Drought Stress Effects and Olive Tree Acclimation under a Changing Climate
Abstract
1. Introduction
2. Olive Tree Growth Conditions and Distribution
3. Implication of the Change in Environmental Conditions for the Olive Tree
4. Drought Effects in Plants Morphological, Physiological and Biochemical Mechanisms
4.1. Influence on Water Status, Growth and Plant Morphology
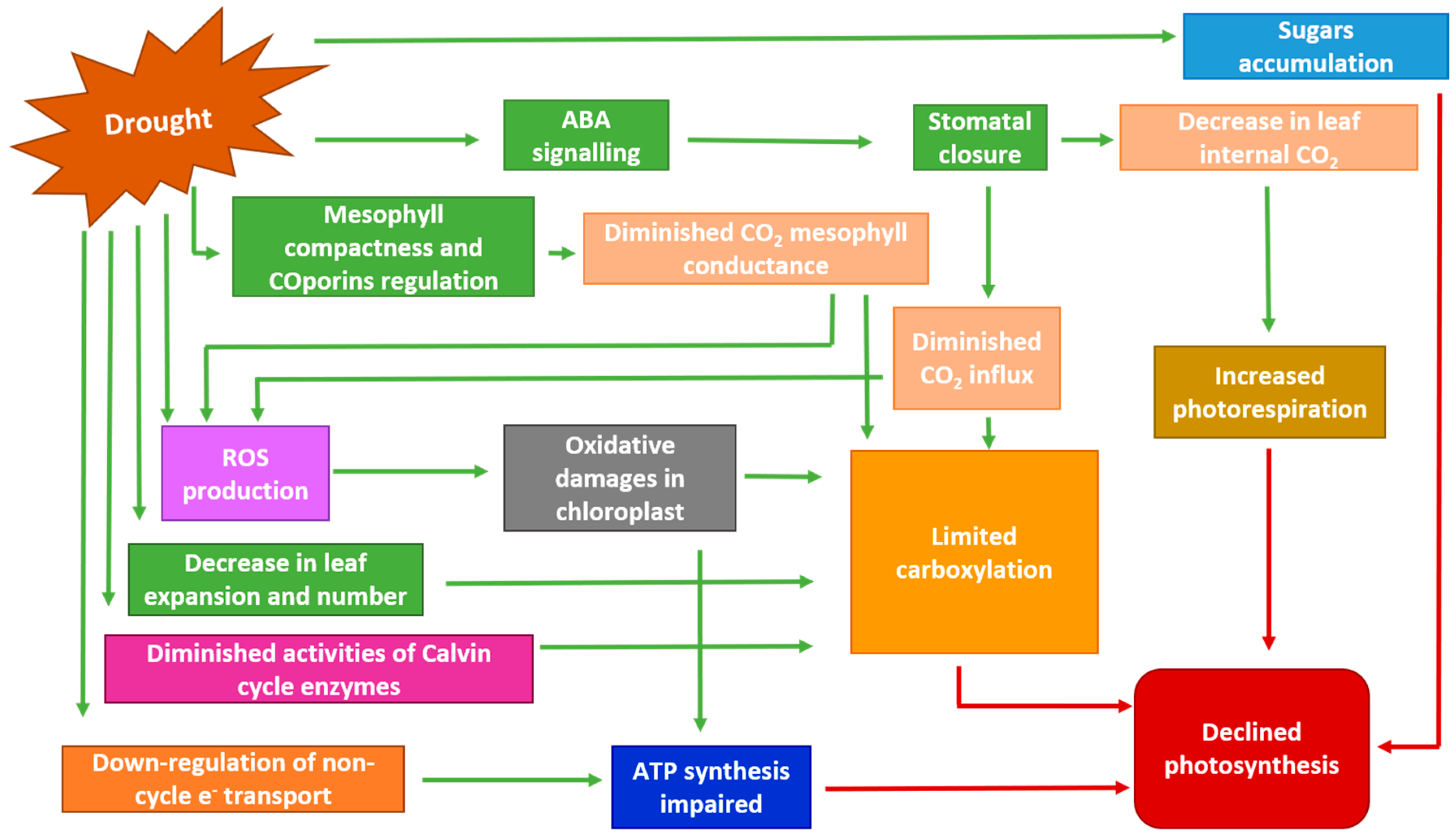
4.2. Influence on Stomatal and Mesophyll Conductance, Photosynthesis, Respiration and Water Use Efficiency
4.3. Influence on Minerals Uptake and Allocation
4.4. Influence on Redox Status
4.5. Influence on Hormonal Dynamics
5. Drought Influence on Olive Crop Yield and Quality
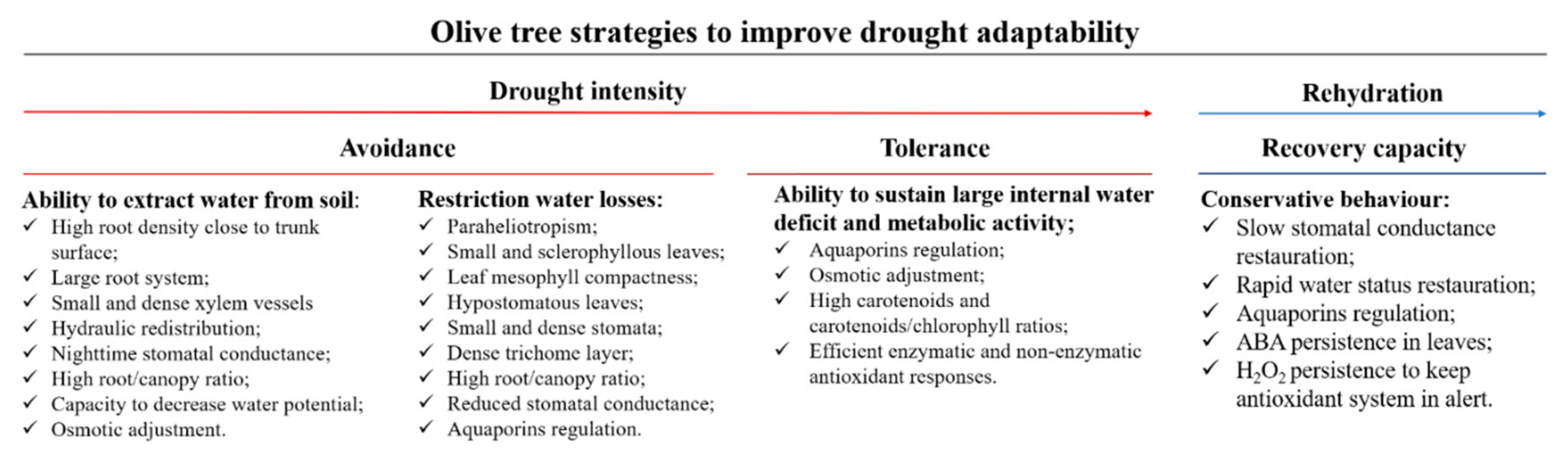
6. Olive Tree Strategies to Withstand Drought
7. Cultivars’ Response to Drought
8. Concluding Remarks and Future Challenges
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations and Symbols
| A | net photosynthetic rate |
| A/gs | intrinsic water use efficiency |
| ABA | abscisic acid |
| AQPs | aquaporins |
| ATP | adenosine triphosphate |
| Aux | auxins |
| CA | carbonic anhydrase |
| CO2 | carbon dioxide |
| DNA | deoxyribonucleic acid |
| Enight | night-time transpiration |
| ET | ethylene |
| ETR | photosynthetic electron transport rate |
| F’v/F’m | capture efficiency of excitation energy by open PSII reaction centres |
| Fv/Fm | maximal quantum efficiency of photosystem II |
| GA | gibberellins |
| gm | mesophyll conductance |
| gnight | night-time stomatal conductance |
| gs | stomatal conductance |
| H2O2 | hydrogen peroxide |
| HCO3− | bicarbonate anion |
| K232: | excitation coefficient at 232 nm |
| K270 | excitation coefficient at 270 nm |
| Kleaf | leaf hydraulic conductance |
| NPQ | non-photochemical quenching |
| O2 | oxygen |
| OA | osmotic adjustment |
| PPFD | photosynthetic photon flux density |
| PSII | photosystem II a |
| qP | photochemical quenching |
| R | respiration |
| ROS | reactive oxygen species |
| WUEi | intrinsic water use efficiency |
| WUEWP | whole plant water use efficiency |
| ΔK | variation of the specific extinction |
| ΦPSII | effective quantum efficiency of photosystem II |
| Ψ | leaf water potential |
References
- International Olive Concil (IOC). Available online: http://www.internationaloliveoil.org (acessed on 5 June 2019).
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Climate Change 2013: The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change 2013; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Bacelar, E.A.; Santos, D.L.; Moutinho-Pereira, J.M.; Gonçalves, B.C.; Ferreira, H.F.; Correia, C.M. Immediate responses and adaptative strategies of three olive cultivars under contrasting water availability regimes: Changes on structure and chemical composition of foliage and oxidative damage. Plant Sci. 2006, 170, 596–605. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Santos, D.L.; Moutinho-Pereira, J.M.; Lopes, J.I.; Gonçalves, B.C.; Ferreira, T.C.; Correia, C.M. Physiological behaviour, oxidative damage and antioxidative protection of olive trees grown under different irrigation regimes. Plant Soil 2007, 292, 1–12. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Moutinho-Pereira, J.M.; Gonçalves, B.C.; Ferreira, H.F.; Correia, C.M. Changes in growth, gas exchange, xylem hydraulic properties and water use efficiency of three olive cultivars under contrasting water availability regimes. Environ. Exp. Bot. 2007, 60, 183–192. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Meijón, M.; Ferreira, H.; Pinto, G.; Moutinho-Pereira, J.; Correia, C. Salicylic acid modulates olive tree physiological and growth responses to drought and rewatering events in a dose dependent manner. J. Plant Physiol. 2018, 230, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Dinis, L.-T.; Ferreira, H.; Moutinho-Pereira, J.; Correia, C. The role of nighttime water balance on Olea europaea plants subjected to contrasting water regimes. J. Plant Physiol. 2018, 226, 56–63. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Petridis, A.; Therios, I.; Samouris, G.; Koundouras, S.; Giannakoula, A. Effect of water deficit on leaf phenolic composition, gas exchange, oxidative damage and antioxidant activity of four Greek olive (Olea europaea L.) cultivars. Plant Physiol. Biochem. 2012, 60, 1–11. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef]
- Greven, M.; Neal, S.; Green, S.; Dichio, B.; Clothier, B. The effects of drought on the water use, fruit development and oil yield from young olive trees. Agric. Water Manag. 2009, 96, 1525–1531. [Google Scholar] [CrossRef]
- Fernandes-Silva, A.A.; Ferreira, T.C.; Correia, C.M.; Malheiro, A.; Villalobos, F.J. Influence of different irrigation regimes on crop yield and water use efficiency of olive. Plant Soil 2010, 333, 35–47. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Correia, C.M.; Moutinho-Pereira, J.M.; Goncalves, B.C.; Lopes, J.I.; Torres-Pereira, J.M. Sclerophylly and leaf anatomical traits of five field-grown olive cultivars growing under drought conditions. Tree Physiol. 2004, 24, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Bacelar, E.A.; Moutinho-Pereira, J.M.; Gonçalves, B.C.; Lopes, J.I.; Correia, C.M. Physiological responses of different olive genotypes to drought conditions. Acta Physiol. Plant. 2009, 31, 611–621. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.M.; Diaz-Espejo, A.; Morales-Sillero, A.; Martín-Palomo, M.J.; Mayr, S.; Beikircher, B.; Fernández, J.E. Shoot hydraulic characteristics, plant water status and stomatal response in olive trees under different soil water conditions. Plant Soil 2013, 373, 77–87. [Google Scholar] [CrossRef]
- Fernández, J.-E. Understanding olive adaptation to abiotic stresses as a tool to increase crop performance. Environ. Exp. Bot. 2014, 103, 158–179. [Google Scholar] [CrossRef]
- Lopez-Bellido, P.J.; Lopez-Bellido, L.; Fernandez-Garcia, P.; Muñoz-Romero, V.; Lopez-Bellido, F.J. Assessment of carbon sequestration and the carbon footprint in olive groves in Southern Spain. Carbon Manag. 2016, 7, 161–170. [Google Scholar] [CrossRef]
- Therios, I. Olives: Crop Production Science in Horticulture 18; CABI Publishing: Wallingford, UK, 2009. [Google Scholar]
- Connor, D.J.; Fereres, E. The physiology of adaptation and yield expression in olive. Hortic. Rev. 2005, 31, 155–193. [Google Scholar] [CrossRef]
- Böhm, J.; Antunes, M.T. A evolução da espécie olea europaea. In O Grande Livro da Oliveira e do Azeite—Portugal Oleícola; Böhm, J., Ed.; Dinalivro Editora: Lisboa, Portugal, 2013; pp. 34–49. [Google Scholar]
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Tombesi, A.; Tombesi, S. Orchard planning and planting. In Production Techniques in Olive Growing; Sbitri, M.O., Serafini, F., Eds.; International Olive Council: Madrid, Spain, 2007; pp. 17–40. [Google Scholar]
- Fernández, J.E.; Moreno, F. Water use by the olive tree. J. Crop Prod. 1999, 2, 101–162. [Google Scholar] [CrossRef]
- Guerrero, A. Nueva Olivicultura, 5th ed.; Ediciones Mundi-Prensa: Madrid, Spain, 2002. [Google Scholar]
- Ponti, L.; Gutierrez, A.P.; Basso, B.; Neteler, M.; Ruti, P.M.; Dell’Aquila, A.; Iannetta, M. Olive agroecosystems in the Mediterranean Basin: Multitrophic analysis of climate effects with process-based representation of soil water balance. Procedia Environ. Sci. 2013, 19, 122–131. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAOSTAT). Available online: http://www.fao.org/faostat/en/#home (acessed on 4 March 2019).
- Gucci, R.; Fereres, E. Crop Yield Response to Water, FAO Irrigation and Drainage Paper 66; Steduto, P., Hsiao, T.C., Fereres, E.D.R., Eds.; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2012; pp. 300–315. [Google Scholar]
- Viola, F.; Caracciolo, D.; Pumo, D.; Noto, L.V. Olive yield and future climate forcings. Procedia Environ. Sci. 2013, 19, 132–138. [Google Scholar] [CrossRef]
- Viola, F.; Caracciolo, D.; Pumo, D.; Noto, L.; Loggia, G. Future climate forcings and olive yield in a Mediterranean orchard. Water 2014, 6, 1562–1580. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Psarras, G. Global change effects on crop photosynthesis and production in Mediterranean: The case of Crete, Greece. Agric. Ecosyst. Environ. 2005, 106, 147–157. [Google Scholar] [CrossRef]
- Minnocci, A.; Panicucci, A.; Sebastiani, L.; Lorenzini, G.; Vitagliano, C. Physiological and morphological responses of olive plants to ozone exposure during a growing season. Tree Physiol. 1999, 19, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, D.; Ribas, F.; Moriana, A.; Rapoport, H.F.; De Juan, A. Influence of temperature on the growth and development of olive (Olea europaea L.) trees. J. Hortic. Sci. Biotechnol. 2008, 83, 171–176. [Google Scholar] [CrossRef]
- Aguilera, F.; Fornaciari, M.; Ruiz-Valenzuela, L.; Galán, C.; Msallem, M.; Ben Dhiab, A.; Díaz-de la Guardia, C.; Trigo, M.; Bonofiglio, T.; Orlandi, F. Phenological models to predict the main flowering phases of olive (Olea europaea L.) along a latitudinal and longitudinal gradient across the Mediterranean region. Int. J. Biometeorol. 2015, 59, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Pierantozzi, P.; Searles, P.; Rousseaux, M.C.; García-Inza, G.; Miserere, A.; Bodoira, R.; Contreras, C.; Maestri, D. Olive cultivation in the southern hemisphere: Flowering, water requirements and oil quality responses to new crop environments. Front. Plant Sci. 2017, 8, 1830. [Google Scholar] [CrossRef] [PubMed]
- Dag, A.; Harlev, G.; Lavee, S.; Zipori, I.; Kerem, Z. Optimizing olive harvest time under hot climatic conditions of Jordan Valley, Israel. Eur. J. Lipid Sci. Technol. 2014, 116, 169–176. [Google Scholar] [CrossRef]
- Orlandi, F.; Garcia-Mozo, H.; Dhiab, A.B.; Galán, C.; Msallem, M.; Romano, B.; Abichou, M.; Dominguez-Vilches, E.; Fornaciari, M. Climatic indices in the interpretation of the phenological phases of the olive in mediterranean areas during its biological cycle. Clim. Chang. 2013, 116, 263–284. [Google Scholar] [CrossRef]
- Tanasijevic, L.; Todorovic, M.; Pereira, L.S.; Pizzigalli, C.; Lionello, P. Impacts of climate change on olive crop evapotranspiration and irrigation requirements in the Mediterranean region. Agric. Water Manag. 2014, 144, 54–68. [Google Scholar] [CrossRef]
- Rallo, L.; Martin, G.C. The role of chilling in releasing olive floral buds from dormancy. J. Am. Soc. Hortic. Sci. 1991, 116, 1058–1062. [Google Scholar] [CrossRef]
- Fabbri, A.; Benelli, C. Flower bud induction and differentiation in olive. J. Hortic. Sci. Biotec. 2000, 75, 131–141. [Google Scholar] [CrossRef]
- Ramos, A.; Rapoport, H.F.; Cabello, D.; Rallo, L. Chilling accumulation, dormancy release temperature, and the role of leaves in olive reproductive budburst: Evaluation using shoot explants. Sci. Hortic. 2018, 231, 241–252. [Google Scholar] [CrossRef]
- Moriondo, M.; Trombi, G.; Ferrise, R.; Brandani, G.; Dibari, C.; Ammann, C.M.; Lippi, M.M.; Bindi, M. Olive trees as bio-indicators of climate evolution in the Mediterranean Basin. Glob. Ecol. Biogeogr. 2013, 22, 818–833. [Google Scholar] [CrossRef]
- Ponti, L.; Gutierrez, A.P.; Ruti, P.M.; Dell’Aquila, A. Fine-scale ecological and economic assessment of climate change on olive in the Mediterranean Basin reveals winners and losers. Proc. Natl. Acad. Sci. USA 2014, 111, 5598–5603. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2006. [Google Scholar]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought stress in plants: An overview. In Plant Responses to Drought Stress—From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Heidelberg, Germany, 2012; pp. 1–33. [Google Scholar]
- Hernandez-Santana, V.; Rodriguez-Dominguez, C.M.; Fernandez, J.E.; Diaz-Espejo, A. Role of leaf hydraulic conductance in the regulation of stomatal conductance in almond and olive in response to water stress. Tree Physiol. 2016, 36, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Torres-Ruiz, J.M.; Diaz-Espejo, A.; Perez-Martin, A.; Hernandez-Santana, V. Role of hydraulic and chemical signals in leaves, stems and roots in the stomatal behaviour of olive trees under water stress and recovery conditions. Tree Physiol. 2015, 35, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Pantin, F.; Monnet, F.; Jannaud, D.; Costa, J.M.; Renaud, J.; Muller, B.; Simonneau, T.; Genty, B. The dual effect of abscisic acid on stomata. New Phytol. 2013, 197, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Boughalleb, F.; Hajlaoui, H. Physiological and anatomical changes induced by drought in two olive cultivars (cv Zalmati and Chemlali). Acta Physiol. Plant. 2011, 33, 53–65. [Google Scholar] [CrossRef]
- Tomás, M.; Flexas, J.; Copolovici, L.; Galmes, J.; Hallik, L.; Medrano, H.; Ribas-Carbo, M.; Tosens, T.; Vislap, V.; Niinemets, U. Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: Quantitative limitations and scaling up by models. J. Exp. Bot. 2013, 64, 2269–2281. [Google Scholar] [CrossRef]
- Perez-Martin, A.; Flexas, J.; Ribas-Carbo, M.; Bota, J.; Tomás, M.; Infante, J.M.; Diaz-Espejo, A. Interactive effects of soil water deficit and air vapour pressure deficit on mesophyll conductance to CO2 in Vitis vinifera and Olea europaea. J. Exp. Bot. 2009, 60, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Perez-Martin, A.; Michelazzo, C.; Torres-Ruiz, J.M.; Flexas, J.; Fernández, J.E.; Sebastiani, L.; Diaz-Espejo, A. Regulation of photosynthesis and stomatal and mesophyll conductance under water stress and recovery in olive trees: Correlation with gene expression of carbonic anhydrase and aquaporins. J. Exp. Bot. 2014, 65, 3143–3156. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.J.; Guo, C.; Coleman, J.R. Reduction of plastid-localized carbonic anhydrase activity results in reduced arabidopsis seedling survivorship. Plant Physiol. 2008, 147, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Tholen, D.; Zhu, X.-G. The mechanistic basis of internal conductance: A theoretical analysis of mesophyll cell photosynthesis and CO2 diffusion. Plant Physiol. 2011, 156, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Gillon, J.S.; Yakir, D. Internal Conductance to CO2 diffusion and C18OO discrimination in C3 Leaves. Plant Physiol. 2000, 123, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Heckwolf, M.; Pater, D.; Hanson, D.T.; Kaldenhoff, R. The Arabidopsis thaliana aquaporin AtPIP1;2 is a physiologically relevant CO2 transport facilitator. Plant J. 2011, 67, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Moshelion, M.; Halperin, O.; Wallach, R.; Oren, R.; Way, D.A. Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants: Crop water-use efficiency, growth and yield. Plant Cell Environ. 2015, 38, 1785–1793. [Google Scholar] [CrossRef]
- Guerfel, M.; Baccouri, O.; Boujnah, D.; Chaïbi, W.; Zarrouk, M. Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L.) cultivars. Sci. Hortic. 2009, 119, 257–263. [Google Scholar] [CrossRef]
- Sofo, A.; Manfreda, S.; Fiorentino, M.; Dichio, B.; Xiloyannis, C. The olive tree: A paradigm for drought tolerance in Mediterranean climates. Hydrol. Earth Syst. Sci. Discuss. 2008, 12, 293–301. [Google Scholar] [CrossRef]
- Guerfel, M.; Ouni, Y.; Boujnah, D.; Zarrouk, M. Photosynthesis parameters and activities of enzymes of oxidative stress in two young ‘Chemlali’ and ‘Chetoui’ olive trees under water deficit. Photosynthetica 2009, 47, 340. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Methenni, K.; Nouairi, I.; Zarrouk, M.; Youssef, N.B. Drought priming improves subsequent more severe drought in a drought-sensitive cultivar of olive cv. Chétoui. Sci. Hortic. 2017, 221, 43–52. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Trupiano, D.; Polzella, A.; de Zio, E.; Sassi, M.; Scaloni, A.; Zarrouk, M.; Youssef, N.B.; Scippa, G.S. Unraveling physiological, biochemical and molecular mechanisms involved in olive (Olea europaea L. cv. Chétoui) tolerance to drought and salt stresses. J. Plant Physiol. 2018, 220, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Medrano, H. Energy dissipation in C3 plants under drought. Funct. Plant Biol. 2002, 29, 1209–1215. [Google Scholar] [CrossRef]
- Flexas, J.; Gallé, A.; Galmés, J.; Ribas-Carbo, M.; Medrano, H. The response of photosynthesis to soil water stress. In Plant Responses to Drought Stress—From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Heidelberg, Germany, 2012; pp. 129–144. [Google Scholar]
- Caird, M.A.; Richards, J.H.; Donovan, L.A. Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol. 2007, 143, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Scholz, F.G.; Bucci, S.J.; Goldstein, G.; Meinzer, F.C.; Franco, A.C.; Miralles-Wilhelm, F. Removal of nutrient limitations by long-term fertilization decreases nocturnal water loss in savanna trees. Tree Physiol. 2007, 27, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Ogle, K.; Lucas, R.W.; Bentley, L.P.; Cable, J.M.; Barron-Gafford, G.A.; Griffith, A.; Ignace, D.; Jenerette, G.D.; Tyler, A.; Huxman, T.E.; et al. Differential daytime and night-time stomatal behavior in plants from North American deserts. New Phytol. 2012, 194, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Escalona, J.M.; Fuentes, S.; Tomás, M.; Martorell, S.; Flexas, J.; Medrano, H. Responses of leaf night transpiration to drought stress in Vitis vinifera L. Agric. Water Manag. 2013, 118, 50–58. [Google Scholar] [CrossRef]
- Chen, X.; Gao, J.; Zhao, P.; McCarthy, H.R.; Zhu, L.; Ni, G.; Ouyang, L. Tree species with photosynthetic stems have greater nightime sap flux. Front. Plant Sci. 2018, 9, 30. [Google Scholar] [CrossRef]
- Cannell, M.G.R.; Thornley, J.H.M. Modelling the components of plant respiration: Some guiding principles. Ann. Bot. 2000, 85, 45–54. [Google Scholar] [CrossRef]
- Flexas, J.; Galmes, J.; Ribas-Carbo, M.; Medrano, H. The effects of water stress on plant respiration. In Plant Respiration: From Cell to Ecosystem; Lambers, H., Ribas-Carbo, M., Eds.; Springer: Berlin, Germany, 2005; pp. 85–94. [Google Scholar]
- Varone, L.; Gratani, L. Leaf respiration responsiveness to induced water stress in Mediterranean species. Environ. Exp. Bot. 2015, 109, 141–150. [Google Scholar] [CrossRef]
- Paul, M.J.; Pellny, T.K. Carbon metabolite feedback regulation of leaf photosynthesis and development. J. Exp. Bot. 2003, 54, 539–547. [Google Scholar] [CrossRef]
- Flexas, J.; Galmés, J.; Gallé, A.; GulíAs, J.; Pou, A.; Ribas-Carbo, M.; Tomásw, M.; Medrano, H. Improving water use efficiency in grapevines: Potential physiological targets for biotechnological improvement. Aust. J. Grape Wine Res. 2010, 16, 106–121. [Google Scholar] [CrossRef]
- Bacelar, E.; Moutinho-Pereira, J.M.; Gonçalves, B.; Brito, C.; Gomes-Laranjo, J.; Ferreira, H.; Correia, C.M. Water use strategies of plants under drought conditions. In Plant Responses to Drought Stress—From Morphological to Molecular Features; Aroca, R., Ed.; Springer: New York, NY, USA, 2012; pp. 145–170. [Google Scholar]
- Maroco, J.P.; Pereira, J.S.; Manuela Chaves, M. Growth, photosynthesis and water-use efficiency of two C4 sahelian grasses subjected to water deficits. J. Arid Environ. 2000, 45, 119–137. [Google Scholar] [CrossRef]
- Grusak, M.A. Plant macro- and micronutrient minerals. Encyclopedia Life Sci. 2001, 1–5. [Google Scholar] [CrossRef]
- Silva, E.; Nogueira, R.; Silva, M.; Albuquerque, M. Drought stress and plant nutrition. Plant Stress 2011, 5, 32–41. [Google Scholar]
- Sanaullah, M.; Rumpel, C.; Charrier, X.; Chabbi, A. How does drought stress influence the decomposition of plant litter with contrasting quality in a grassland ecosystem? Plant Soil 2012, 352, 277–288. [Google Scholar] [CrossRef]
- Bista, D.R.; Heckathorn, S.A.; Jayawardena, D.M.; Mishra, S.; Boldt, J.K. Effects of drought on nutrient uptake and the levels of nutrient-uptake proteins in roots of drought-sensitive and -tolerant grasses. Plants 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Dinis, L.-T.; Ferreira, H.; Coutinho, J.; Moutinho-Pereira, J.; Correia, C. Salicylic acid increases drought adaptability of young olive trees by changes on redox status and ionome. Plant Physiol. Biochem. 2019, 141, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef]
- De Diego, N.; Rodriguez, J.L.; Dodd, I.C.; Perez-Alfocea, F.; Moncalean, P.; Lacuesta, M. Immunolocalization of IAA and ABA in roots and needles of radiata pine (Pinus radiata) during drought and rewatering. Tree Physiol. 2013, 33, 537–549. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Luzio, A.; Silva, E.; Gonçalves, A.; Meijón, M.; Escandón, M.; Arrobas, M.; Rodrigues, M.A.; Moutinho-Pereira, J.; et al. Kaolin and salicylic acid alleviate summer stress in rainfed olive orchards by modulation of distinct physiological and biochemical responses. Sci. Hortic. 2019, 246, 201–211. [Google Scholar] [CrossRef]
- Xiong, M. Aabscisic acid in plant response and adaptation to drought and salt stress. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Jenks, M., Hasegawa, P., Jain, S., Eds.; Springer Science & Business Media: New York, NY, USA, 2007; pp. 193–221. [Google Scholar]
- Gómez-Cadenas, A.; Tadeo, F.; Talon, M.; Primo-Millo, E. Leaf abscission lnduced by ethylene in water-stressed lntact seedlings of cleopatra mandarin requires previous abscisic acid accumulation in roots. Plant Physiol. 1996, 112, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Lin, Y.; Zu, Y.; Efferth, T.; Li, D.; Tang, Z. Ethylene increases accumulation of compatible solutes and decreases oxidative stress to improve plant tolerance to water stress in Arabidopsis. J. Plant Biol. 2015, 58, 193–201. [Google Scholar] [CrossRef]
- Wilkinson, S.; Kudoyarova, G.R.; Veselov, D.S.; Arkhipova, T.N.; Davies, W.J. Plant hormone interactions: Innovative targets for crop breeding and management. J. Exp. Bot. 2012, 63, 3499–3509. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sano, T.; Tamaoki, M.; Nakajima, N.; Kondo, N.; Hasezawa, S. Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol. 2005, 138, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R.E.; LeNoble, M.E. ABA, ethylene and the control of shoot and root growth under water stress. J. Exp. Bot. 2002, 53, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Man, D.; Bao, Y.-X.; Han, L.-B.; Zhang, X. Drought tolerance associated with proline and hormone metabolism in two tall fescue cultivars. HortScience 2011, 46, 1027–1032. [Google Scholar] [CrossRef]
- Du, H.; Liu, H.; Xiong, L. Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front. Plant Sci. 2013, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- De Diego, N.; Perez-Alfocea, F.; Cantero, E.; Lacuesta, M.; Moncalean, P. Physiological response to drought in radiata pine: Phytohormone implication at leaf level. Tree Physiol. 2012, 32, 435–449. [Google Scholar] [CrossRef]
- Pustovoitova, T.N.; Zhdanova, N.E.; Zholkevich, V.N. Changes in the levels of IAA and ABA in cucumber leaves under progressive soil drought. Russ. J. Plant Physiol. 2004, 51, 513–517. [Google Scholar] [CrossRef]
- Wang, C.; Yang, A.; Yin, H.; Zhang, J. Influence of water stress on endogenous hormone contents and cell damage of maize seedlings. J. Integr. Plant Biol. 2008, 50, 427–434. [Google Scholar] [CrossRef]
- Shen, C.; Bai, Y.; Wang, S.; Zhang, S.; Wu, Y.; Chen, M.; Jiang, D.; Qi, Y. Expression profile of PIN, AUX/LAX and PGP auxin transporter gene families in Sorghum bicolor under phytohormone and abiotic stress. FEBS J. 2010, 277, 2954–2969. [Google Scholar] [CrossRef] [PubMed]
- Shojaie, B.; Mostajeran, A.; Esmaeili, A. Different drought conditions could modulate growth responses of Arabidopsis thaliana through regulation of mRNA expression of genes encoding plasma membrane PIN proteins. Int. J. Adv. Res. Biol. Sci. 2015, 2, 241–254. [Google Scholar]
- Sharma, E.; Sharma, R.; Borah, P.; Jain, M.; Khurana, J.P. Emerging Roles of Auxin in Abiotic Stress Responses. In Elucidation of Abiotic Stress Signaling in Plants: Functional Genomics Perspectives; Pandey, G.K., Ed.; Springer: New York, NY, USA, 2015; pp. 299–328. [Google Scholar]
- Peleg, Z.M.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Patumi, M.; d’Andria, R.; Marsilio, V.; Fontanazza, G.; Morelli, G.; Lanzac, B. Olive and olive oil quality after intensive monocone olive growing (Olea europaea L., cv. Kalamata) in different irrigation regimes. Food Chem. 2002, 77, 27–34. [Google Scholar] [CrossRef]
- Bartolini, S.; Leccese, A.; Andreini, L. Influence of canopy fruit location on morphological, histochemical and biochemical changes in two oil olive cultivars. Plant Biosyst. 2014, 148, 1221–1230. [Google Scholar] [CrossRef]
- Machado, M.; Felizardo, C.; Fernandes-Silva, A.A.; Nunes, F.M.; Barros, A. Polyphenolic compounds, antioxidant activity and l-phenylalanine ammonia-lyase activity during ripening of olive cv. “Cobrançosa” under different irrigation regimes. Food. Res. Int. 2013, 51, 412–421. [Google Scholar] [CrossRef]
- Bucelli, P.; Costantini, E.A.C.; Barbetti, R.; Franchini, E. Soil water availability in rainfed cultivations affects more than cultivar some nutraceutical components and the sensory profile of virgin olive oil. J. Agric. Food. Chem. 2011, 59, 8304–8313. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Guccia, R.; Urbani, S.; Esposto, S.; Taticchi, A.; di Maio, I.; Selvaggini, R.; Maurizio Servili, M. Effect of different irrigation volumes during fruit development onquality of virgin olive oil of cv. Frantoio. Agric. Water Manag. 2014, 134, 94–103. [Google Scholar] [CrossRef]
- Gómez del Campo, M.; García, J.M. Summer deficit-irrigation strategies in a hedgerow olive cv. Arbequina orchard: Effect on oil quality. J. Agric. Food Chem. 2013, 61, 8899–8905. [Google Scholar] [CrossRef]
- García, J.M.; Morales-Sillero, A.; Pérez-Rubio, A.G.; Diaz-Espejo, A.; Montero, A.; Fernández, J.E. Virgin olive oil quality of hedgerow ‘Arbequina’ olive trees under deficit irrigation. J. Sci. Food Agric. 2017, 97, 1018–1026. [Google Scholar] [CrossRef]
- Houliston, A.; Vanhanen, L.; Savage, G. Frost protection of olives using glycine betaine. Sci. Thecnol. 2007, 108, 31–40. [Google Scholar]
- Morelló, J.-R.; Motilva, M.-J.; Ramo, T.; Romero, M.-P. Effect of freeze injuries in olive fruit on virgin olive oil composition. Food Chem. 2003, 81, 547–553. [Google Scholar] [CrossRef]
- Morelló, J.R.; Romero, M.P.; Motilva, M.J. Influence of seasonal conditions on the composition and quality parameters of monovarietal virgin olive oils. J. Am. Oil Chem. Soc. 2006, 83, 683–690. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Silva, E.; Gonçalves, A.; Matos, C.; Rodrigues, M.A.; Moutinho-Pereira, J.; Barros, A.; Correia, C. Kaolin and salicylic acid foliar application modulate yield, quality and phytochemical composition of olive pulp and oil from rainfed trees. Sci. Hortic. 2018, 237, 176–183. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Cao, B.; Cao, D.; Leng, G.; Li, H.; Yin, L.; Shan, L.; Deng, X. Genotypic variation in growth and physiological response to drought stress and re-watering reveals the critical role of recovery in drought adaptation in maize seedlings. Front. Plant Sci. 2016, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ennajeh, M.; Vadel, A.M.; Cochard, H.; Khemira, H. Comparative impacts of water stress on the leaf anatomy of a drought-resistant and a drought-sensitive olive cultivar. J. Hortic. Sci. Biotechnol. 2010, 85, 289–294. [Google Scholar] [CrossRef]
- Tognetti, R.; Giovannelli, A.; Lavini, A.; Morelli, G.; Fragnito, F.; d’Andria, R. Assessing environmental controls over conductances through the soil–plant–atmosphere continuum in an experimental olive tree plantation of southern Italy. Agric. For. Meteorol. 2009, 149, 1229–1243. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Patakas, A.; Bosabalidis, A.M. Changes in water relations, photosynthesis and leaf anatomy induced by intermittent drought in two olive cultivars. Environ. Exp. Bot. 1999, 42, 113–120. [Google Scholar] [CrossRef]
- Mediavilla, S.; Escudero, A.; Heilmeier, H. Internal leaf anatomy and photosynthetic resource-use efficiency: Interspecific and intraspecific comparisons. Tree Physiol. 2001, 21, 251–259. [Google Scholar] [CrossRef]
- Savé, R.; Biel, C.; de Herralde, F. Leaf pubescence, water relations and chlorophyll fluorescence in two subspecies of Lotus Creticus L. Biol. Plant. 2000, 43, 239–244. [Google Scholar] [CrossRef]
- Veihmeyer, F.J.; Hendrickson, A.H. Soil moisture at permanent wilting of plants. Plant Physiol. 1928, 3, 355–357. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xiloyannis, C.; Dichio, B. Irrigazione. In Olea: Trattato di Olivicoltura; Fiorino, P., Ed.; Il Sole 24 ORE Edagricole Srl: Bologna, Italy, 2003; pp. 365–389. [Google Scholar]
- Dichio, B.; Xiloyannis, C.; Angelopoulos, K.; Nuzzo, V.; Bufo, S.A.; Celano, G. Drought-induced variations of water relations parameters in Olea europaea. Plant Soil 2003, 257, 381–389. [Google Scholar] [CrossRef]
- Dichio, B.; Xiloyannis, C.; Sofo, A.; Montanaro, G. Osmotic regulation in leaves and roots of olive trees during a water deficit and rewatering. Tree Physiol. 2006, 26, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Moriana, A.; Orgaz, F.; Pastor, M.; Fereres, E. Yield responses of a mature olive orchard to water deficits. J. Am. Soc. Hortic. Sci. 2003, 128, 425–431. [Google Scholar] [CrossRef]
- Orgaz, F.; Fereres, E.; Barranco, D.; Fernández-Escobar, R.; Rallo, L. El Cultivo del Olivo, 6th ed.; Ediciones Mundi-Prensa y Junta de Andalucía: Madrid, Spain, 2008; pp. 337–362. [Google Scholar]
- Dichio, B.; Montanaro, G.; Sofo, A.; Xiloyannis, C. Stem and whole-plant hydraulics in olive (Olea europaea) and kiwifruit (Actinidia deliciosa). Trees 2013, 27, 183–191. [Google Scholar] [CrossRef]
- Fernández Luque, J.E.; Aranda, M.; Moreno Lucas, F.; Rapoport, H.F. Anatomical response of olive roots to dry and irrigated soils. Adv. Hortic. Sci. 1994, 8, 141–144. [Google Scholar]
- Nadezhdina, N.; Ferreira, M.I.; Conceição, N.; Pacheco, C.A.; Häusler, M.; David, T.S. Water uptake and hydraulic redistribution under a seasonal climate: Long-term study in a rainfed olive orchard. Ecohydrology 2015, 8, 387–397. [Google Scholar] [CrossRef]
- Dichio, B.; Margiotta, G.; Xiloyannis, C.; Bufo, S.A.; Sofo, A.; Cataldi, T.R.I. Changes in water status and osmolyte contents in leaves and roots of olive plants (Olea europaea L.) subjected to water deficit. Trees 2009, 23, 247–256. [Google Scholar] [CrossRef]
- Sanders, G.J.; Arndt, S.K. Osmotic adjustment under drought conditions. In Plant Responses to Drought Stress—From Morphological to Molecular Features; Aroca, R., Ed.; Springer: New York, NY, USA, 2012; pp. 199–230. [Google Scholar]
- Patakas, A.; Nikolaou, N.; Zioziou, E.; Radoglou, K.; Noitsakis, B. The role of organic solute and ion accumulation in osmotic adjustment in drought-stressed grapevines. Plant Sci. 2002, 163, 361–367. [Google Scholar] [CrossRef]
- Joly, R.J.; Zaerr, J.B. Alteration of cell-wall water content and elasticity in Douglas-fir during periods of water deficit. Plant Physiol. 1987, 83, 418–422. [Google Scholar] [CrossRef]
- Zargar, S.M.; Nagar, P.; Deshmukh, R.; Nazir, M.; Wani, A.A.; Masoodi, K.Z.; Agrawal, G.K.; Rakwal, R. Aquaporins as potential drought tolerance inducing proteins: Towards instigating stress tolerance. J. Proteom. 2017, 169, 233–238. [Google Scholar] [CrossRef]
- Šurbanovski, N.; Grant, O.M. The emerging role of aquaporins in plant tolerance. In Emerging Technologies and Management of Crop Stress Tolerance, Volume 2: A Sustainable Approach; Ahmad, P., Rasool, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 431–447. [Google Scholar]
- Secchi, F.; Lovisolo, C.; Schubert, A. Expression of OePIP2.1 aquaporin gene and water relations of Olea europaea twigs during drought stress and recovery. Ann. Appl. Biol. 2007, 150, 163–167. [Google Scholar] [CrossRef]
- Secchi, F.; Lovisolo, C.; Uehlein, N.; Kaldenhoff, R.; Schubert, A. Isolation and functional characterization of three aquaporins from olive (Olea europaea L.). Planta 2007, 225, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Rogiers, S.Y.; Greer, D.H.; Hatfield, J.M.; Hutton, R.J.; Clarke, S.J.; Hutchinson, P.A.; Somers, A. Stomatal response of an anisohydric grapevine cultivar to evaporative demand, available soil moisture and abscisic acid. Tree Physiol. 2012, 32, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Naor, A.; Schneider, D.; Ben-Gal, A.; Zipori, I.; Dag, A.; Kerem, Z.; Birger, R.; Peres, M.; Gal, Y. The effects of crop load and irrigation rate in the oil accumulation stage on oil yield and water relations of ‘Koroneiki’ olives. Irrig. Sci. 2013, 31, 781–791. [Google Scholar] [CrossRef]
- Mattos, L.; Moretti, C. Oxidative stress in plants under drought conditions and the role of different enzymes. Enzyme Eng. 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Ahmadipour, S.; Arji, I.; Ebadi, A.; Abdossi, V. Physiological and biochemical responses of some olive cultivars (Olea europaea L.) to water stress. Cell Mol. Biol. 2018, 64, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Rahemi, M.; Rostami, A.A.; Sedaghat, S. Drought effects on growth, water content and osmoprotectants in four olive cultivars with different drought tolerance. Int. J. Fruit Sci. 2018, 18, 254–267. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, C.; Dinis, L.-T.; Moutinho-Pereira, J.; Correia, C.M. Drought Stress Effects and Olive Tree Acclimation under a Changing Climate. Plants 2019, 8, 232. https://doi.org/10.3390/plants8070232
Brito C, Dinis L-T, Moutinho-Pereira J, Correia CM. Drought Stress Effects and Olive Tree Acclimation under a Changing Climate. Plants. 2019; 8(7):232. https://doi.org/10.3390/plants8070232
Chicago/Turabian StyleBrito, Cátia, Lia-Tânia Dinis, José Moutinho-Pereira, and Carlos M. Correia. 2019. "Drought Stress Effects and Olive Tree Acclimation under a Changing Climate" Plants 8, no. 7: 232. https://doi.org/10.3390/plants8070232
APA StyleBrito, C., Dinis, L.-T., Moutinho-Pereira, J., & Correia, C. M. (2019). Drought Stress Effects and Olive Tree Acclimation under a Changing Climate. Plants, 8(7), 232. https://doi.org/10.3390/plants8070232







