Diversion of Carbon Flux from Sugars to Lipids Improves the Growth of an Arabidopsis Starchless Mutant
Abstract
1. Introduction
2. Results
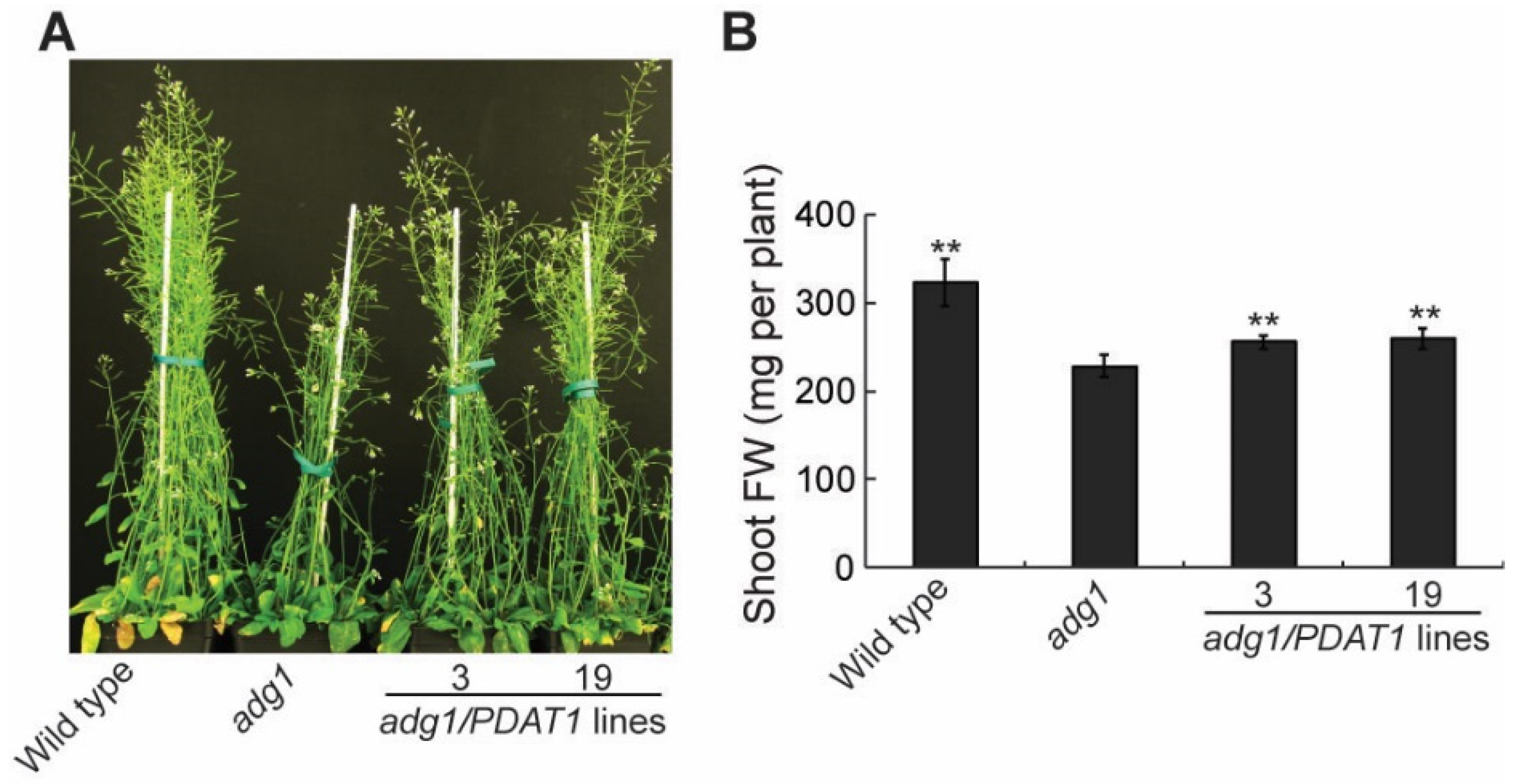
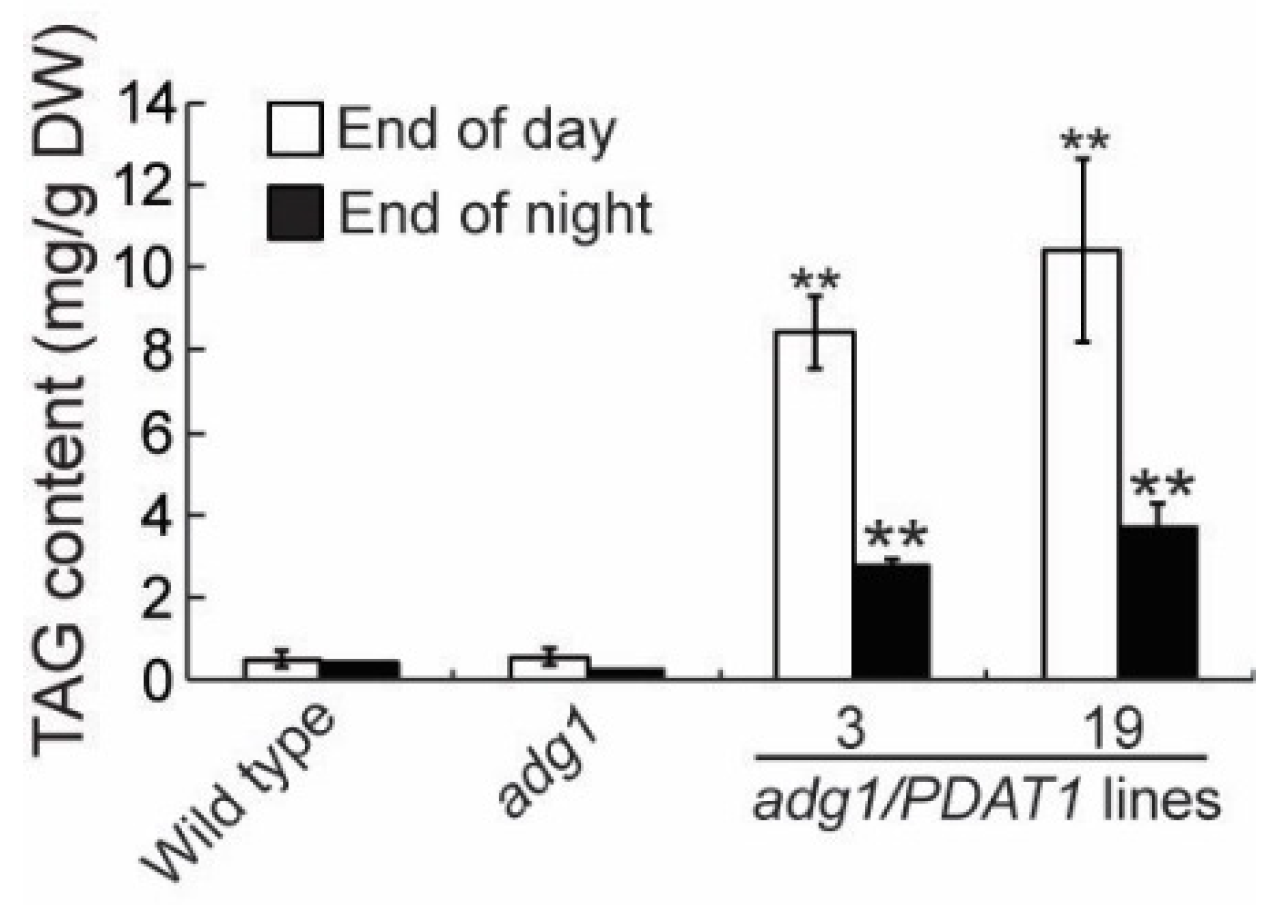
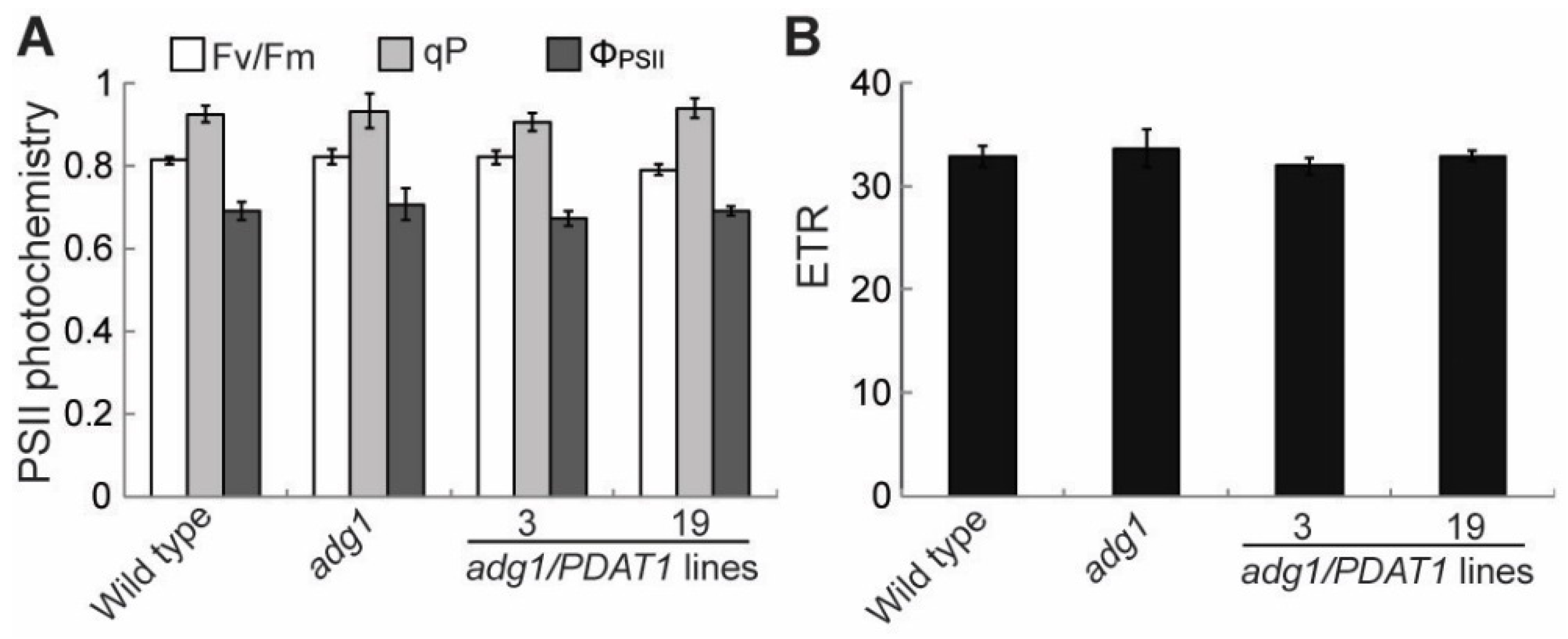
2.1. Overexpressing PDAT1 Improves the Growth of adg1
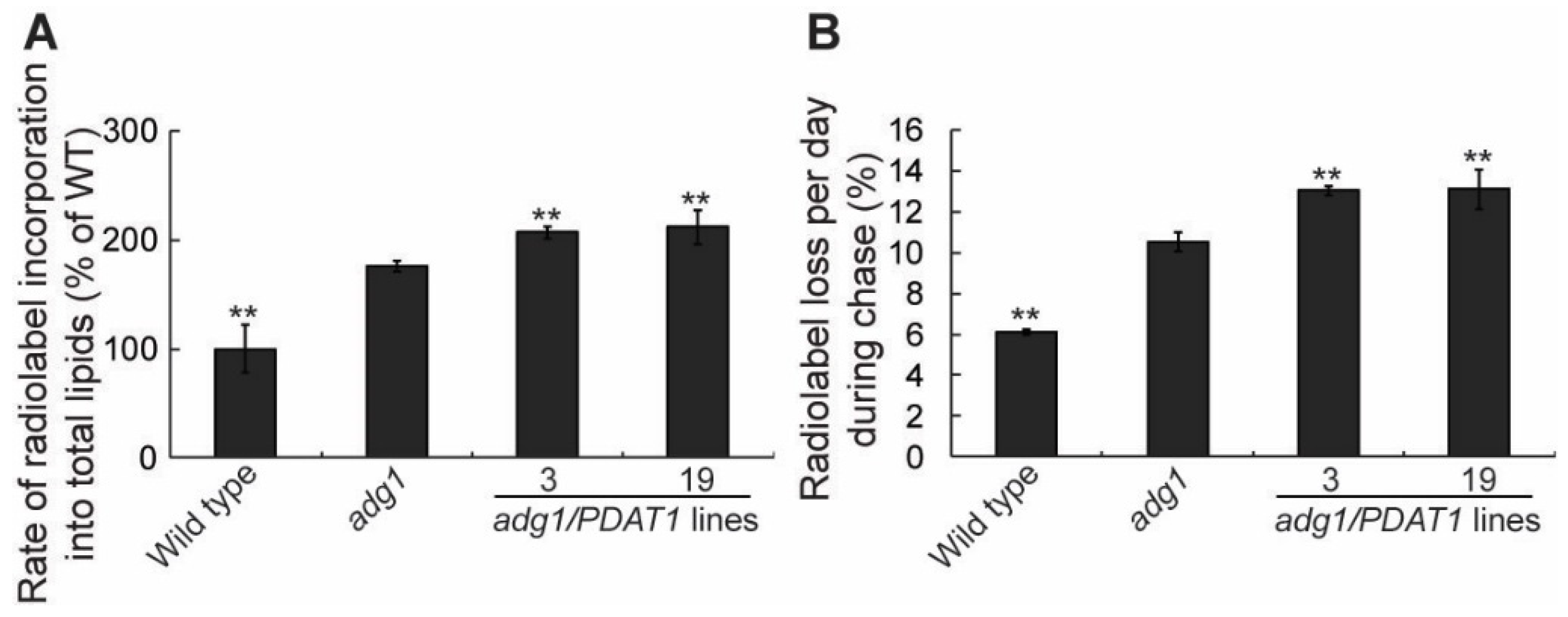
2.2. Overexpressing PDAT1 Enhance the Synthesis and Turnover of Fatty Acids
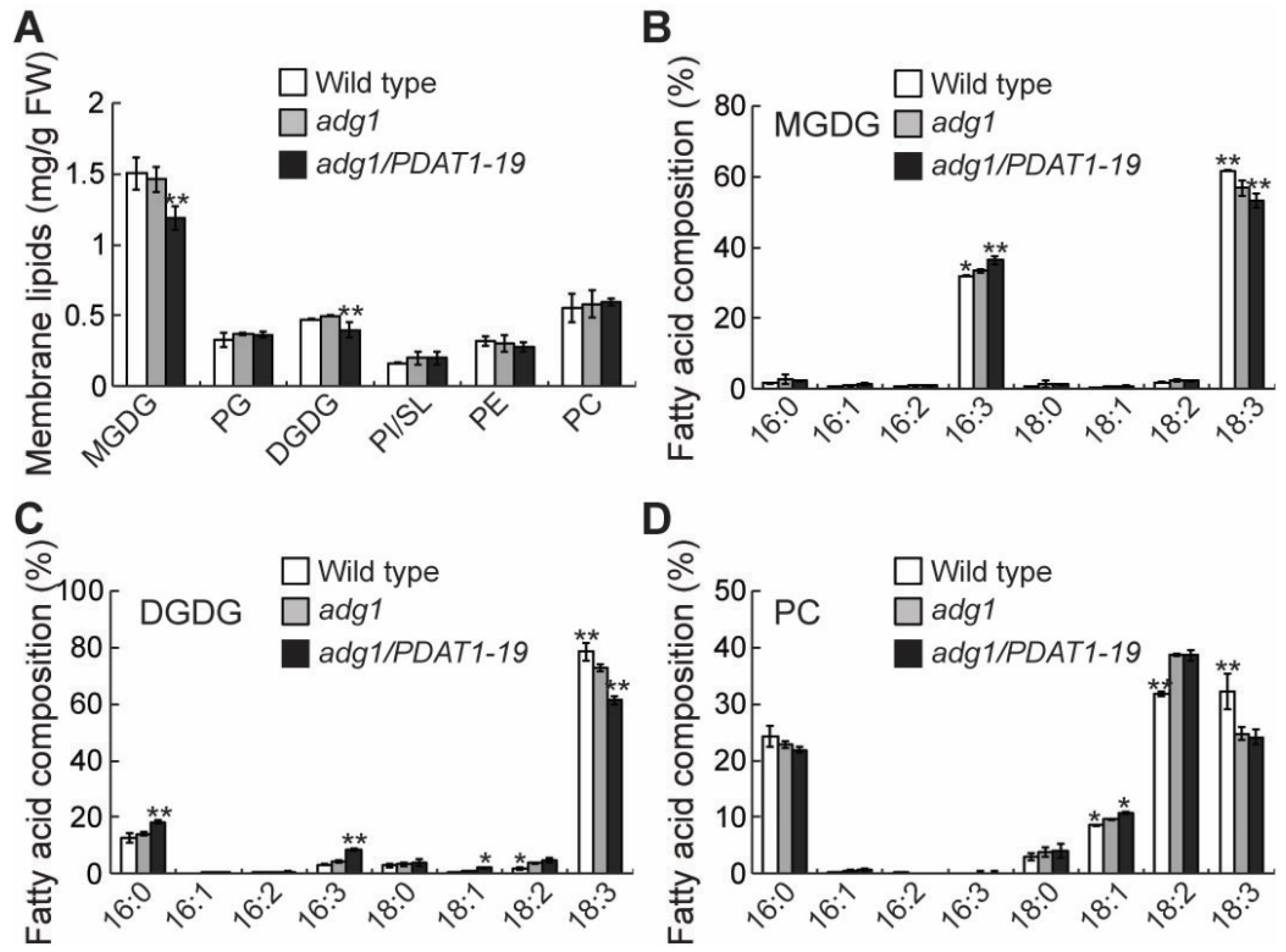
2.3. Overexpressing PDAT1 Diverts Fatty Acid Flux from Membrane Lipids to TAG
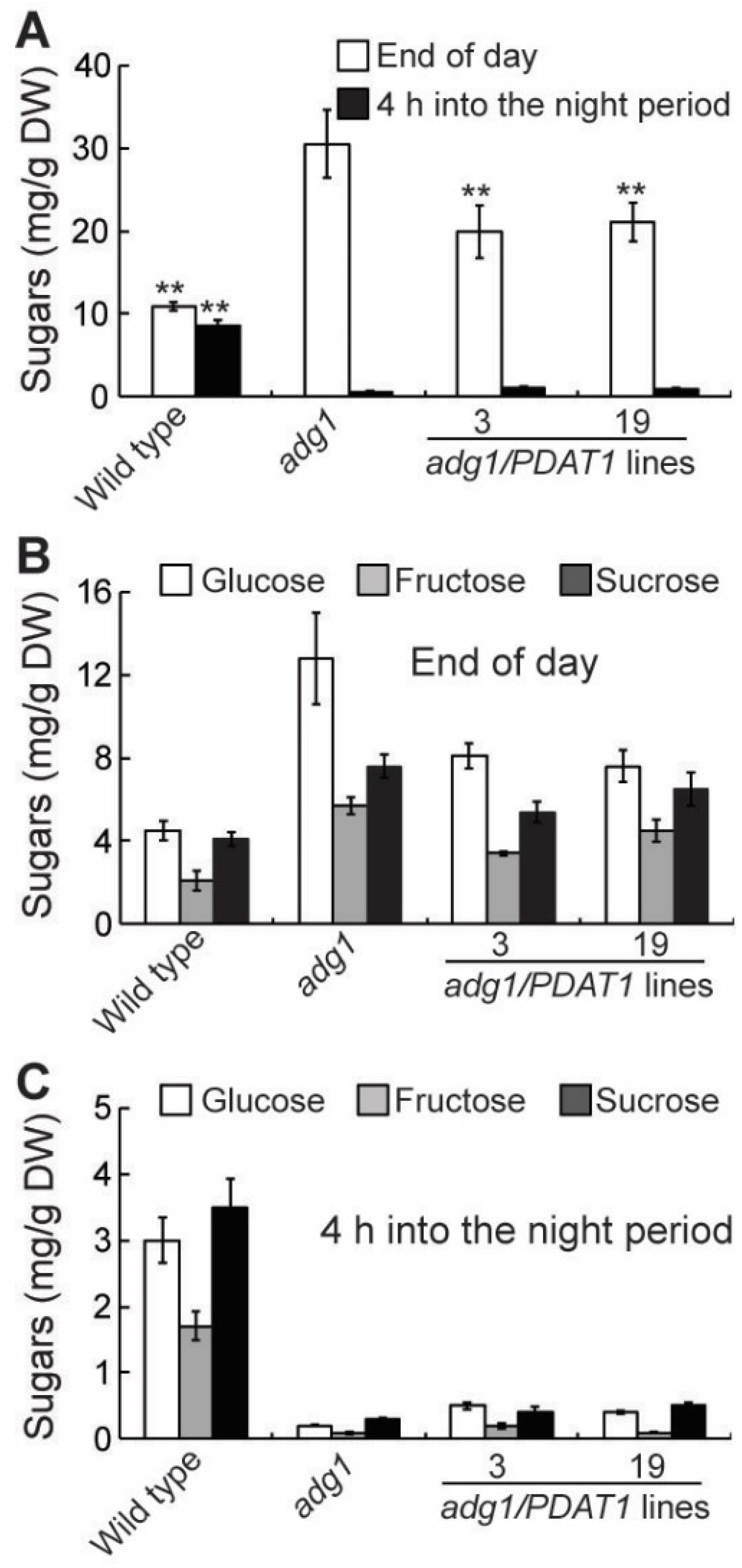
2.4. Overexpressing PDAT1 Diverts Carbon Flux from Sugars to Lipids
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Generation of the Construct and Plant Transformation
4.3. Lipid Analysis
4.4. Radiotracer Labeling
4.5. Measurement of Chlorophyll Fluorescence Parameters
4.6. Quantification of Sugars
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, C.; Shanklin, J. Triacylglycerol metabolism, function, and accumulation in plant vegetative tissues. Annu. Rev. Plant Biol. 2016, 67, 179–206. [Google Scholar] [CrossRef]
- Geigenberger, P. Regulation of starch biosynthesis in response to a fluctuating environment. Plant Physiol. 2011, 155, 1566–1577. [Google Scholar] [CrossRef] [PubMed]
- Graf, A.; Smith, A.M. Starch and the clock: The dark side of plant productivity. Trends Plant Sci. 2011, 16, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Stitt, M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007, 30, 1126–1149. [Google Scholar] [CrossRef]
- Stitt, M.; Zeeman, S.C. Starch turnover: Pathways, regulation and role in growth. Curr. Opin. Plant Biol. 2012, 15, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.P.; Caspar, T.; Somerville, C.; Preiss, J. Isolation and characterization of a starchless mutant of Arabidopsis thaliana (L.) lacking ADPglucose pyrophosphorylase activity. Plant Physiol. 1988, 86, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Caspar, T.; Huber, S.C.; Somerville, C. Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L) deficient in chloroplast phosphoglucomutase activity. Plant Physiol. 1985, 79, 11–17. [Google Scholar] [CrossRef]
- Kotting, O.; Santelia, D.; Edner, C.; Eicke, S.; Marthaler, T.; Gentry, M.S.; Comparot-Moss, S.; Chen, J.; Smith, A.M.; Steup, M.; et al. STARCH-EXCESS4 is a laforin-like phosphoglucan phosphatase required for starch degradation in Arabidopsis thaliana. Plant Cell 2009, 21, 334–346. [Google Scholar] [CrossRef]
- Yu, T.S.; Kofler, H.; Hausler, R.E.; Hille, D.; Flugge, U.I.; Zeeman, S.C.; Smith, A.M.; Kossmann, J.; Lloyd, J.; Ritte, G.; et al. The Arabidopsis sex1 mutant is defective in the R1 protein, a general regulator of starch degradation in plants, and not in the chloroplast hexose transporter. Plant Cell 2001, 13, 1907–1918. [Google Scholar] [CrossRef]
- Brauner, K.; Hormiller, I.; Nagele, T.; Heyer, A.G. Exaggerated root respiration accounts for growth retardation in a starchless mutant of Arabidopsis thaliana. Plant J. 2014, 79, 82–91. [Google Scholar] [CrossRef]
- Sun, J.D.; Okita, T.W.; Edwards, G.E. Modification of carbon partitioning, photosynthetic capacity, and O2 sensitivity in Arabidopsis plants with low ADP-glucose pyrophosphorylase activity. Plant Physiol. 1999, 119, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Ohlrogge, J.; Browse, J. Lipid biosynthesis. Plant Cell 1995, 7, 957–970. [Google Scholar] [PubMed]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; Debono, A.; Durrett, T.P.; et al. Acyl-lipid metabolism. Arab. Book 2013, 11, e0161. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yan, C.; Zhang, X.; Xu, C. Dual role for phospholipid:diacylglycerol acyltransferase: Enhancing fatty acid synthesis and diverting fatty acids from membrane lipids to triacylglycerol in Arabidopsis leaves. Plant Cell 2013, 25, 3506–3518. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.L.; Ohlrogge, J.B. Turnover of fatty acids during natural senescence of Arabidopsis, Brachypodium, and Switchgrass and in Arabidopsis β-xidation mutants. Plant Physiol. 2009, 150, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Dahlqvist, A.; Stahl, U.; Lenman, M.; Banas, A.; Lee, M.; Sandager, L.; Ronne, H.; Stymne, S. Phospholipid:diacylglycerol acyltransferase: An enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc. Natl. Acad. Sci. USA 2000, 97, 6487–6492. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.A.; van Erp, H.; Quettier, A.L.; Shaw, E.; Menard, G.; Kurup, S.; Eastmond, P.J. The SUGAR-DEPENDENT1 lipase limits triacylglycerol accumulation in vegetative tissues of Arabidopsis. Plant Physiol. 2013, 162, 1282–1289. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, J.; Taylor, D.C.; Ohlrogge, J.B. DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell 2009, 21, 3885–3901. [Google Scholar] [CrossRef]
- Eastmond, P.J. SUGAR-DEPENDENT1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. Plant Cell 2006, 18, 665–675. [Google Scholar] [CrossRef]
- Graham, I.A. Seed storage oil mobilization. Annu. Rev. Plant Biol. 2008, 59, 115–142. [Google Scholar] [CrossRef]
- Yu, L.H.; Fan, J.L.; Yan, C.S.; Xu, C.C. Starch deficiency enhances lipid biosynthesis and turnover in leaves. Plant Physiol. 2018, 178, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Browse, J.; Somerville, C. Glycerolipid synthesis: Biochemistry and regulation. Annu. Rev. Plant Physiol. 1991, 42, 467–506. [Google Scholar] [CrossRef]
- Bao, X.; Focke, M.; Pollard, M.; Ohlrogge, J. Understanding in vivo carbon precursor supply for fatty acid synthesis in leaf tissue. Plant J. 2000, 22, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Gibon, Y.; Blasing, O.E.; Palacios-Rojas, N.; Pankovic, D.; Hendriks, J.H.M.; Fisahn, J.; Hohne, M.; Gunther, M.; Stitt, M. Adjustment of diurnal starch turnover to short days: Depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J. 2004, 39, 847–862. [Google Scholar]
- Heyneke, E.; Fernie, A.R. Metabolic regulation of photosynthesis. Biochem. Soc. Trans. 2018, 46, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.J.; Foyer, C.H. Sink regulation of photosynthesis. J. Exp. Bot. 2001, 52, 1383–1400. [Google Scholar] [CrossRef]
- Foyer, C.H. Feedback inhibition of photosynthesis through source-sink regulation in leaves. Plant Physiol. Biochem. 1988, 26, 483–492. [Google Scholar]
- Ohlrogge, J.B.; Jaworski, J.G. Regulation of fatty acid synthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 109–136. [Google Scholar] [CrossRef]
- Durrett, T.P.; Benning, C.; Ohlrogge, J. Plant triacylglycerols as feedstocks for the production of biofuels. Plant J. 2008, 54, 593–607. [Google Scholar] [CrossRef]
- Alonso, J.M. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 2003, 301, 653–657. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Koo, A.J.K.; Fulda, M.; Browse, J.; Ohlrogge, J.B. Identification of a plastid acyl-acyl carrier protein synthetase in Arabidopsis and its role in the activation and elongation of exogenous fatty acids. Plant J. 2005, 44, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.M.; Baker, N.R. The Relationship between the quantum yield of photosynthetic electrontransport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1998, 990, 87–92. [Google Scholar] [CrossRef]
- Stitt, M.; Lilley, R.M.; Gerhardt, R.; Heldt, H.W. Metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol. 1989, 174, 518–552. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Zhou, C.; Yu, L.; Li, P.; Shanklin, J.; Xu, C. Diversion of Carbon Flux from Sugars to Lipids Improves the Growth of an Arabidopsis Starchless Mutant. Plants 2019, 8, 229. https://doi.org/10.3390/plants8070229
Fan J, Zhou C, Yu L, Li P, Shanklin J, Xu C. Diversion of Carbon Flux from Sugars to Lipids Improves the Growth of an Arabidopsis Starchless Mutant. Plants. 2019; 8(7):229. https://doi.org/10.3390/plants8070229
Chicago/Turabian StyleFan, Jilian, Chao Zhou, Linhui Yu, Ping Li, John Shanklin, and Changcheng Xu. 2019. "Diversion of Carbon Flux from Sugars to Lipids Improves the Growth of an Arabidopsis Starchless Mutant" Plants 8, no. 7: 229. https://doi.org/10.3390/plants8070229
APA StyleFan, J., Zhou, C., Yu, L., Li, P., Shanklin, J., & Xu, C. (2019). Diversion of Carbon Flux from Sugars to Lipids Improves the Growth of an Arabidopsis Starchless Mutant. Plants, 8(7), 229. https://doi.org/10.3390/plants8070229




