamines in grapevine. In Molecular Biology & Biotechnology of the Grapevine; Roubelakis-Angelakis, K.A., Ed.; Springer: Dordrecht, The Netherlands, 2001; pp. 109–151. [Google Scholar]
- Wang, W.; Paschalidis, K.; Feng, J.C.; Song, J.; Liu, J.H. Polyamine catabolism in plants: A universal process with diverse functions. Front. Plant Sci. 2019, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, A.K.; Paschalidis, K.A.; Roubelakis-Angelakis, K.A. Biosynthesis profile and endogenous titers of polyamines differ in totipotent and recalcitrant plant protoplasts. Physiol. Plant 2005, 125, 10–20. [Google Scholar] [CrossRef]
- Papadakis, A.K.; Roubelakis-Angelakis, K.A. Polyamines inhibit nadph oxidase-mediated superoxide generation and putrescine prevents programmed cell death induced by polyamine oxidase-generated hydrogen peroxide. Planta 2005, 220, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Paschalidis, K.A.; Roubelakis-Angelakis, K.A. Sites and regulation of polyamine catabolism in the tobacco plant. Correlations with cell division/expansion, cell cycle progression, and vascular development. Plant Physiol. 2005b, 138, 2174–2184. [Google Scholar] [CrossRef] [PubMed]
- Paschalidis, K.A.; Roubelakis-Angelakis, K.A. Spatial and temporal distribution of polyamine levels and polyamine anabolism in different organs/tissues of the tobacco plant. Correlations with age, cell division/expansion, and differentiation. Plant Physiol. 2005a, 138, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Moschou, P.N.; Delis, I.D.; Paschalidis, K.A.; Roubelakis-Angelakis, K.A. Transgenic tobacco plants overexpressing polyamine oxidase are not able to cope with oxidative burst generated by abiotic factors. Physiol. Plant 2008a, 133, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Moschou, P.N.; Paschalidis, K.A.; Delis, I.D.; Andriopoulou, A.H.; Lagiotis, G.D.; Yakoumakis, D.I.; Roubelakis-Angelakis, K.A. Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plant Cell 2008b, 20, 1708–1724. [Google Scholar] [CrossRef] [PubMed]
- Moschou, P.N.; Sanmartin, M.; Andriopoulou, A.H.; Rojo, E.; Sanchez-Serrano, J.J.; Roubelakis-Angelakis, K.A. Bridging the gap between plant and mammalian polyamine catabolism: A novel peroxisomal polyamine oxidase responsible for a full back-conversion pathway in arabidopsis. Plant Physiol. 2009b, 147, 1845–1857. [Google Scholar] [CrossRef]
- Moschou, P.N.; Sarris, P.F.; Skandalis, N.; Andriopoulou, A.H.; Paschalidis, K.A.; Panopoulos, N.J.; Roubelakis-Angelakis, K.A. Engineered polyamine catabolism preinduces tolerance of tobacco to bacteria and oomycetes. Plant Physiol. 2009a, 149, 1970–1981. [Google Scholar] [CrossRef]
- Paschalidis, K.; Moschou, P.N.; Aziz, A.; Toumi, I.; Roubelakis-Angelakis, Κ.A. Polyamines in grapevine: An update. In Grapevine Molecular Physiology & Biotechnology; Roubelakis-Angelakis, K.A., Ed.; Springer: Dordrecht, The Netherlands, 2009a; pp. 207–228. [Google Scholar]
- Toumi, I.; Moschou, P.N.; Paschalidis, K.A.; Bouamama, B.; Ben Salem-Fnayou, A.; Ghorbel, A.W.; Mliki, A.; Roubelakis-Angelakis, K.A. Abscisic acid signals reorientation of polyamine metabolism to orchestrate stress responses via the polyamine exodus pathway in grapevine. J. Plant Physiol. 2010, 167, 519–525. [Google Scholar] [CrossRef]
- Paschalidis, K.A.; Toumi, I.; Moschou, P.N.; Roubelakis-Angelakis, K.A. Aba-dependent amine oxidases-derived h2o2 affects stomata conductance. Plant Signal. Behav. 2010, 5, 1153–1156. [Google Scholar]
- Skopelitis, D.S.; Paranychianakis, N.V.; Paschalidis, K.A.; Pliakonis, E.D.; Delis, I.D.; Yakoumakis, D.I.; Kouvarakis, A.; Stephanou, E.; Papadakis, A.K.; Roubelakis-Angelakis, K.A. Abiotic stress generates ros that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 2006, 18, 2767–2781. [Google Scholar] [CrossRef] [PubMed]
- Andronis, E.A.; Moschou, P.N.; Roubelakis-Angelakis, K.A. Peroxisomal polyamine oxidase and nadph-oxidase cross-talk for ros homeostasis which affects respiration rate in arabidopsis thaliana. Front. Plant Sci. 2014, 5, 132. [Google Scholar] [CrossRef] [PubMed]
- Gemes, K.; Kim, Y.J.; Park, K.Y.; Moschou, P.N.; Andronis, E.; Valassaki, C.; Roussis, A.; Roubelakis-Angelakis, K.A. An nadph-oxidase/polyamine oxidase feedback loop controls oxidative burst under salinity. Plant Physiol. 2016, 172, 1418–1431. [Google Scholar] [CrossRef] [PubMed]
- Gemes, K.; Mellidou, I.; Karamanoli, K.; Beris, D.; Park, K.Y.; Matsi, T.; Haralampidis, K.; Constantinidou, H.I.; Roubelakis-Angelakis, K.A. Deregulation of apoplastic polyamine oxidase affects development and salt response of tobacco plants. J. Plant Physiol. 2017, 211, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mellidou, I.; Karamanoli, K.; Beris, D.; Haralampidis, K.; Constantinidou, H.A.; Roubelakis-Angelakis, K.A. Underexpression of apoplastic polyamine oxidase improves thermotolerance in Nicotiana tabacum. J. Plant Physiol. 2017, 218, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Mellidou, I.; Moschou, P.N.; Pankou, C.; Valassakis, C.; Ioannidis, N.; Gémes, K.; Andronis, E.A.; Roussis, A.; Beris, D.; Haralampidis, K.; et al. Nicotiana tabacum plants with silenced s-adenosyl -l-methionine decarboxylase (samdc) reveal a pa-dependent trade-off between growth and tolerance responses. Front. Plant Sci. 2016, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lea, P.J.; Miflin, B.J. Alternative route for nitrogen assimilation in higher plants. Nat. Rev. Genet. 1974, 251, 614–616. [Google Scholar] [CrossRef]
- Skopelitis, D.S.; Paranychianakis, N.V.; Kouvarakis, A.; Spyros, A.; Stephanou, E.G.; Roubelakis-Angelakis, K.A. The isoenzyme 7 of tobacco nad(h)-dependent glutamate dehydrogenase exhibits high deaminating and low aminating activities in vivo. Plant Physiol. 2007, 145, 1726–1734. [Google Scholar] [CrossRef]
- Moschou, P.N.; Roubelakis-Angelakis, K.A. Polyamines and programmed cell death. J. Exp. Bot. 2014, 65, 1285–1296. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Singhal, P.; Jan, A.T.; Azam, M.; Haq, Q.M.R. Plant abiotic stress: A prospective strategy of exploiting promoters as alternative to overcome the escalating burden. Front. Life Sci. 2016, 9, 52–63. [Google Scholar] [CrossRef]
- Shabala, S.; Bose, J.; Fuglsang, A.T.; Pottosin, I. On a quest for stress tolerance genes: Membrane transporters in sensing and adapting to hostile soils. J. Exp. Bot. 2016, 67, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Kosová, K.; Vítámvás, P.; Urban, M.O.; Prášil, I.T.; Renaut, J. Plant abiotic stress proteomics: The major factors determining alterations in cellular proteome. Front. Plant Sci. 2018, 9, 1–22. [Google Scholar] [CrossRef]
- Romero, F.M.; Maiale, S.J.; Rossi, F.R.; Marina, M.; Ruiz, O.A.; Garriz, A. Polyamine metabolism responses to biotic and abiotic stress. Methods Mol. Biol. 2018, 1694, 37–49. [Google Scholar] [PubMed]
- Gago, C.; Drosou, V.; Paschalidis, K.; Guerreiro, A.; Miguel, G.; Antunes, D.; Hilioti, Z. Targeted gene disruption coupled with metabolic screen approach to uncover the leafy cotyledon1-like4 (l1l4) function in tomato fruit metabolism. Plant Cell Rep. 2017, 36, 1065–1082. [Google Scholar] [CrossRef] [PubMed]
- Manganaris, G.A.; Drogoudi, P.; Goulas, V.; Tanou, G.; Georgiadou, E.C.; Pantelidis, G.E.; Paschalidis, K.A.; Fotopoulos, V.; Manganaris, A. Deciphering the interplay among genotype, maturity stage and low-temperature storage on phytochemical composition and transcript levels of enzymatic antioxidants in prunus persica fruit. Plant Physiol. Biochem. 2017, 119, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Dhima, Κ.; Vasilakoglou, Ι.; Stefanou, S.; Gatsis, T.; Paschalidis, K.; Aggelopoulos, S.; Eleftherohorinos, I. Differential competitive and allelopathic ability of cyperus rotundus on solanum lycopersicum, solanum melongena and capsicum annuum. Arch. Agron. Soil Sci. 2016, 62, 1250–1263. [Google Scholar] [CrossRef]
- Dhima, K.; Vasilakoglou, I.; Paschalidis, K.A.; Gatsis, T.; Keco, R. Productivity and phytotoxicity of six sunflower hybrids and their residues effects on rotated lentil and ivy-leaved speedwell. Field Crop. Res. 2012, 136, 42–51. [Google Scholar] [CrossRef]
- Goumenaki, E.; Karidis, Z.; Paschalidis, K.A. Assessment of tropospheric ozone impact on crops in crete (greece) using snap bean as bioindicator. Acta Hortic. 2012, 938, 401–407. [Google Scholar] [CrossRef]
- Makky, M.; Paschalidis, K.A.; Dhima, K.; Manganaris, A. A new rapid gas chromatographic method for ethylene, respirational, and senescent gaseous production of climacteric fruits stored in prolonged low temperature. Proc. Int. Conf. Agric. Environ. Biol. Sci. (AEBS-2014) 2014a, 1, 24–25. [Google Scholar]
- Makky, M.; Paschalidis, K.A.; Dhima, K.; Mangganaris, A. Tomato fruits (solanaceae lycopersicon esculentum mill.) feedback mechanism in the presence of exogenous ethylene under prolonged chilling temperature storage. J. Nutr. Pharm. Res. 2015, 1, 4–12. [Google Scholar]
- Ninou, E.G.; Paschalidis, K.A.; Mylonas, I.G.; Vasilikiotis, C.; Mavromatis, A.G. The effect of genetic variation and nitrogen fertilization on productive characters of greek oregano. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2017b, 67, 372–379. [Google Scholar] [CrossRef]
- Vasilakoglou, I.; Dhima, K.; Paschalidis, K.; Gatsis, T.; Zacharis, K.; Galanis, M. Field bindweed (convolvulus arvensis l.) and redroot pigweed (amaranthus retroflexus l.) control in potato by pre-or post-emergence applied flumioxazin and sulfosulfuron. Chil. J. Agric. Res. 2013, 73, 24–30. [Google Scholar] [CrossRef]
- Mantzorou, A.; Navakoudis, E.; Paschalidis, K.; Ververidis, F. Microalgae: A potential tool for remediating aquatic environments from toxic metals. Int. J. Environ. Sci. Technol. 2018, 15, 1815–1830. [Google Scholar] [CrossRef]
- Paschalidis, K.A.; Moschou, P.N.; Toumi, I.; Roubelakis-Angelakis, K.A. Polyamine anabolic/catabolic regulation along the woody grapevine plant axis. J. Plant Physiol. 2009b, 166, 1508–1519. [Google Scholar] [CrossRef]
- Mougiou, Ν.; Trikka, F.; Trantas, E.; Ververidis, F.; Makris, A.; Argiriou, A.; Vlachonasios, K.E. Expression of hydroxytyrosol and oleuropein biosynthetic genes are correlated with metabolite accumulation during fruit development in olive, olea europaea, cv. Koroneiki. Plant Physiol. Biochem. 2018, 128, 41–49. [Google Scholar] [CrossRef]
- Papadakis, I.E.; Tsiantas, P.I.; Tsaniklidis, G.; Landi, M.; Psychoyou, M.; Fasseas, C. Changes in sugar metabolism associated to stem bark thickening partially assist young tissues of eriobotrya japonica seedlings under boron stress. J Plant Physiol. 2018, 231, 337–345. [Google Scholar] [CrossRef]
- Trantas, A.E.; Sarris, P.F.; Mpalantinaki, E.; Papadimitriou, M.; Ververidis, F.; Goumas, D.E. First report of xanthomonas hortorum pv. Hederae causing bacterial leaf spot on ivy in greece. Plant Disease 2016, 100, 1–10. [Google Scholar] [CrossRef]
- Trantas, E.A.; Mpalantinaki, E.; Pagoulatou, M.; Sarris, P.F.; Ververidis, F.; Goumas, D.E. First report of bacterial apical necrosis of mango caused by pseudomonas syringae pv. Syringae in greece. Plant Disease 2017, 101, 1541. [Google Scholar] [CrossRef]
- Wang, W.; Wu, H.; Liu, J.H. Genome-wide identification and expression profiling of copper-containing amine oxidase genes in sweet orange (citrus sinensis). Tree Genet. Genomes 2017, 13, 31. [Google Scholar] [CrossRef]
- Wu, H.; Fu, B.; Sun, P.; Xiao, C.; Liu, J.H. A nac transcription factor represses putrescine biosynthesis and affects drought tolerance. Plant Physiol. 2016, 172, 1532–1547. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Zhang, J.; Hu, J.; Wang, W.; Wu, H.; Zhang, Q.; Liu, J.H. Fcwrky70, a wrky protein of fortunella crassifolia, functions in drought tolerance and modulates putrescine synthesis by regulating arginine decarboxylase gene. Plant Cell Environ. 2015, 38, 2248–2262. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.S.; Zhang, Q.; Zhu, D.; Fu, X.; Wang, M.; Zhang, Q.; Moriguchi, T.; Liu, J.H. Ice1 of poncirus trifoliata functions in cold tolerance by modulating polyamine levels through interacting with arginine decarboxylase. J. Exp. Bot. 2015, 66, 3259–3274. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.H.; Yu, A.; Xiang, Y.; Kurosawa, T.; Nada, K.; Ban, Y. Cloning, biochemical identification, and expression analysis of a gene encoding s-adenosylmethionine decarboxylase in citrus sinensis osbeck. J. Hortic. Sci. Biotechnol. 2010, 85, 219–226. [Google Scholar] [CrossRef]
- Fu, X.Z.; Chen, C.W.; Wang, Y.; Liu, J.H.; Moriguchi, T. Ectopic expression of mdspds1 in sweet orange (citrus sinensis osbeck) reduces canker susceptibility: Involvement of H2O2 production and transcriptional alteration. BMC Plant Biol. 2011, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Fu, X.Z.; Peng, T.; Huang, X.S.; Fan, Q.J.; Liu, J.H. Spermine pretreatment confers dehydration tolerance of citrus in vitro plants via modulation of antioxidative capacity and stomatal response. Tree Physiol. 2010, 30, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, M.; Hu, J.; Wang, W.; Fu, X.; Liu, J.H. Ptrabf of poncirus trifoliata functions in dehydration tolerance by reducing stomatal density and maintaining reactive oxygen species homeostasis. J. Exp. Bot. 2015, 66, 5911–5927. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Wang, M.; Zhang, H.Y.; Liu, J.H. The mir396b of poncirus trifoliata functions in cold tolerance by regulating acc oxidase gene expression and modulating ethylene-polyamine homeostasis. Plant Cell Physiol. 2016, 57, 1865–1878. [Google Scholar] [CrossRef]
- Liu, J.-H.; Wang, W.; Wu, H.; Gong, X.; Moriguchi, T. Polyamines function in stress tolerance: From synthesis to regulation. Front. Plant Sci. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Moschou, P.N.; Wu, J.; Cona, A.; Tavladoraki, P.; Angelini, R.; Roubelakis-Angelakis, K.A. The polyamines and their catabolic products are significant players in the nitrogenous turnover in plants. J. Exp. Bot. 2012, 63, 5003–5015. [Google Scholar] [CrossRef] [PubMed]
- Loulakakis, K.A.; Primikirios, N.I.; Nikolantonakis, M.A.; Roubelakis-Angelakis, K.A. Immunocharacterization of vitis vinifera l. Ferredoxin-dependent glutamate synthase, and its spatial and temporal changes during leaf development. Planta 2002, 215, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Syntichaki, K.M.; Loulakakis, K.A.; Roubelakis-Angelakis, K.A. The amino-acid sequence similarity of plant glutamate dehydrogenase to the extremophilic archaeal enzyme conforms to its stress-related function. Gene 1996, 168, 87–92. [Google Scholar] [CrossRef]
- Loulakakis, K.A.; Roubelakis-Angelakis, K.A.; Kanellis, A.K. Regulation of glutamate dehydrogenase and glutamine synthetase in avocado fruit during development and ripening. Plant Physiol. 1994, 106, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Loulakakis, K.A.; Roubelakis-Angelakis, K.A. Plant nad(h)-glutamate dehydrogenase consists of two subunit polypeptides and their participation in the seven isoenzymes occurs in an ordered ratio. Plant Physiol. 1991, 97, 104–111. [Google Scholar] [CrossRef]
- Majumdar, R.; Barchi, B.; Turlapati, S.A.; Gagne, M.; Minocha, R.; Long, S.; Minocha, S.C. Glutamate, ornithine, arginine, proline, and polyamine metabolic interactions: The pathway is regulated at the post-transcriptional level. Front. Plant Sci. 2016, 7, 78. [Google Scholar] [CrossRef]
- Ninou, E.; Paschalidis, K.; Mylonas, I. Essential oil responses to water stress in greek oregano populations. J. Essent. Oil Bear. Plants 2017a, 20, 12–23. [Google Scholar] [CrossRef]
- Makky, M.; Paschalidis, K.A.; Dhima, K.; Manganaris, A. Harnessing untapped bio-ethylene sources from tomatoes climacteric effluent. Proc. Int. Conf. Agric. Environ. Biol. Sci. (AEBS-2014) 2014b, 1, 24–25. [Google Scholar]
- Serapiglia, M.J.; Minocha, R.; Minocha, S.C. Changes in polyamines, inorganic ions and glutamine synthetase activity in response to nitrogen availability and form in red spruce (picea rubens). Tree Physiol. 2008, 28, 1793–1803. [Google Scholar] [CrossRef][Green Version]
- Tong, W.; Imai, A.; Tabata, R.; Shigenobu, S.; Yamaguchi, K.; Yamada, M.; Hasebe, M.; Sawa, S.; Motose, H.; Takahashi, T. Polyamine resistance is increased by mutations in a nitrate transporter gene nrt1.3 (atnpf6.4) in arabidopsis thaliana. Front. Plant Sci. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Ma, S.Z.; Zhang, W.W.; Yao, K.B.; Chen, L.; Zhao, F.; Zhuang, Y.Q. Accumulating pathways of gamma-aminobutyric acid during anaerobic and aerobic sequential incubations in fresh tea leaves. Food Chem. 2018, 240, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, H.; Lv, X.; Liu, D.; Wen, X.; Liao, Y. Effect of polyamines on the grain filling of wheat under drought stress. Plant Physiol. Biochem. 2016, 100, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Tanou, G.; Ziogas, V.; Belghazi, M.; Christou, A.; Filippou, P.; Job, D.; Fotopoulos, V.; Molassiotis, A. Polyamines reprogram oxidative and nitrosative status and the proteome of citrus plants exposed to salinity stress. Plant Cell Environ. 2014, 37, 864–885. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.C.; Lin, K.H.; Ho, S.L.; Chiang, C.M.; Yang, C.M. Enhancing the abiotic stress tolerance of plants: From chemical treatment to biotechnological approaches. Physiol. Plant 2018, 164, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Peng, Y.; Lin, J.; Du, C.; Yang, Y.; Wang, D.; Liu, C.; Yan, L.; Zhao, X.; Li, X.; et al. Ectopic expression of fungal ecgdh improves nitrogen assimilation and grain yield in rice. J. Integr. Plant Biol. 2018, 60, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Ban, Y.; Wen, X.P.; Nakajima, I.; Moriguchi, T. Molecular cloning and expression analysis of an arginine decarboxylase gene from peach (prunus persica). Gene 2009, 429, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Moriguchi, T. Changes in free polyamine titers and expression of polyamine biosynthetic genes during growth of peach in vitro callus. Plant Cell Rep. 2007, 26, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Nada, K.; Honda, C.; Kitashiba, H.; Wen, X.P.; Pang, X.M.; Moriguchi, T. Polyamine biosynthesis of apple callus under salt stress: Importance of the arginine decarboxylase pathway in stress response. J. Exp. Bot. 2006, 57, 2589–2599. [Google Scholar] [CrossRef]
- Liu, J.H.; Nakajima, I.; Moriguchi, T. Effects of salt and osmotic stresses on free polyamine content and expression of polyamine biosynthetic genes in vitis vinifera. Biol. Plant. 2011, 55, 340–344. [Google Scholar] [CrossRef]
- Sun, P.; Zhu, X.; Huang, X.; Liu, J.H. Overexpression of a stress-responsive myb transcription factor of poncirus trifoliata confers enhanced dehydration tolerance and increases polyamine biosynthesis. Plant Physiol. Biochem. 2014, 78, 71–79. [Google Scholar] [CrossRef]
- Wang, B.Q.; Zhang, Q.F.; Liu, J.H.; Li, G.H. Overexpression of ptadc confers enhanced dehydration and drought tolerance in transgenic tobacco and tomato: Effect on ros elimination. Biochem. Biophys. Res. Commun. 2011b, 413, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, P.P.; Chen, C.L.; Wang, Y.; Fu, X.Z.; Liu, J.H. An arginine decarboxylase gene ptadc from poncirus trifoliata confers abiotic stress tolerance and promotes primary root growth in arabidopsis. J. Exp. Bot. 2011a, 62, 2899–2914. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, J.H. Genome-wide identification and expression analysis of the polyamine oxidase gene family in sweet orange (citrus sinensis). Gene 2015, 555, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, J.H. Cspao4 of citrus sinensis functions in polyamine terminal catabolism and inhibits plant growth under salt stress. Sci. Rep. 2016, 6, 31384. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qu, H.; Shang, Z.; Jiang, X.; Moschou, P.N.; Roubelakis-Angelakis, K.A.; Zhang, S. Spermidine oxidase-derived H2O2 activates downstream ca2+ channels which signal pollen tube growth in pyruspyrifolia. Plant J. 2010, 63, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Zarza, X.; Atanasov, K.E.; Marco, F.; Arbona, V.; Carrasco, P.; Kopka, J.; Fotopoulos, V.; Munnik, T.; Gomez-Cadenas, A.; Tiburcio, A.F.; et al. Polyamine oxidase 5 loss-of-function mutations in arabidopsis thaliana trigger metabolic and transcriptional reprogramming and promote salt stress tolerance. Plant Cell Environ. 2017, 40, 527–542. [Google Scholar] [CrossRef]
- Fincato, P.; Moschou, P.N.; Ahou, A.; Angelini, R.; Roubelakis-Angelakis, K.A.; Federico, R.; Tavladoraki, P. The members of arabidopsis thaliana pao gene family exhibit distinct tissue- and organ-specific expression pattern during seedling growth and flower development. Amino Acids 2012, 42, 831–841. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paschalidis, K.; Tsaniklidis, G.; Wang, B.-Q.; Delis, C.; Trantas, E.; Loulakakis, K.; Makky, M.; Sarris, P.F.; Ververidis, F.; Liu, J.-H. The Interplay among Polyamines and Nitrogen in Plant Stress Responses. Plants 2019, 8, 315. https://doi.org/10.3390/plants8090315
Paschalidis K, Tsaniklidis G, Wang B-Q, Delis C, Trantas E, Loulakakis K, Makky M, Sarris PF, Ververidis F, Liu J-H. The Interplay among Polyamines and Nitrogen in Plant Stress Responses. Plants. 2019; 8(9):315. https://doi.org/10.3390/plants8090315
Chicago/Turabian StylePaschalidis, Konstantinos, Georgios Tsaniklidis, Bao-Quan Wang, Costas Delis, Emmanouil Trantas, Konstantinos Loulakakis, Muhammad Makky, Panagiotis F. Sarris, Filippos Ververidis, and Ji-Hong Liu. 2019. "The Interplay among Polyamines and Nitrogen in Plant Stress Responses" Plants 8, no. 9: 315. https://doi.org/10.3390/plants8090315
APA StylePaschalidis, K., Tsaniklidis, G., Wang, B.-Q., Delis, C., Trantas, E., Loulakakis, K., Makky, M., Sarris, P. F., Ververidis, F., & Liu, J.-H. (2019). The Interplay among Polyamines and Nitrogen in Plant Stress Responses. Plants, 8(9), 315. https://doi.org/10.3390/plants8090315







