Bletilla striata (Orchidaceae) Seed Coat Restricts the Invasion of Fungal Hyphae at the Initial Stage of Fungal Colonization
Abstract
:1. Introduction
2. Results
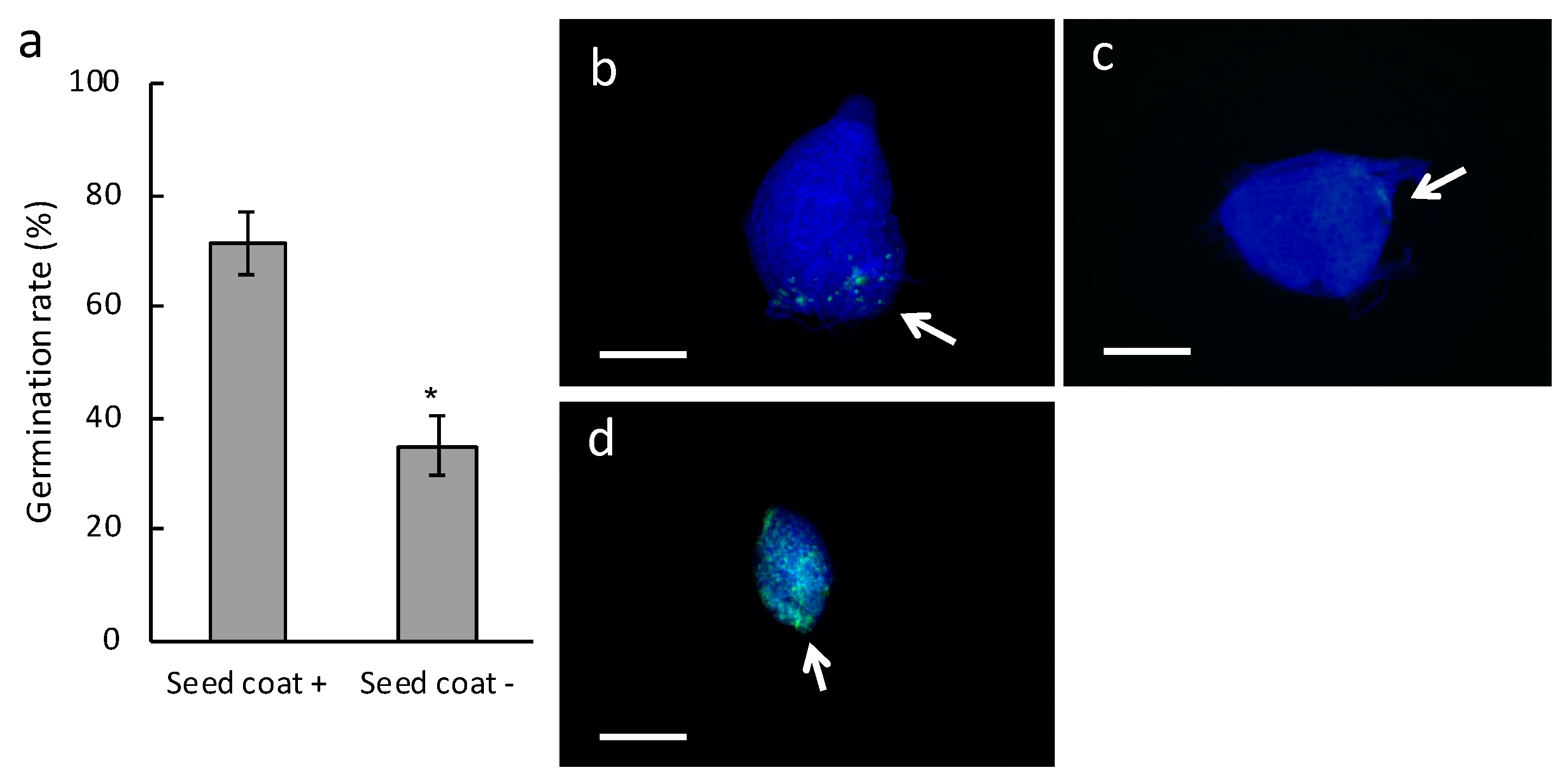
2.1. Effect on Germination of Inoculating Bletilla Striata Seeds Stripped of Seed Coat with the Symbiotic Fungus Tulasnella sp. Strain HR1-1
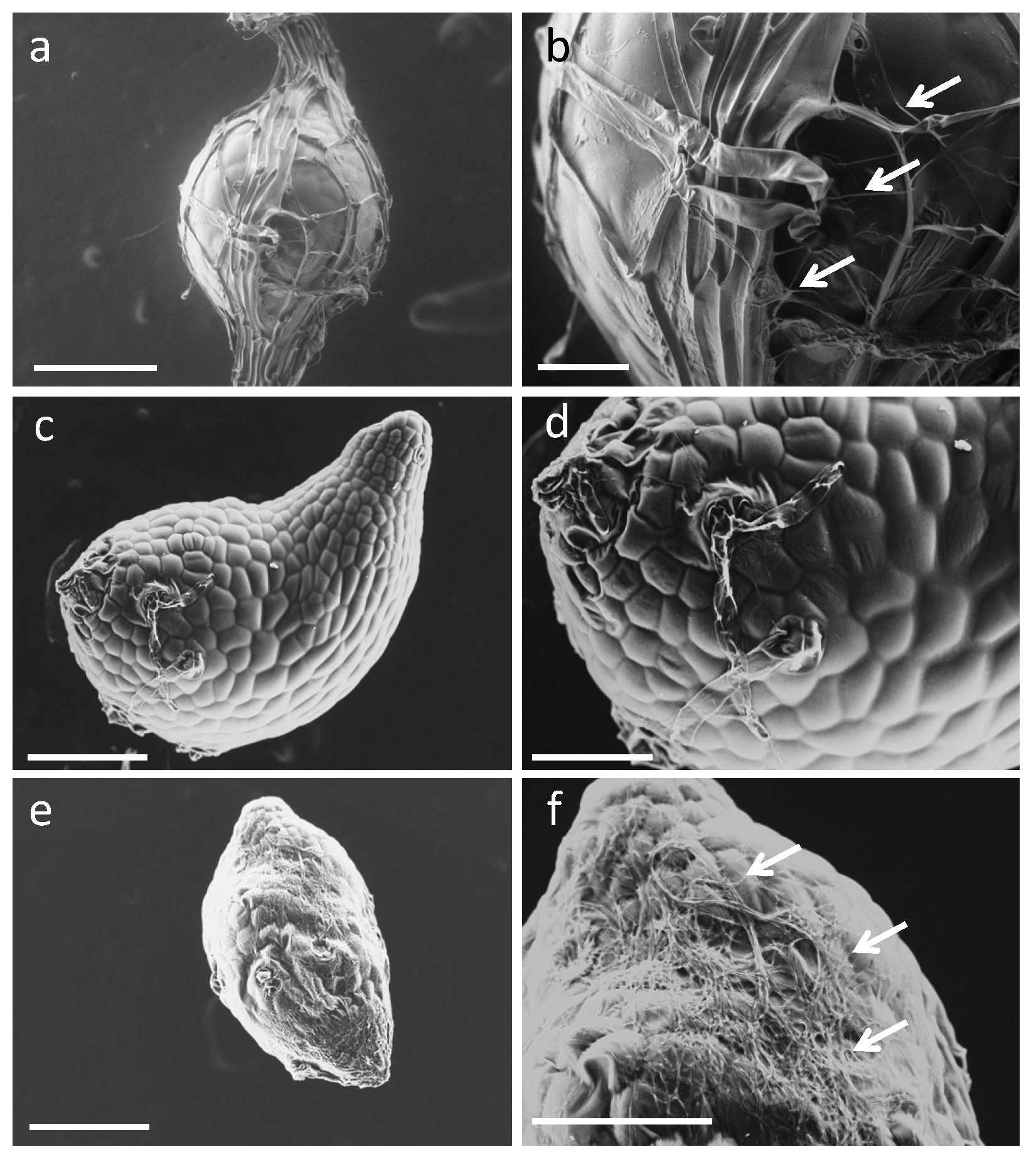
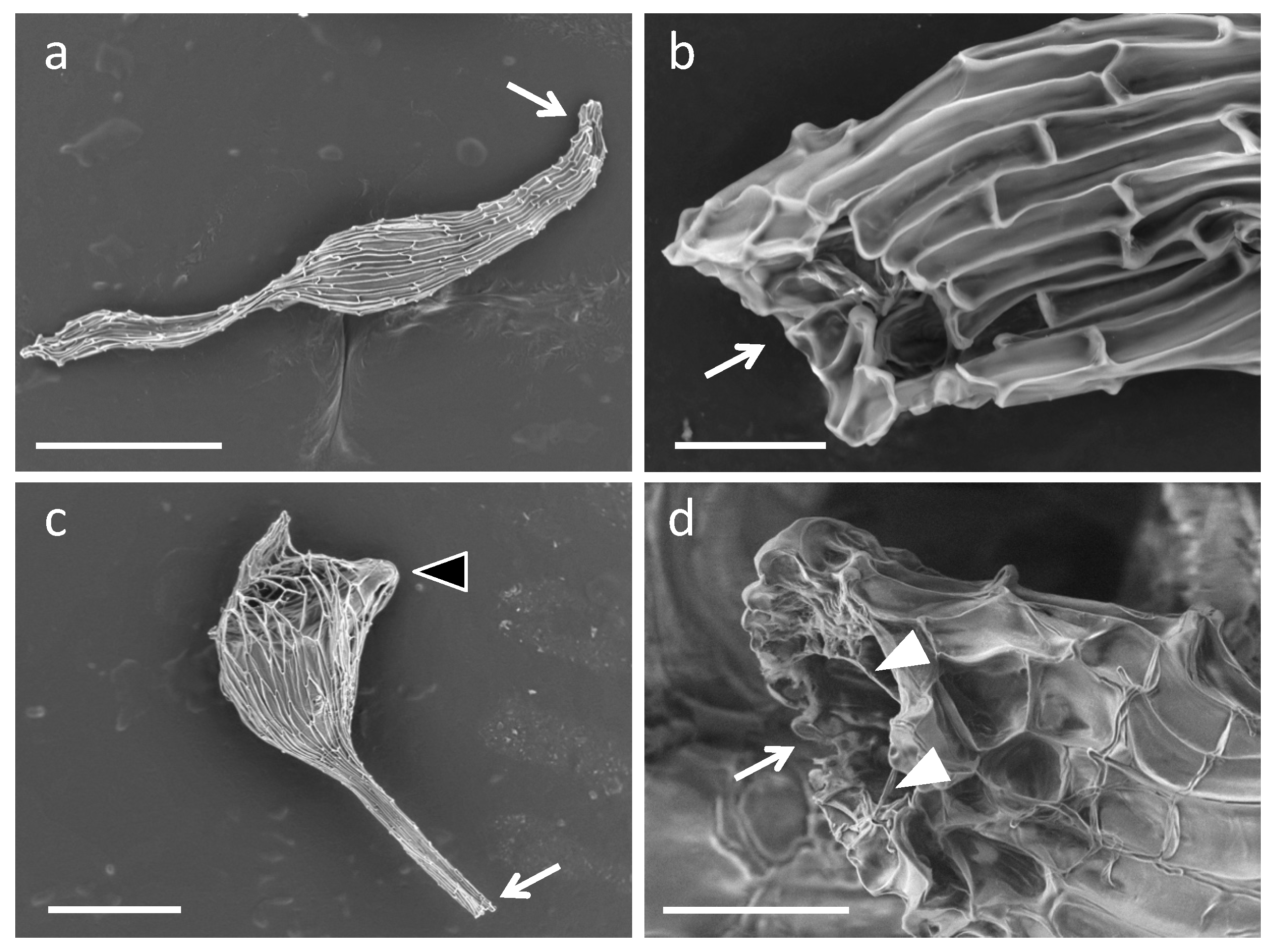
2.2. Germination Rate and Fine Observations of Seed Coat-Stripped Seeds of Bletilla striata Inoculated with the Symbiotic Fungus Sebacina Vermifera
2.3. Infection Rate of Seed Coat-Stripped Seeds of Bletilla striata Inoculated with Pathogenic Fungi Rhizoctonia Solani and Fusarium Oxysporum
2.4. Fine Observation of the Bletilla striata Seeds Inoculated with Symbiotic Fungi
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Fungal Strains
4.2. Seed Inoculation
4.3. Evaluation of Seed Germination and Fungal Infection
4.4. Fluorescent Staining of Fungal Hyphae
4.5. Scanning Electron Microscope Observations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dressler, R.L. Phylogeny and Classification of the Orchid Family; Dressler, R.L., Ed.; Dioscorides Press: Portland, OR, USA, 1993; Volume 31. [Google Scholar]
- Cribb, P.J.; Kell, S.P.; Dixon, K.W.; Barrett, R.L. Orchid conservation: A global perspective. In Orchid Conservation; Dixon, K.W., Kell, S.P., Barrett, R.L., Cribb, P.J., Eds.; Natural History Publications: Sabah, Malaysia, 2003; pp. 1–24. ISBN 9838120782. [Google Scholar]
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; van den Berg, C.; Schuiteman, A. An updated classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Arditti, J.; Ghani, A.K.A. Tansley review No. 110: Numerical and physical properties of orchid seeds and their biological implications. New Phytol. 2000, 145, 367–421. [Google Scholar] [CrossRef]
- Healey, P.L.; Michaud, J.D.; Arditti, J. Morphometry of Orchid seeds. III. Native California and related species of Goodyera, Piperia, Platanthera and Spiranthes. Am. J. Bot. 1980, 67, 508–518. [Google Scholar] [CrossRef]
- Barthlott, W.; Große-Veldmann, B.; Korotkova, N. Orchid Seed Diversity A Scanning Electron Microscopy Survey; Botanischer Garten und Botanisches Museum: Berlin, Germany, 2014; ISBN 9783921800928. [Google Scholar]
- Prutsch, J.; Schardt, A.; Schill, R. Adaptations of an orchid seed to water uptake and-storage. Plant. Syst. Evol. 2000, 220, 69–75. [Google Scholar] [CrossRef]
- Barsberg, S.T.; Lee, Y.I.; Rasmussen, H.N. Development of C-lignin with G/S-lignin and lipids in orchid seed coats-an unexpected diversity exposed by ATR-FT-IR spectroscopy. Seed Sci. Res. 2018, 28, 41–51. [Google Scholar] [CrossRef]
- McCormick, M.K.; Whigham, D.F.; Canchani-Viruet, A. Mycorrhizal fungi affect orchid distribution and population dynamics. New Phytol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.L.; Currah, R.S. Synthesis of mycorrhizae between protocorms of Goodyera repens (Orchidaceae) and Ceratobasidium cereale. Can. J. Bot. 1990, 68, 1117–1125. [Google Scholar] [CrossRef]
- Richardson, K.A.; Peterson, R.L.; Currah, R.S. Seed reserves and early symbiotic protocorm development of Platanthera hyperborea (Orchidaceae). Can. J. Bot. 1992, 70, 291–300. [Google Scholar] [CrossRef]
- Rasmussen, H.N.; Rasmussen, F.N. Orchid mycorrhiza: Implications of a mycophagous life style. Oikos 2009, 118, 334–345. [Google Scholar] [CrossRef]
- Williamson, B.; Hadley, G. Penetration and infection of orchid protocorms by Thanatephorus cucumeris and other Rhizoctonia isolates. Phytopathology 1970, 60, 1092–1096. [Google Scholar] [CrossRef]
- Shimura, H.; Koda, Y. Enhanced symbiotic seed germination of Cypripedium macranthos var. rebunense following inoculation after cold treatment. Physiol. Plant. 2005, 123, 281–287. [Google Scholar] [CrossRef]
- Miura, C.; Yamaguchi, K.; Miyahara, R.; Yamamoto, T.; Fuji, M.; Yagame, T.; Imaizumi-Anraku, H.; Yamato, M.; Shigenobu, S.; Kaminaka, H. The mycoheterotrophic symbiosis between orchids and mycorrhizal fungi possesses major components shared with mutualistic plant-mycorrhizal symbioses. Mol. Plant Microbe Interact. 2018, 31, 1032–1047. [Google Scholar] [CrossRef]
- Kuga, Y.; Sakamoto, N.; Yurimoto, H. Stable isotope cellular imaging reveals that both live and degenerating fungal pelotons transfer carbon and nitrogen to orchid protocorms. New Phytol. 2014, 202, 594–605. [Google Scholar] [CrossRef]
- Beyrle, H.F.; Smith, S.E.; Franco, C.M.M.; Peterson, R.L. Colonization of Orchis morio protocorms by a mycorrhizal fungus: Effects of nitrogen nutrition and glyphosate in modifying the responses. Can. J. Bot. 1995, 73, 1128–1140. [Google Scholar] [CrossRef]
- Harvais, G.; Hadley, G. The development of Orchis purpurella in asymbiotic and inoculated cultures. New Phytol. 1967, 66, 217–230. [Google Scholar] [CrossRef]
- Lahrmann, U.; Strehmel, N.; Langen, G.; Frerigmann, H.; Leson, L.; Ding, Y.; Scheel, D.; Herklotz, S.; Hilbert, M.; Zuccaro, A. Mutualistic root endophytism is not associated with the reduction of saprotrophic traits and requires a noncompromised plant innate immunity. New Phytol. 2015, 207, 841–857. [Google Scholar] [CrossRef]
- Hadley, G. Non-specificity of symbotic infection in orchid mycorrhiza. New Phytol. 1970, 69, 1015–1023. [Google Scholar] [CrossRef]
- Blakeman, J.P.; Mokahel, M.A.; Hadley, G. Effect of mycorrhizal infection on respiration and activity of some oxidase enzymes of orchid protocorms. New Phytol. 1976, 77, 697–704. [Google Scholar] [CrossRef]
- Shimura, H.; Matsuura, M.; Takada, N.; Koda, Y. An antifungal compound involved in symbiotic germination of Cypripedium macranthos var. rebunense (Orchidaceae). Phytochemistry 2007, 68, 1442–1447. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Yamamoto, T.; Miura, C.; Fuji, M.; Nagata, S.; Otani, Y.; Yagame, T.; Yamato, M.; Kaminaka, H. Quantitative evaluation of protocorm growth and fungal colonization in Bletilla striata (Orchidaceae) reveals less-productive symbiosis with a non-native symbiotic fungus. BMC Plant Biol. 2017, 17, 50. [Google Scholar] [CrossRef]
- Fesel, P.H.; Zuccaro, A. Dissecting endophytic lifestyle along the parasitism/mutualism continuum in Arabidopsis. Curr. Opin. Microbiol. 2016, 32, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Dalling, J.W.; Davis, A.S.; Schutte, B.J.; Elizabeth Arnold, A. Seed survival in soil: Interacting effects of predation, dormancy and the soil microbial community. J. Ecol. 2011, 99, 89–95. [Google Scholar] [CrossRef]
- Yeung, E.C.; Zee, S.Y.; Ye, X.L. Embryology of Cymbidium sinense: Embryo development. Ann. Bot. 1996, 78, 105–110. [Google Scholar] [CrossRef]
- Chen, F.; Tobimatsu, Y.; Havkin-Frenkel, D.; Dixon, R.A.; Ralph, J. A polymer of caffeyl alcohol in plant seeds. Proc. Natl. Acad. Sci. USA 2012, 109, 1772–1777. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Tobimatsu, Y.; Jackson, L.; Nakashima, J.; Ralph, J.; Dixon, R.A. Novel seed coat lignins in the Cactaceae: Structure, distribution and implications for the evolution of lignin diversity. Plant J. 2013, 73, 201–211. [Google Scholar] [CrossRef]
- Miyoshi, K.; Mii, M. Ultrasonic treatment for enhancing seed germination of terrestrial orchid, Calanthe discolor, in asymbiotic culture. Sci. Hortic. 1988, 35, 127–130. [Google Scholar] [CrossRef]
- Molvray, M.; Kores, P.J. Character analysis of the seed coat in Spiranthoideae and Orchidoideae, with special reference to the Diurideae (Orchidaceae). Am. J. Bot. 1995, 82, 1443–1454. [Google Scholar] [CrossRef]
- Chaudhary, B.; Chattopadhyay, P.; Banerjee, N. Modulations in seed micromorphology reveal signature of adaptive species-diversification in Dendrobium (Orchidaceae). Open J. Ecol. 2014, 4, 33–42. [Google Scholar] [CrossRef]
- Güler, N. Seed micromorphology of Orchis Tourn. ex L. (Orchidaceae) and allied genera growing in Edirne province, Turkey. PhytoKeys 2016, 68, 9–25. [Google Scholar] [CrossRef]
- Kurzweil, H. Seed morphology in Southern African Orchidoideae (Orchidaceae). Plant Syst. Evol. 1993, 185, 229–247. [Google Scholar] [CrossRef]
- Nishimura, G.; Yukawa, T. Dark material accumulation and sclerotization during seed coat formation in Vanilla planifolia Jacks. ex Andrews (Orchidaceae). Bull. Natl. Mus. Nat. Sci. Ser. B 2010, 36, 33–37. [Google Scholar]
- Yang, C.K.; Lee, Y.I. The seed development of a mycoheterotrophic orchid, Cyrtosia javanica blume. Bot. Stud. 2014, 55, 44. [Google Scholar] [CrossRef]
- Suetsugu, K.; Kawakita, A.; Kato, M. Avian seed dispersal in a mycoheterotrophic orchid Cyrtosia septentrionalis. Nat. Plants 2015, 1, 15052. [Google Scholar] [CrossRef]
- Chase, M.W.; Pippen, J.S. Seed morphology in the Oncidiinae and related subtribes (Orchidaceae). Syst. Bot. 1988, 13, 313. [Google Scholar] [CrossRef]
- Whigham, D.F.; O’Neill, J.P.; Rasmussen, H.N.; Caldwell, B.A.; McCormick, M.K. Seed longevity in terrestrial orchids—Potential for persistent in situ seed banks. Biol. Conserv. 2006, 129, 24–30. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Brys, R.; Vandepitte, K.; Honnay, O.; Roldán-Ruiz, I.; Wiegand, T. A spatially explicit analysis of seedling recruitment in the terrestrial orchid Orchis purpurea. New Phytol. 2007, 176, 448–459. [Google Scholar] [CrossRef]
- Rasmussen, H.N.; Dixon, K.W.; Jersáková, J.; Těšitelová, T. Germination and seedling establishment in orchids: A complex of requirements. Ann. Bot. 2015, 116, 391–402. [Google Scholar] [CrossRef]
- Warcup, J.H. Mycorrhizal associations of isolates of Sebacina vermifera. New Phytol. 1988, 110, 227–231. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miura, C.; Saisho, M.; Yagame, T.; Yamato, M.; Kaminaka, H. Bletilla striata (Orchidaceae) Seed Coat Restricts the Invasion of Fungal Hyphae at the Initial Stage of Fungal Colonization. Plants 2019, 8, 280. https://doi.org/10.3390/plants8080280
Miura C, Saisho M, Yagame T, Yamato M, Kaminaka H. Bletilla striata (Orchidaceae) Seed Coat Restricts the Invasion of Fungal Hyphae at the Initial Stage of Fungal Colonization. Plants. 2019; 8(8):280. https://doi.org/10.3390/plants8080280
Chicago/Turabian StyleMiura, Chihiro, Miharu Saisho, Takahiro Yagame, Masahide Yamato, and Hironori Kaminaka. 2019. "Bletilla striata (Orchidaceae) Seed Coat Restricts the Invasion of Fungal Hyphae at the Initial Stage of Fungal Colonization" Plants 8, no. 8: 280. https://doi.org/10.3390/plants8080280





