Mathematical Modelling of Hydrophilic Ionic Fertiliser Diffusion in Plant Cuticles: Lipophilic Surfactant Effects
Abstract
1. Introduction
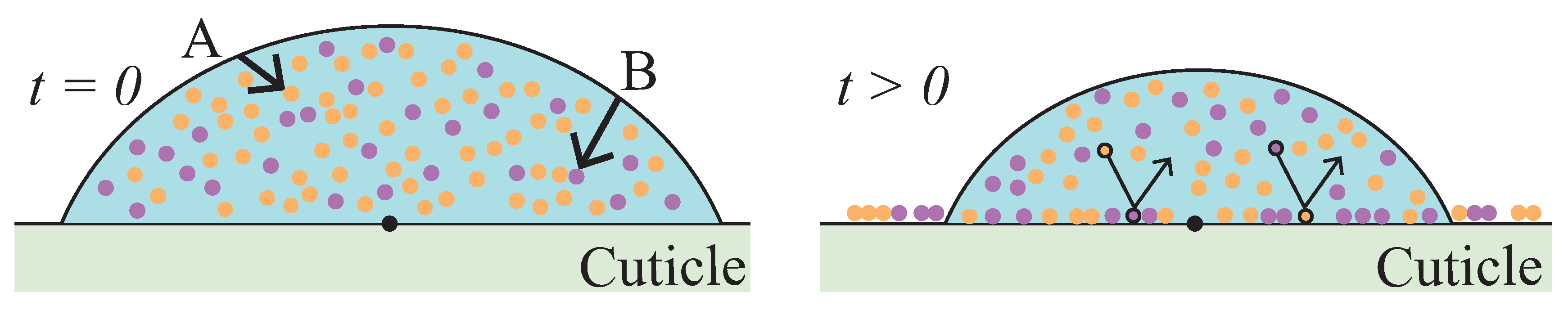
2. Model Framework
Numerical Solution Procedure
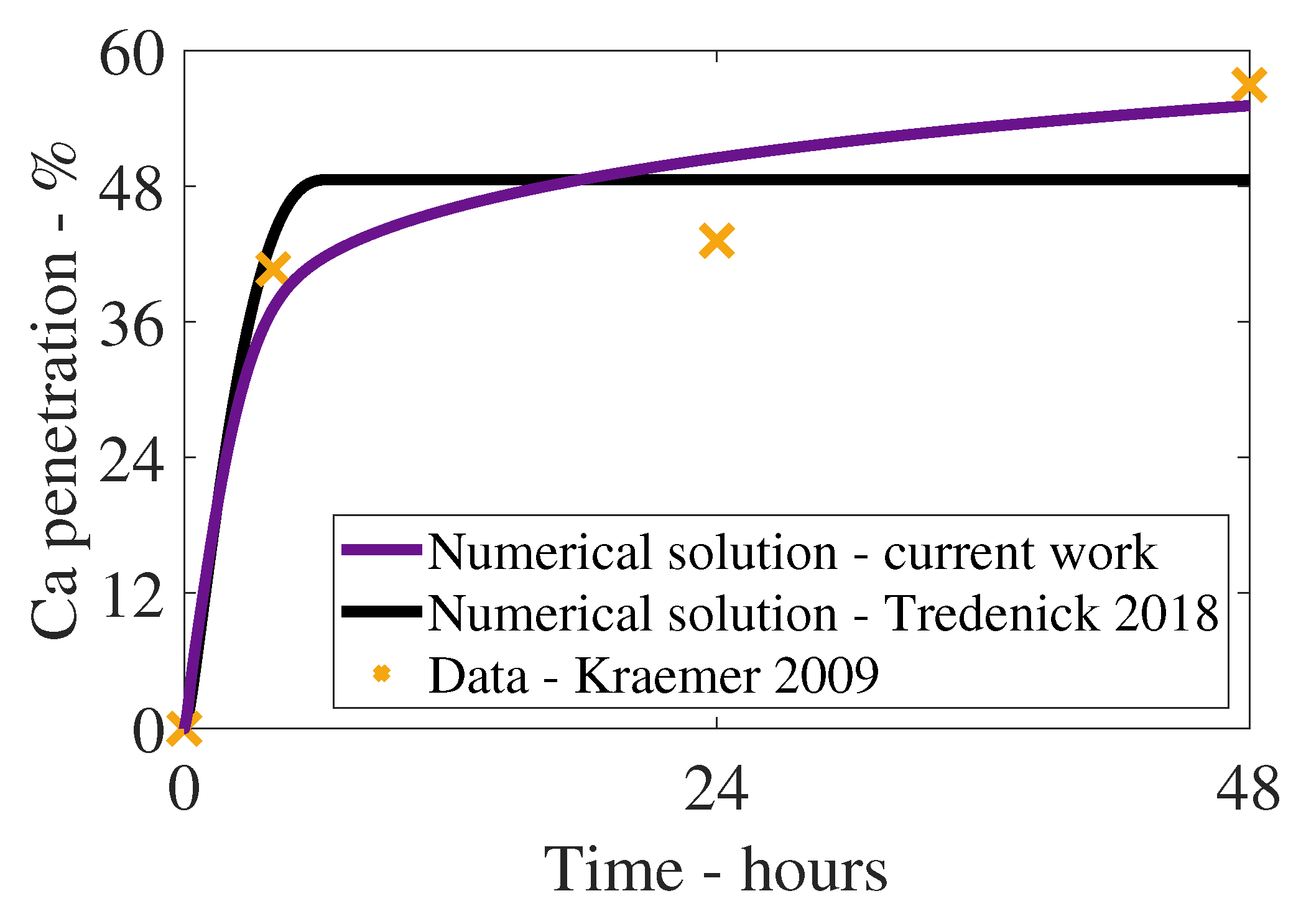
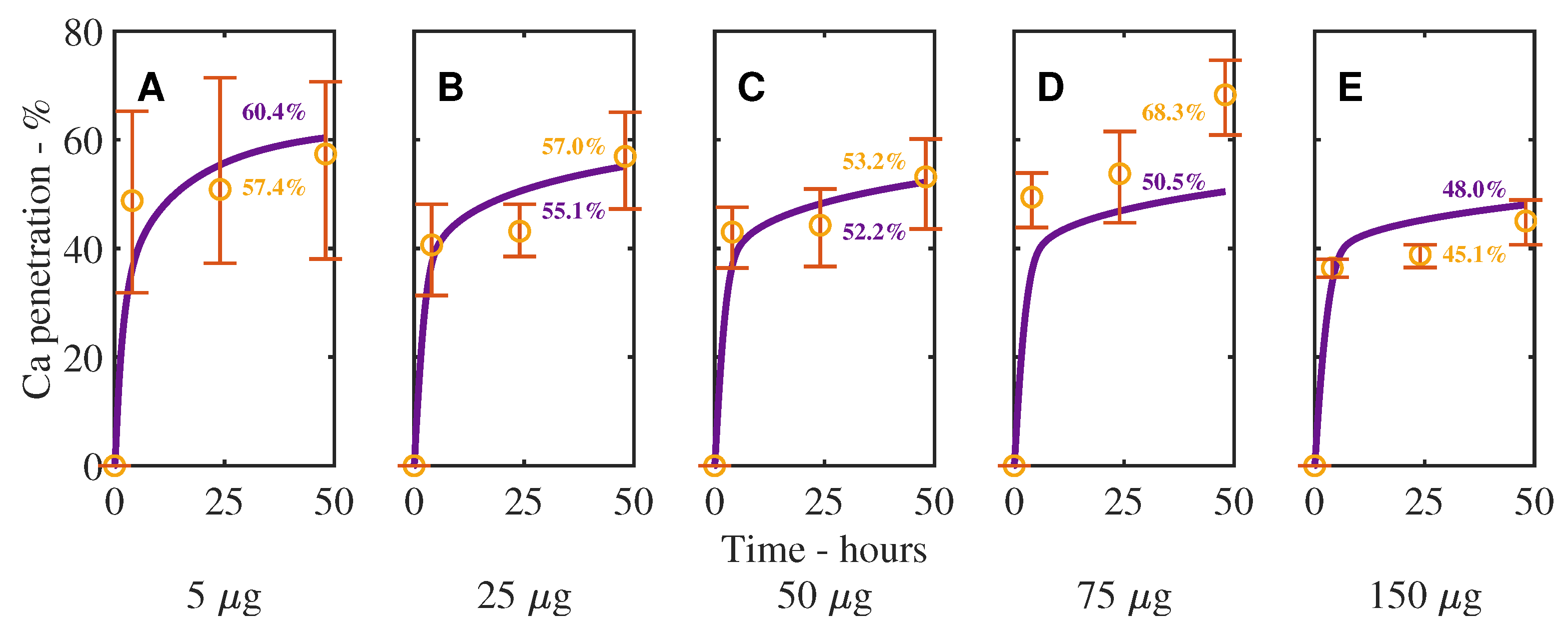
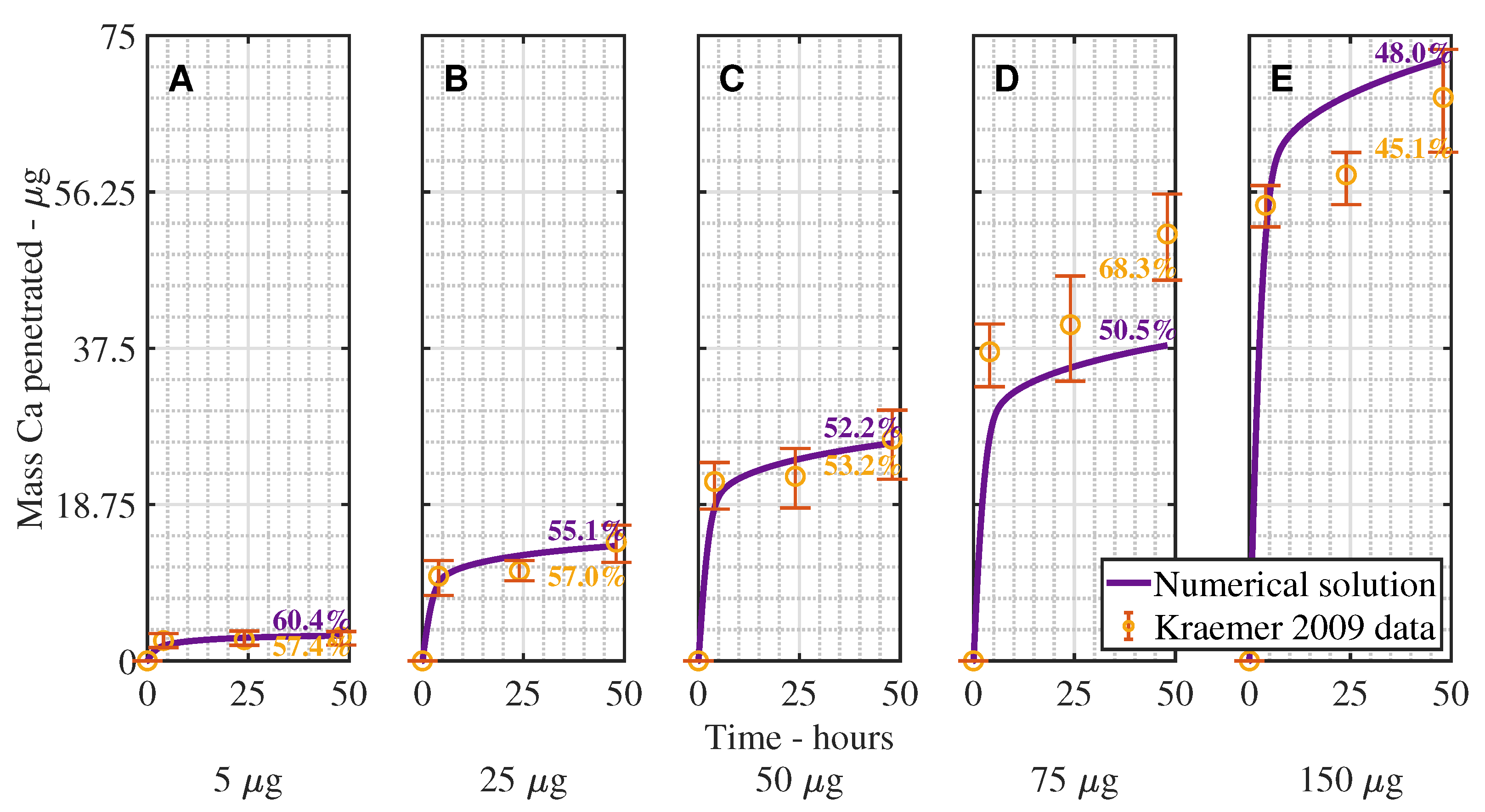
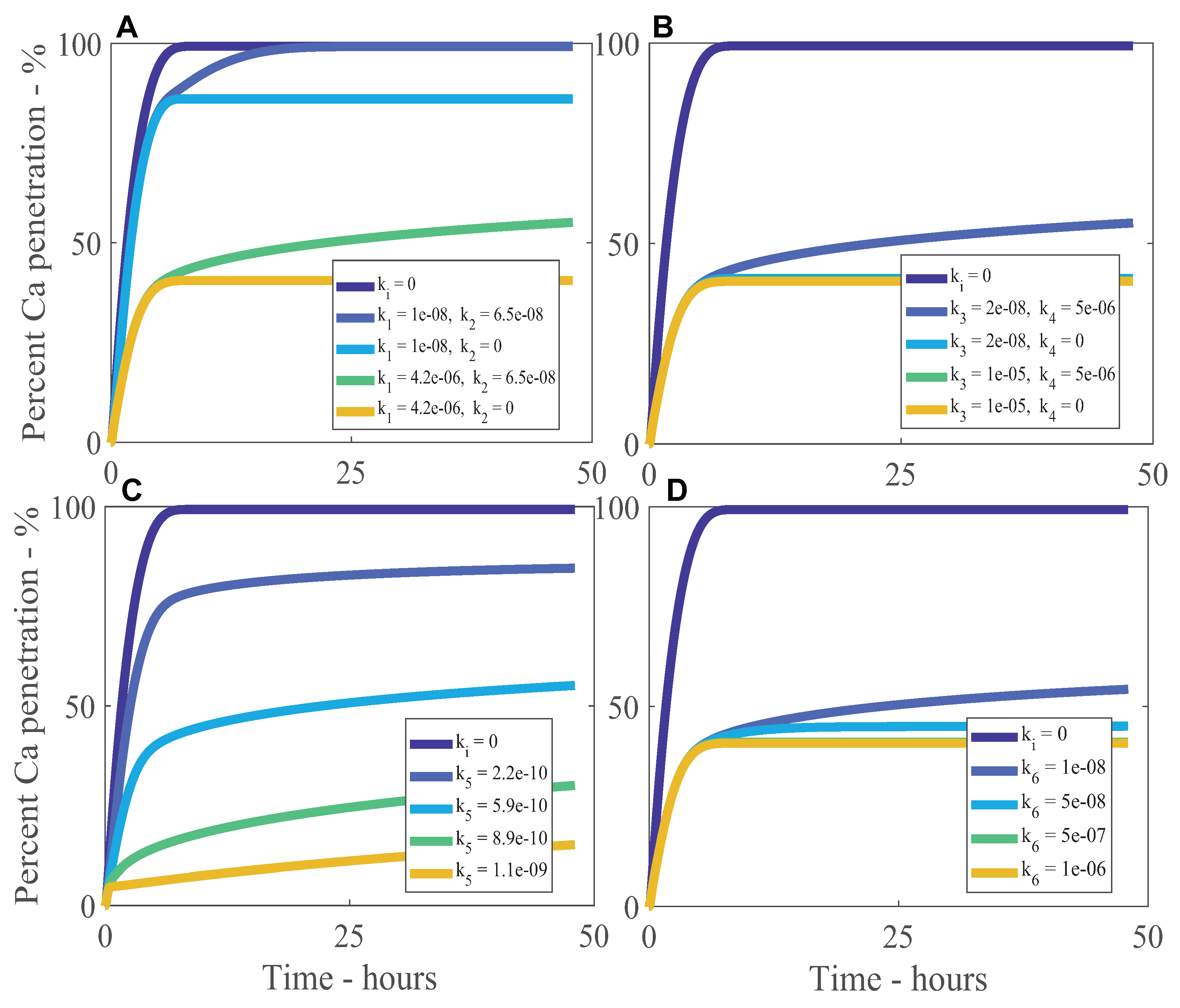
3. Results and Discussion
Sensitivity Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Additional Droplet Evaporation Equations
References
- Shaner, D.L.; Beckie, H.J. The future for weed control and technology. Pest Manag. Sci. 2014, 70, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Knoche, M. Effect of droplet size and carrier volume on performance of foliage-applied herbicides. Crop Prot. 1994, 13, 163–178. [Google Scholar] [CrossRef]
- Balneaves, J.; Gaskin, R.; Zabkiewicz, J. The effect of varying rates of glyphosate and an organosilicone surfactant on the control of gorse. Ann. Appl. Biol. 1993, 122, 531–536. [Google Scholar] [CrossRef]
- Schönherr, J. Characterization of aqueous pores in plant cuticles and permeation of ionic solutes. J. Exp. Bot. 2006, 57, 2471–2491. [Google Scholar] [CrossRef] [PubMed]
- McKenna, C.; Gaskin, R.; Horgan, D.; Dobson, S.; Jia, Y. Efficacy of a postharvest spirotetramat spray against armoured scale insects on kiwifruit vines. N. Z. J. Crop Horticult. Sci. 2013, 41, 105–116. [Google Scholar] [CrossRef]
- Schönherr, J.; Riederer, M. Foliar Penetration and Accumulation of Organic Chemicals in Plant Cuticles. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer: New York, NY, USA, 1989; Volume 108, pp. 1–70. [Google Scholar] [CrossRef]
- Riederer, M.; Schreiber, L. Protecting against water loss: Analysis of the barrier properties of plant cuticles. J. Exp. Bot. 2001, 52, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Mays, T. A new classification of pore sizes. Stud. Surf. Sci. Catal. 2007, 160, 57–62. [Google Scholar] [CrossRef]
- Baur, P. Surfactant Effects on Cuticular Penetration of Neutral Polar Compounds: Dependence on Humidity and Temperature. J. Agric. Food Chem. 1999, 47, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, L. Review of sorption and diffusion of lipophilic molecules in cuticular waxes and the effects of accelerators on solute mobilities. J. Exp. Bot. 2006, 57, 2515–2523. [Google Scholar] [CrossRef]
- Schreiber, L. Polar paths of diffusion across plant cuticles: New evidence for an old hypothesis. Ann. Bot. 2005, 95, 1069–1073. [Google Scholar] [CrossRef]
- Tredenick, E.C.; Farrell, T.W.; Forster, W.A.; Psaltis, S.T.P. Nonlinear Porous Diffusion Modeling of Hydrophilic Ionic Agrochemicals in Astomatous Plant Cuticle Aqueous Pores: A Mechanistic Approach. Front. Plant Sci. 2017, 8, 746. [Google Scholar] [CrossRef] [PubMed]
- Tredenick, E.C.; Farrell, T.W.; Forster, W.A. Mathematical Modeling of Diffusion of a Hydrophilic Ionic Fertilizer in Plant Cuticles: Surfactant and Hygroscopic Effects. Front. Plant Sci. 2018, 9, 1888. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.; Bahamonde, H.A.; Javier Peguero-Pina, J.; Gil-Pelegrín, E.; Sancho-Knapik, D.; Gil, L.; Goldbach, H.E.; Eichert, T. Physico-chemical properties of plant cuticles and their functional and ecological significance. J. Exp. Bot. 2017, 68, 5293–5306. [Google Scholar] [CrossRef] [PubMed]
- Jeffree, C.E. The fine structure of the plant cuticle. In Annual Plant Reviews, Biology of the Plant Cuticle; Riederer, M., Muller, C., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 23, pp. 11–126. [Google Scholar] [CrossRef]
- Schreiber, L.; Elshatshat, S.; Koch, K.; Lin, J.; Santrucek, J. AgCl precipitates in isolated cuticular membranes reduce rates of cuticular transpiration. Planta 2006, 223, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Santier, S.; Chamel, A. Penetration of glyphosate and diuron into and through isolated plant cuticles. Weed Res. 1992, 32, 337–347. [Google Scholar] [CrossRef]
- Yamada, Y.; Wittwer, S.; Bukovac, M. Penetration of ions through isolated cuticles. Plant Physiol. 1964, 39, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Tang, I.N.; Tridico, A.C.; Fung, K.H. Thermodynamic and optical properties of sea salt aerosols. J. Geophy. Res. 1997, 102, 23269–23275. [Google Scholar] [CrossRef]
- OxyChem. Calcium Chloride Properties. 2014. Available online: http://www.oxy.com/OurBusinesses/Chemicals/Products/Documents/CalciumChloride/173-01791.pdf (accessed on 30 September 2018).
- Hunsche, M.; Noga, G. Effects of relative humidity and substrate on the spatial association between glyphosate and ethoxylated seed oil adjuvants in the dried deposits of sessile droplets. Pest Manag. Sci. 2012, 68, 231–239. [Google Scholar] [CrossRef]
- Kraemer, T.; Hunsche, M.; Noga, G. Cuticular calcium penetration is directly related to the area covered by calcium within droplet spread area. Sci. Horticult. 2009, 120, 201–206. [Google Scholar] [CrossRef]
- Agnique RSO 30. 2016. Available online: https://e-applications.basf-ag.de/data/basf-pcan/pds2/pds2-web.nsf/41CC0BAF3EDDB063C125771B00551C8D/$File/AGNIQUE_r__RSO_30_E.pdf (accessed on 2 May 2018).
- Hess, F.D.A.N.; Foy, C.L. Interaction of Surfactants with Plant Cuticles. Weed Technol. 2000, 14, 807–813. [Google Scholar] [CrossRef]
- Wang, C.J.; Liu, Z.Q. Foliar uptake of pesticides-Present status and future challenge. Pestic. Biochem. Physiol. 2007, 87, 1–8. [Google Scholar] [CrossRef]
- Stock, D.; Holloway, P.J. Possible mechanisms for surfactant-induced foliar uptake of agrochemicals. Pest Manag. Sci. 1993, 38, 165–177. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, W.G. The effect of surfactants on the deliquescence of sodium chloride. J. Environ. Sci. Health Part A 2001, 36, 229–242. [Google Scholar] [CrossRef]
- Gaskin, R.E.; Steele, K.D.; Forster, W.A. Characterising plant surfaces for spray adhesion and retention. N. Z. Plant Prot. 2005, 58, 179–183. [Google Scholar]
- Zhang, C.; Zhao, X.; Lei, J.; Ma, Y.; Du, F. The wetting behavior of aqueous surfactant solutions on wheat (Triticum aestivum) leaf surfaces. Soft Matter 2017, 13, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.A.; Shadizadeh, S. Experimental and Theoretical Study of a New Plant Derived Surfactant Adsorption on Quartz Surface: Kinetic and Isotherm Methods. J. Dispers. Sci. Technol. 2015, 36, 441–452. [Google Scholar] [CrossRef]
- Bilaowas, E.; Hreczuch, W.; Szymanowski, J.; Trathnigg, B. Static and Dynamic Surface Tension of Ethoxylated Rapeseed Fatty Acid Methyl Esters. In Proceedings of the 5th World Conference on Detergents. Reiventing the Industry: Opportunities and Challenges, AOCS, Motreux, Switzerland, 13–17 October 2003; pp. 166–172. [Google Scholar]
- Peirce, C.A.E.; Priest, C.; McBeath, T.M.; McLaughlin, M.J. Uptake of phosphorus from surfactant solutions by wheat leaves: Spreading kinetics, wetted area, and drying time. Soft Matter 2016, 12, 209–218. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Han, F. The spreading and superspeading behavior of new glucosamide-based trisiloxane surfactants on hydrophobic foliage. Colloids Surf. A Physicochem. Eng. Asp. 2006, 276, 100–106. [Google Scholar] [CrossRef]
- Schreiber, L.; Skrabs, M.; Hartmann, K.D.; Diamantopoulos, P.; Simanova, E.; Santrucek, J. Effect of humidity on cuticular water permeability of isolated cuticular membranes and leaf disks. Planta 2001, 214, 274–282. [Google Scholar] [CrossRef]
- Buchholz, A. Characterization of the diffusion of non-electrolytes across plant cuticles: Properties of the lipophilic pathway. J. Exp. Bot. 2006, 57, 2501–2513. [Google Scholar] [CrossRef]
- Kirkwood, R.C. Recent developments in our understanding of the plant cuticle as a barrier to the foliar uptake of pesticides. Pestic. Sci. 1999, 55, 69–77. [Google Scholar] [CrossRef]
- Schönherr, J.; Baur, P.; Buchholz, A. Modelling foliar penetration: Its role in optimising pesticide delivery. In Pesticide Chemistry and Bioscience, the Food-Environment Challenge; Brooks, G.T., Roberts, T., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 134–151. [Google Scholar]
- Bukovac, M.J.; Petracek, P.D. Characterizing pesticide and surfactant penetration with isolated plant cuticles. Pest Manag. Sci. 1993, 37, 179–194. [Google Scholar] [CrossRef]
- Schreiber, L.; Schönherr, J. Uptake of organic chemicals in conifer needles: Surface adsorption and permeability of cuticles. Environ. Sci. Technol. 1992, 26, 153–159. [Google Scholar] [CrossRef]
- Schönherr, J.; Riederer, M. Desorption of chemicals from plant cuticles: Evidence for asymmetry. Arch. Environ. Contam. Toxicol. 1988, 17, 13–19. [Google Scholar] [CrossRef]
- Schönherr, J. Calcium chloride penetrates plant cuticles via aqueous pores. Planta 2000, 212, 112–118. [Google Scholar] [CrossRef]
- Schönherr, J. Cuticular penetration of calcium salts: Effects of humidity, anions, and adjuvants. J. Plant Nutr. Soil Sci. 2001, 164, 225–231. [Google Scholar] [CrossRef]
- Riederer, M.; Burghardt, M.; Mayer, S.; Obermeier, H.; Schoenherr, J. Sorption of Monodisperse Alcohol Ethoxylates and Their Effects on the Mobility of 2,4-D in Isolated Plant Cuticles. J. Agric. Food Chem. 1995, 43, 1067–1075. [Google Scholar] [CrossRef]
- Buchholz, A.; Schönherr, J. Thermodynamic analysis of diffusion of non-electrolytes across plant cuticles in the presence and absence of the plasticiser tributyl phosphate. Planta 2000, 212, 103–111. [Google Scholar] [CrossRef]
- Gauvrit, C.; Müller, T.; Milius, A.; Trouvé, G. Ethoxylated rapeseed oil derivatives as non-ionic adjuvants for glyphosate. Pest Manag. Sci. 2007, 63, 707–713. [Google Scholar] [CrossRef]
- Coret, J.M.; Chamel, A.R. Influence of some nonionic surfactants on water sorption by isolated tomato fruit cuticles in relation to cuticular penetration of glyphosate. Pestic. Sci. 1993, 38, 27–32. [Google Scholar] [CrossRef]
- Forster, W.A.; Zabkiewicz, J.A.; Riederer, M. Mechanisms of cuticular uptake of xenobiotics into living plants: 1. Influence of xenobiotic dose on the uptake of three model compounds applied in the absence and presence of surfactants into Chenopodium album, Hedera helix and Stephanotis floribunda lea. Pest Manag. Sci. 2004, 60, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Trapp, S. Plant uptake and transport models for neutral and ionic chemicals. Environ. Sci. Pollut. Res. Int. 2004, 11, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.A.V.; Ockrent, C. Studies in Electrocapillarity. III. J. Phys. Chem. 1929, 34, 2841–2859. [Google Scholar] [CrossRef]
- Markham, E.C.; Benton, A.F. The Adsorption of Gas Mixtures by Silica. J. Am. Chem. Soc. 1931, 53, 497–507. [Google Scholar] [CrossRef]
- Cameron, D.; Klute, A. Convective-dispersive solute transport with a combined equilibrium and kinetic adsorption model. Water Resour. Res. 1977, 13, 183–188. [Google Scholar] [CrossRef]
- Keymeulen, R.; Schamp, N.; Van Langenhove, H. Uptake of gaseous toluene in plant leaves: A two compartment model. Chemosphere 1995, 31, 3961–3975. [Google Scholar] [CrossRef]
- Trapp, S. Fruit Tree model for uptake of organic compounds from soil and air. SAR QSAR Environ. Res. 2007, 18, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Riederer, M.; Daiß, A.; Gilbert, N.; Köhle, H. Semi-volatile organic compounds at the leaf/atmosphere interface: Numerical simulation of dispersal and foliar uptake. J. Exp. Bot. 2002, 53, 1815–1823. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brazee, R.D.; Bukovac, M.J.; Zhu, H. Diffusion model for plant cuticular penetration by spray-applied weak organic acid bioregulator in presence or absence of ammonium nitratte. Am. Soc. Agric. Eng. 2004, 47, 629–636. [Google Scholar] [CrossRef]
- Schönherr, J.; Baur, P. Modelling penetration of plant cuticles by crop protection agents and effects of adjuvants on their rates of penetration. Pestic. Sci. 1994, 42, 185–208. [Google Scholar] [CrossRef]
- Legind, C.; Kennedy, C.; Rein, A.; Snyder, N.; Trapp, S. Dynamic plant uptake model applied for drip irrigation of an insecticide to pepper fruit plants. Pest Manag. Sci. 2011, 67, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Erbil, H.Y.; McHale, G.; Newton, M.I. Drop evaporation on solid surfaces: Constant contact angle mode. Langmuir 2002, 18, 2636–2641. [Google Scholar] [CrossRef]
- Chamel, A.; Pineri, M.; Escoubes, M. Quantitative determination of water sorption by plant cuticles. Plant Cell Environ. 1991, 14, 87–95. [Google Scholar] [CrossRef]
- Yuan-Hui, L.; Gregory, S. Diffusion of ions in sea water and in deep-sea sediments. Geochim. Cosmochim. Acta 1974, 38, 703–714. [Google Scholar] [CrossRef]
- Holz, M.; Heil, S.R.; Sacco, A. Temperature-dependent self-diffusion coefficients of water and six selected molecular liquids for calibration in accurate 1H NMR PFG measurements. Phys. Chem. Chem. Phys. 2000, 2, 4740–4742. [Google Scholar] [CrossRef]
- Semenov, S.; Trybala, A.; Agogo, H.; Kovalchuk, N.; Ortega, F.; Rubio, R.G.; Starov, V.M.; Velarde, M.G. Evaporation of droplets of surfactant solutions. Langmuir ACS J. Surf. Coll. 2013, 29, 10028–10036. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Nie, Y. Fractal scaling of effective diffusion coefficient of solute in porous media. J. Environ. Sci. 2001, 13, 170–172. [Google Scholar]
- Popov, Y.O. Evaporative deposition patterns: Spatial dimensions of the deposit. Phys. Rev. E 2005, 71, 036313. [Google Scholar] [CrossRef]
- Dash, S.; Garimella, S.V. Droplet evaporation dynamics on a superhydrophobic surface with negligible hysteresis. Langmuir 2013, 29, 10785–10795. [Google Scholar] [CrossRef]
- Anastopoulos, G.; Zannikou, Y.; Stournas, S.; Kalligeros, S. Transesterification of Vegetable Oils with Ethanol and Characterization of the Key Fuel Properties of Ethyl Esters. Energies 2009, 2, 362–376. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 84th ed.; CRC press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Kolthoff, I.M.; Sandell, E.B.; Meehan, E.; Bruckenstein, S. Quantitative Chemical Analysis; Macmillan: London, UK, 1969; Volume 826. [Google Scholar]
- Schreiber, L.; Schönherr, J. Water and Solute Permeability of Plant Cuticles: Measurement and Data Analysis; Springer: Berlin, Germany, 2009; Volume 616. [Google Scholar] [CrossRef]
- Oakes, C.S.; Simonson, J.M.; Bodnar, R.J. Apparent molar volumes of aqueous calcium chloride to 250 C, 400 bars, and from molalities of 0.242 to 6.150. J. Solut. Chem. 1995, 24, 897–916. [Google Scholar] [CrossRef]
- Zen, E. Partial molar volumes of some salts in aqueous solutions. Geochim. Cosmochim. Acta 1957, 12, 103–122. [Google Scholar] [CrossRef]
- Erbil, H.Y. Evaporation of pure liquid sessile and spherical suspended drops: A review. Adv. Coll. Interface Sci. 2012, 170, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Weast, R.; Lide, D. (Eds.) CRC Handbook of Chemistry and Physics, 70th ed.; CRC Press, Inc.: Boca Raton, FL, USA, 1989. [Google Scholar]
- Dow Chemical Company. Calcium Chloride Handbook. A Guide to Properties, Forms, Storage and Handling; Dow Chemical Company: Midland, MI, USA, 2003. [Google Scholar]
- Schönherr, J.; Luber, M. Cuticular penetration of potassium salts: Effects of humidity, anions, and temperature. Plant Soil 2001, 236, 117–122. [Google Scholar] [CrossRef]
- Kraemer, T.; Hunsche, M.; Noga, G. Selected Calcium Salt Formulations: Interactions between Spray Deposit Characteristics and Ca Penetration with Consequences for Rain-Induced Wash-Off. J. Plant Nutr. 2009, 32, 1718–1730. [Google Scholar] [CrossRef]
- Tredenick, E.C. Mathematical Modelling of Ionic Agrochemical Diffusion in Plant Cuticles: A Mechanistic Approach. Ph.D. Thesis, Queensland University of Technology, Brisbane City, QLD, Australia, 2019. [Google Scholar] [CrossRef]
- Shampine, L.F. Solving 0= f (t, y (t), y(t)) in Matlab. J. Numer. Math. 2002, 10, 291–310. [Google Scholar] [CrossRef]
- MATLAB. Version 9.3 (R2017b); The MathWorks Inc.: Natick, MA, USA, 2017. [Google Scholar]
- Baur, P. Lognormal distribution of water permeability and organic solute mobility in plant cuticles. Plant Cell Environ. 1997, 20, 167–177. [Google Scholar] [CrossRef]
- Laidler, K.J. The development of the Arrhenius equation. J. Chem. Edu. 1984, 61, 494. [Google Scholar] [CrossRef]
- Sattelmacher, B. The apoplast and its significance for plant mineral nutrition. New Phytol. 2001, 149, 167–192. [Google Scholar] [CrossRef]
- Liu, Z.; Gaskin, R.E. Visualisation of the uptake of two model xenobiotics into bean leaves by confocal laser scanning microscopy: Diffusion pathways and implication in phloem translocation. Pest Manag. Sci. 2004, 60, 434–439. [Google Scholar] [CrossRef]
- Riederer, M.; Schreiber, L. Waxes: The transport barriers of plant cuticles. In Waxes: Chemistry, Molecular Biology and Functions; Hamilton, R., Ed.; The Oily Press: Dundee, Scotland, UK, 1995; Volume 6, pp. 131–156. [Google Scholar]
- Schönherr, J.; Fernández, V.; Schreiber, L. Rates of cuticular penetration of chelated Fe-III: Role of humidity, concentration, adjuvants, temperature, and type of chelate. J. Agric. Food Chem. 2005, 53, 4484–4492. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, T.K.; Schönherr, J.; Schreiber, L. Rates of foliar penetration of chelated Fe (III): Role of light, stomata, species, and leaf age. J. Agric. Food Chem. 2006, 54, 6809–6813. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Definition | Value and Units | Comments |
|---|---|---|---|
| Control volume area | m | ||
| Drop surface contact area | m | Surface contact area of drop on cuticle surface | |
| Initial drop surface contact area | m | Surface contact area of drop on cuticle surface, [58] | |
| AI | Active ingredient | ||
| b | Thickness of cuticle | m | [59] |
| Concentration of AI in drop at | mol/m | [22] | |
| Concentration of adjuvant in drop at | 1 mol/m (1 g/L) | ||
| Pure water concentration at and | 55,409.78 mol/m | calculated | |
| Point of deliquescence concentration | mol/m | [13] | |
| CCR | Constant contact radius evaporation mode | ||
| CCA | Constant contact angle evaporation mode | ||
| Concentration of component i | mol/m | ||
| Self/bulk diffusion coefficient of AI | 7.93 ms | For CaCl, Ca diffuses the slowest, so Ca value is used, [60] | |
| Self/bulk diffusion coefficient of water | 2.299 ms | [61] | |
| Self/bulk diffusion coefficient of adjuvant | 7.93 ms | (fitted) | |
| Diffusivity of water in air | 2.4 ms | [62] | |
| Diffusivity of component i | ms | [63] | |
| Fractal scaling dimension | 1.15 (-) | (fitted) | |
| Functional variation of | [64] | ||
| Functional of | [64,65] | ||
| H | Relative humidity | 0.7 (70%) | [22] |
| HLB | Hydrophilic Lipophilic Balance | ||
| i | Component AI (CaCl), or ADJ (RSO 5) | ||
| Adsorption rate constant ionic AI | 4.2 m(s mol) | (fitted) | |
| Desorption rate constant ionic AI | 6.5 m(s mol) | (fitted) | |
| Adsorption rate constant adjuvant | 2 m(s mol) | (fitted) | |
| Desorption rate constant adjuvant | 5 m | (fitted) | |
| Kinetic rate constant ionic AI | 5.9 m | (fitted) | |
| Kinetic rate constant adjuvant | 8 m | (fitted) | |
| Kinetic rate constant ionic AI | mol | calculated | |
| Kinetic rate constant adjuvant | mol | calculated | |
| L | Control volume length | 1 m | |
| Molecular weight of CaCl2 | 110.98 g/mol | ||
| Molecular weight of RSO 5 | 992 g/mol | [66] | |
| Molecular weight HO | g/mol | ||
| Equilibrium mass of water absorbed per CaCl2 applied | gg | [13] | |
| Avogadro constant | mol | ||
| Number of aqueous pores on area (1 m) of cuticle | (-) | ||
| Saturated water vapour pressure in air at | 2338.8 Pa | [67] | |
| RH for CaCl2 [68] and RH for CaCl2 with RSO 5 | |||
| R | Gas constant | 8.3145 PamK/mol | |
| RSO | Rapeseed oil surfactant | ||
| Initial drop contact radius | m | Contact radius of drop on cuticle surface [58] | |
| Van der Waals radius of a water molecule | m | [16] | |
| Maximum radius of aqueous pores | m | For tomato fruit cuticle, ([69] p. 87) | |
| Relative humidity in text | |||
| t | Time | s | |
| T | Temperature | 293.15 K () | [22] |
| u | Bound integration variable | ||
| Volume of droplet at | 1 m | [22] | |
| Volume of water in droplet at time t | |||
| Deliquescent droplet volume | m | ||
| Partial molar volume CaCl | mmol | [70] | |
| Partial molar volume adjuvant | mmol | ||
| Partial molar volume water | mmol | [71] | |
| x | Length | m | |
| Aqueous pore pathway porosity | |||
| Lipophilic pathway porosity | 0.03 | (fitted) | |
| Concentration adsorbed ionic AI per droplet area on cuticle surface | mol/m | ||
| Concentration adsorbed adjuvant per droplet area on cuticle surface | mol/m | ||
| Saturated adsorbed molecules per droplet area | 400 mol/m | (fitted) | |
| Density of aqueous pores in cuticle | m | [13] | |
| Evaporation constants as a function of relative humidity | ms | ||
| Saturated water vapour concentration as a function of relative humidity | g/m | [72] | |
| Point of deliquescence humidity shifting factor for choosing the humidity in and | |||
| Liquid density HO at | g/m | [73] | |
| Liquid density of CaCl2 at | g/m | [74] | |
| Contact angle of drop on cuticle surface that changes with time | rads | ||
| Contact angle of drop on cuticle surface at | rads | See Table 1 in [13] | |
| Receding contact angle of drop on cuticle surface | rads | See Table 1 in [13] | |
| Logistic decay evaporation constant | 0.0428 Lg | [13] | |
| Logistic decay evaporation term (a constant) as a function of initial concentration of ionic AI | calculated | ||
| Point of deliquescence humidity shifting factor to incorporate with the addition of adjuvants | RH | [13] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tredenick, E.C.; Farrell, T.W.; Forster, W.A. Mathematical Modelling of Hydrophilic Ionic Fertiliser Diffusion in Plant Cuticles: Lipophilic Surfactant Effects. Plants 2019, 8, 202. https://doi.org/10.3390/plants8070202
Tredenick EC, Farrell TW, Forster WA. Mathematical Modelling of Hydrophilic Ionic Fertiliser Diffusion in Plant Cuticles: Lipophilic Surfactant Effects. Plants. 2019; 8(7):202. https://doi.org/10.3390/plants8070202
Chicago/Turabian StyleTredenick, Eloise C., Troy W. Farrell, and W. Alison Forster. 2019. "Mathematical Modelling of Hydrophilic Ionic Fertiliser Diffusion in Plant Cuticles: Lipophilic Surfactant Effects" Plants 8, no. 7: 202. https://doi.org/10.3390/plants8070202
APA StyleTredenick, E. C., Farrell, T. W., & Forster, W. A. (2019). Mathematical Modelling of Hydrophilic Ionic Fertiliser Diffusion in Plant Cuticles: Lipophilic Surfactant Effects. Plants, 8(7), 202. https://doi.org/10.3390/plants8070202





