Polyamine Oxidases Play Various Roles in Plant Development and Abiotic Stress Tolerance
Abstract
1. Introduction
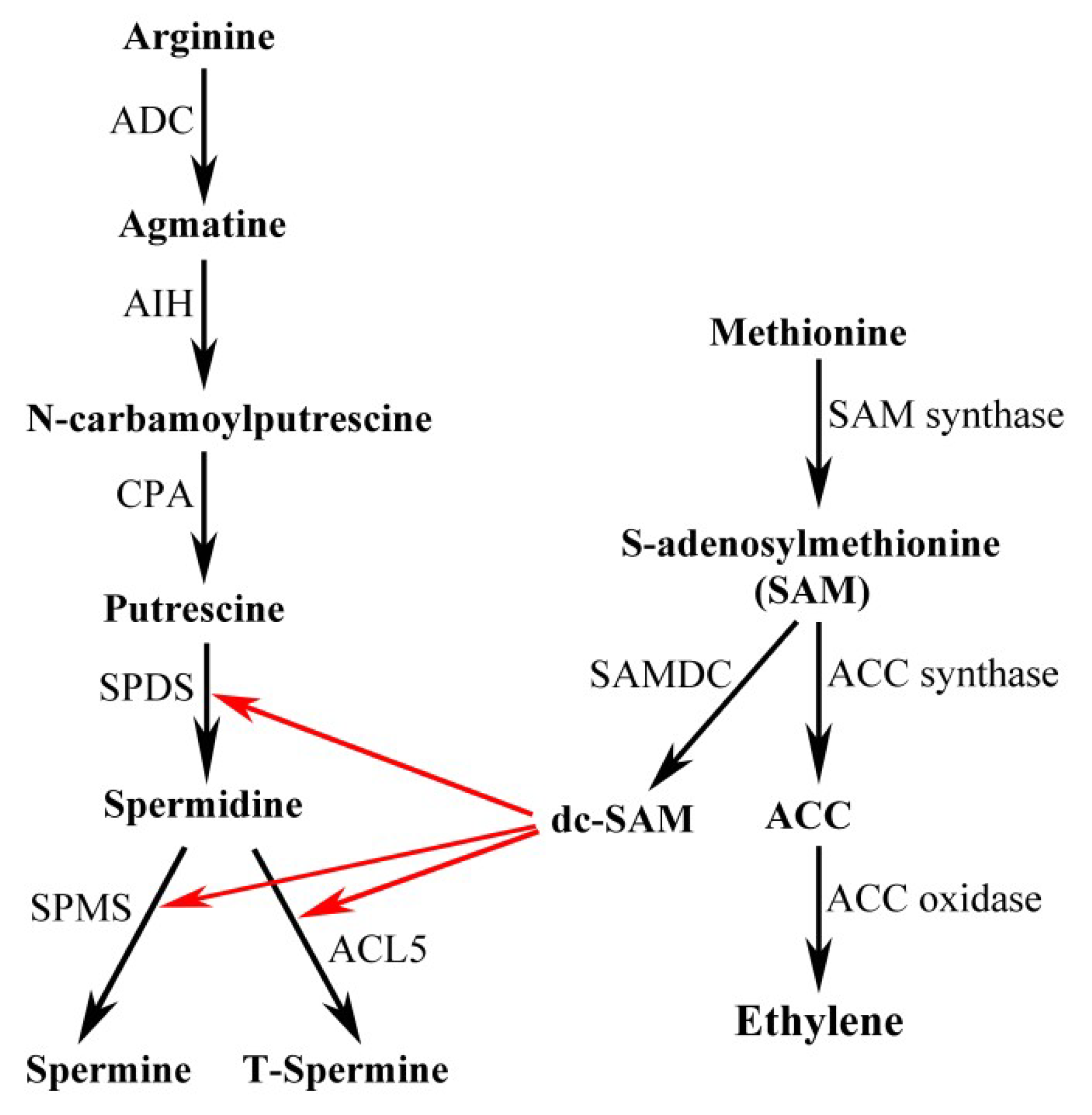
2. PA Biosynthesis in Plants
3. PA Catabolism in Plants
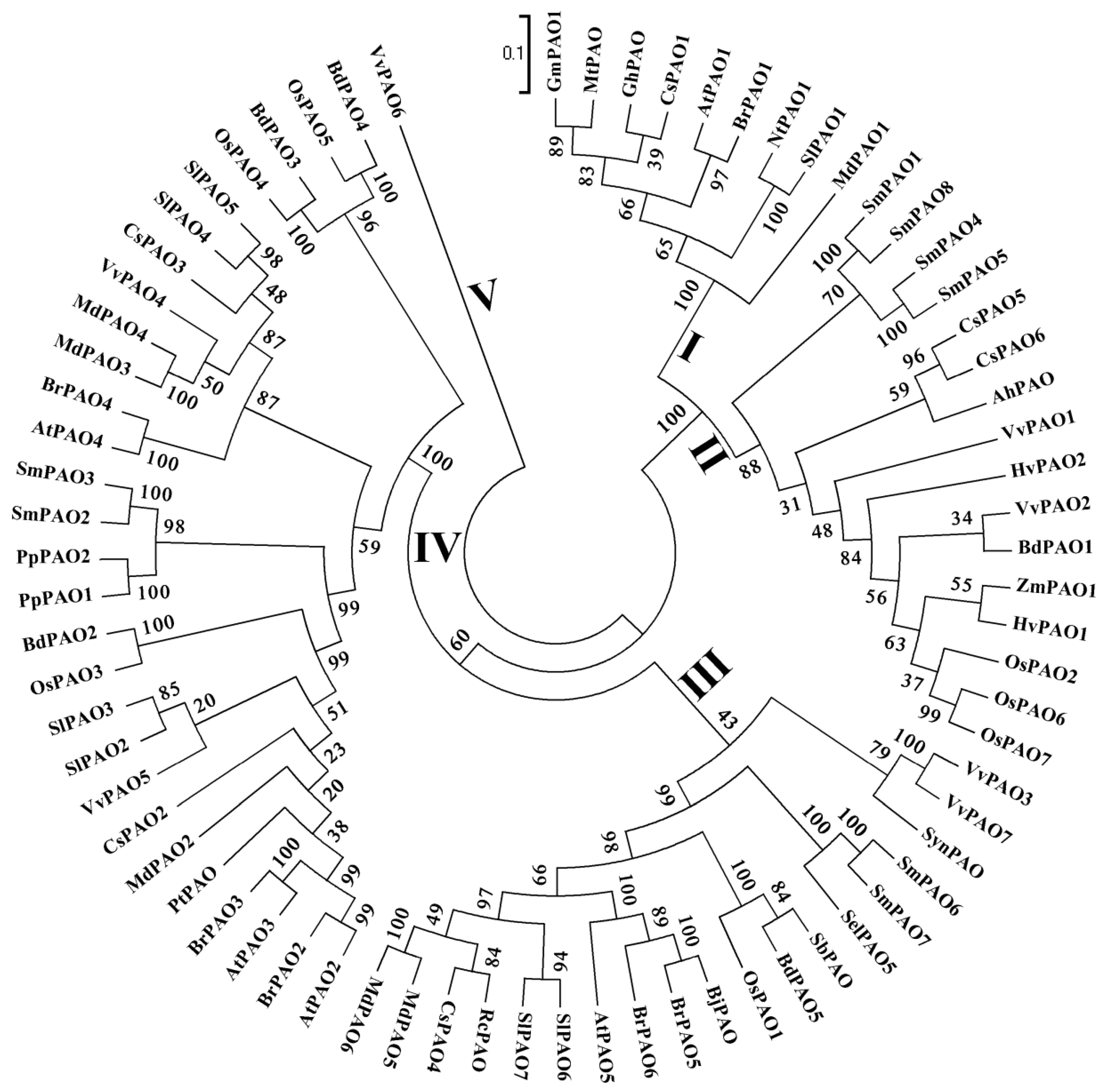
4. PAOs in Plants
4.1. Rice PAOs
4.2. Arabidopsis PAOs
4.3. Tomato PAOs
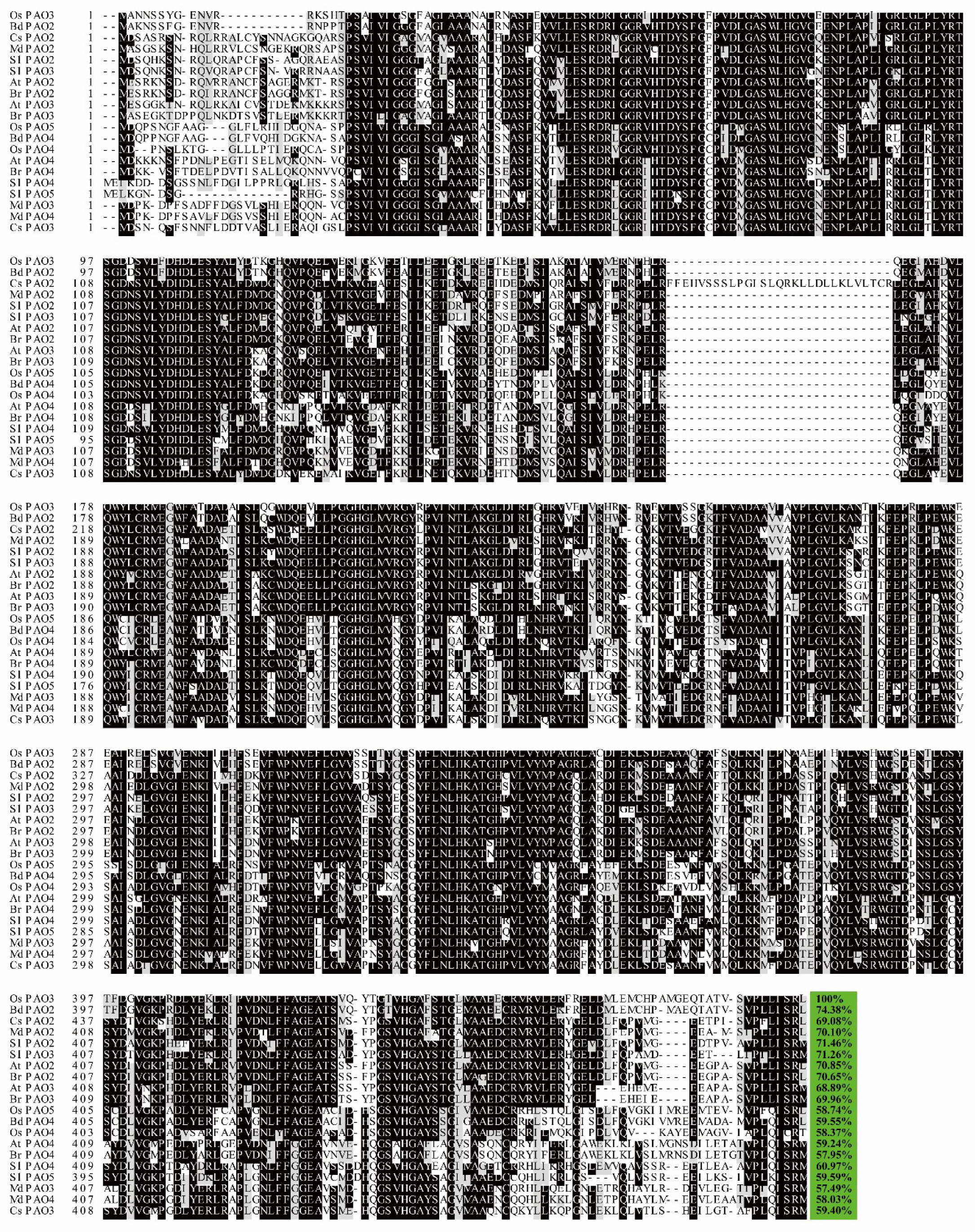
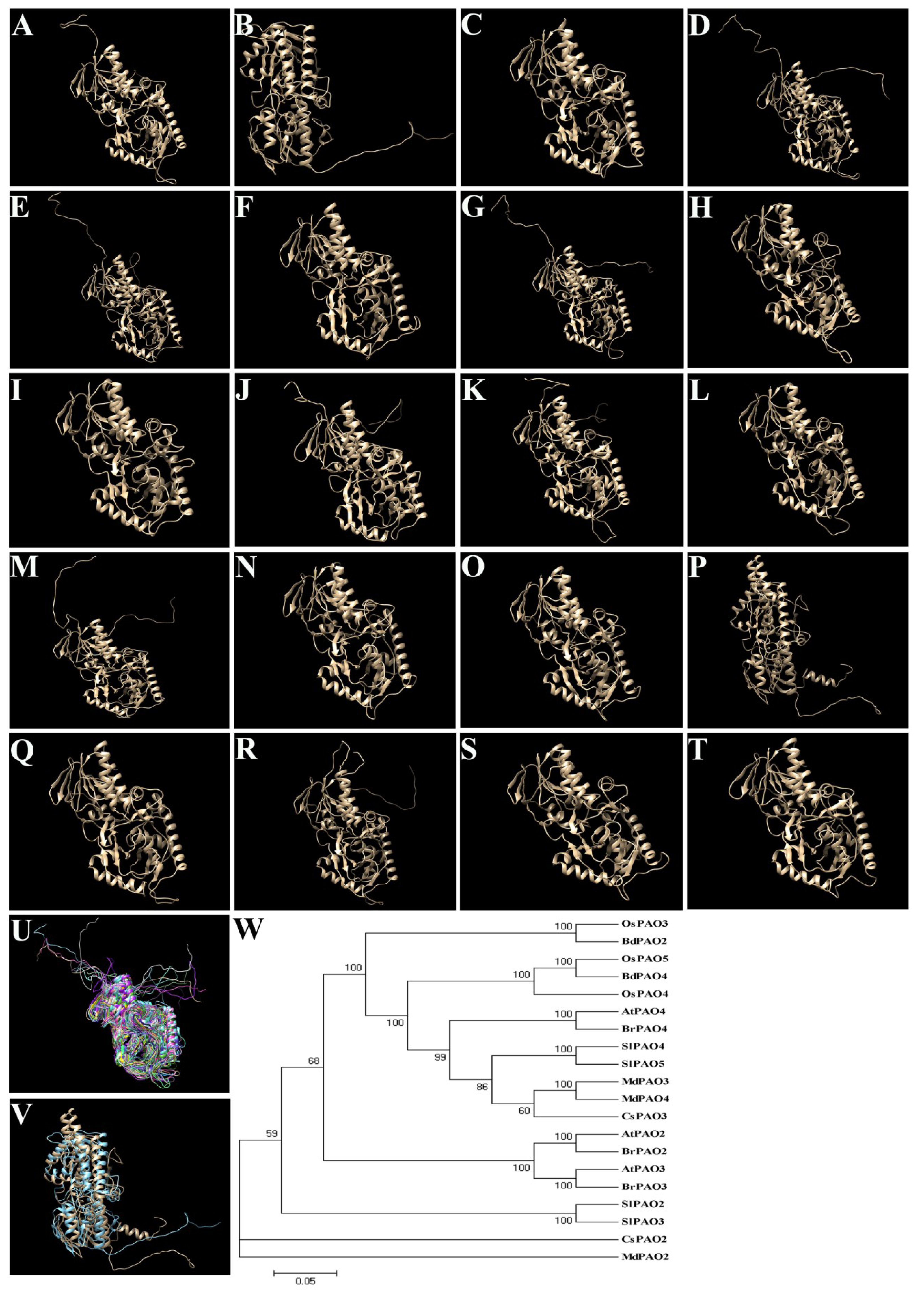
4.4. PAOs in Other Plants
4.5. Peroxisomal PAOs in Plants
5. Conclusions and Future Perspective of PAOs Research in Plants
Author Contributions
Funding
Conflicts of Interest
References
- Cohen, S.S. A Guide to the Polyamines; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Tabor, C.W.; Tabor, H. Polyamines. Annu. Rev. Plant. Biol. 1984, 53, 749–790. [Google Scholar] [CrossRef] [PubMed]
- Knott, J.M.; Römer, P.; Sumper, M. Putative spermine synthases from Thalassiosira pseudonana and Arabidopsis thaliana synthesize thermospermine rather than spermine. FEBS Lett. 2007, 581, 3081–3086. [Google Scholar] [CrossRef] [PubMed]
- Kusano, T.; Berberich, T.; Tateda, C.; Takahashi, Y. Polyamines, essential factors for growth and survival. Planta 2008, 228, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Handa, A.K.; Mattoo, A. Differential and functional interactions emphasize the multiple roles of polyamines in plants. Plant Physiol. Biochem. 2010, 48, 540–546. [Google Scholar] [CrossRef]
- Mattoo, A.K.; Minocha, S.C.; Minocha, R.; Handa, A.K. Polyamines and cellular metabolism in plants, transgenic approaches reveal different responses to diamine putrescine versus higher polyamines spermidine and spermine. Amino Acids 2010, 38, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Takano, A.; Kakehi, J.; Takahashi, T. Thermospermine is not a minor polyamine in the plant kingdom. Plant Cell Physiol. 2012, 53, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Hanzawa, Y.; Takahashi, T.; Komeda, Y. ACL5, an Arabidopsis gene required for internodal elongation after flowering. Plant J. 1997, 12, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Hanzawa, Y.; Takahashi, T.; Michael, A.J.; Burtin, D.; Long, D.; Pineiro, M.; Coupland, G.; Komeda, Y. ACAULIS5, an Arabidopsis gene required for stem elongation, encodes a spermine synthase. EMBO J. 2000, 19, 4248–4256. [Google Scholar] [CrossRef] [PubMed]
- Bouchereau, A.; Aziz, A.; Larher, F.; Martin, T.J. Polyamines and environmental challenges, recent development. Plant Sci. 1999, 140, 103–125. [Google Scholar] [CrossRef]
- Minocha, R.; Majumdar, R.; Minocha, S.C. Polyamines and abiotic stress in plants: A complex relationship. Front. Plant Sci. 2014, 5, 175. [Google Scholar] [CrossRef]
- Moschou, P.N.; Sanmartin, M.; Andriopoulou, A.H.; Rojo, E.; Sanchez-Serrano, J.J.; Roubelakis-Angelakis, K.A. Bridging the gap between plant and mammalian polyamine catabolism, a novel peroxisomal polyamine oxidase responsible for a full back-conversion pathway in Arabidopsis. Plant Physiol. 2008, 147, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Moschou, P.N.; Paschalidis, K.A.; Delis, I.D.; Andriopoulou, A.H.; Lagiotis, G.D.; Yakoumakis, D.I.; Roubelakis-Angelakis, K.A. Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plant Cell. 2008, 20, 1708–1724. [Google Scholar] [CrossRef] [PubMed]
- Moschou, P.N.; Roubelakis-Angelakis, K.A. Polyamines and programmed cell death. J. Exp. Bot. 2014, 65, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Kakehi, J.I.; Kuwashiro, Y.; Niitsu, M.; Takahashi, T. Thermospermine is required for stem elongation in Arabidopsis thaliana. Plant Cell Physiol. 2008, 49, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.A.; Maiale, S.J.; Menendez, A.B.; Ruiz, O.A. Polyamine oxidase activity contributes to sustain maize leaf elongation under saline stress. J. Exp. Bot. 2009, 60, 4249–4262. [Google Scholar] [CrossRef] [PubMed]
- Naka, Y.; Watanabe, K.; Sagor, G.H.M.; Niitsu, M.; Pillai, M.A.; Kusano, T.; Takahashi, Y. Quantitative analysis of plant polyamines including thermospermine during growth and salinity stress. Plant Physiol. Biochem. 2010, 48, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Groppa, M.D.; Benavides, M.P. Polyamines and abiotic stress, recent advances. Amino Acids 2008, 34, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Alcazar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines, molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Marina, M.; Sirera, F.V.; Rambla, J.L.; Gonzalez, M.E.; Blazquez, M.A.; Carbonell, J.; Pieckenstain, F.L.; Ruiz, O.A. Thermospermine catabolism increases Arabidopsis thaliana resistance to Pseudomonas viridiflava. J. Exp. Bot. 2013, 64, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Hatmi, S.; Gruau, C.; Trotel-Aziz, P.; Villaume, S.; Rabenoelina, F.; Baillieul, F.; Eullaffroy, P.; Clément, C.; Ferchichi, A.; Aziz, A. Drought stress tolerance in grapevine involves activation of polyamine oxidation contributing to improved immune response and low susceptibility to Botrytis cinerea. J. Exp. Bot. 2015, 66, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Sobieszczuk-Nowicka, E.; Aloisi, I.; Fattorini, L.; Serafini-Fracassini, D.; Del Duca, S. Polyamines are common players in different facets of plant programmed cell death. Amino Acids 2015, 47, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Wang, W.; Wu, H.; Gong, X.Q.; Moriguchi, T. Polyamines function in stress tolerance, from synthesis to regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-S.; Zhang, Q.; Zhu, D.; Fu, X.; Wang, M.; Zhang, Q.; Moriguchi, T.; Liu, J.-H. ICE1 of Poncirus trifoliata functions in cold tolerance by modulating polyamine levels by interacting with arginine decarboxylase. J. Exp. Bot. 2015, 68, 3259–3274. [Google Scholar] [CrossRef] [PubMed]
- Ebeed, H.T.; Hassan, N.M.; Aljarani, A.M. Exogenous applications of Polyamines modulate drought responses in wheat through osmolytes accumulation, increasing free polyamine levels and regulation of polyamine biosynthetic genes. Plant Physiol. Biochem. 2017, 118, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.B.; Li, Y.; Yin, Y.P.; Qin, Z.L.; Zheng, M.J.; Chen, J.; Luo, Y.L.; Pang, D.W.; Jiang, W.W.; Li, Y.; et al. Involvement of ethylene and polyamines biosynthesis and abdominal phloem tissues characters of wheat caryopsis during grain filling under stress conditions. Sci. Rep. 2017, 7, 46020. [Google Scholar] [CrossRef]
- Park, J.Y.; Kang, B.R.; Ryu, C.M.; Anderson, A.J.; Kim, Y.C. Polyamine is a critical determinant of Pseudomonas chlororaphis O6 for GacS-dependent bacterial cell growth and biocontrol capacity. Mol. Plant Pathol. 2018, 19, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Wuddineh, W.; Minocha, R.; Minocha, S.C. Polyamines in the Context of Metabolic Networks; Alcázar, R., Tiburcio, A., Eds.; Humana Press: New York, NY, USA, 2018; pp. 1–23. [Google Scholar]
- Gémes, K.; Mellidou, I.; Karamanoli, K.; Beris, D.; Park, K.Y.; Matsi, T.; Haralampidis, K.; Constantinidou, H.I.; Roubelakis-Angelakis, K.A. Deregulation of apoplastic polyamine oxidase affects development and salt response of tobacco plants. J. Plant Physiol. 2017, 211, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mellidou, I.; Karamanoli, K.; Beris, D.; Haralampidis, K.; Constantinidou, H.A.; Roubelakis-Angelakis, K.A. Underexpression of apoplastic polyamine oxidase improves thermotolerance in Nicotiana tabacum. J. Plant Physiol. 2017, 218, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-X.; Li, W.-Y.; Gao, Y.-T.; Chen, Z.-J.; Zhang, W.-N.; Liu, Q.-J.; Chen, Z. Involvement of polyamine oxidase-produced hydrogen peroxide during coleorhiza-limited germination of rice seeds. Front. Plant Sci. 2016, 7, 1219. [Google Scholar] [CrossRef] [PubMed]
- Sobieszczuk-Nowicka, E. Polyamine catabolism adds fuel to leaf senescence. Amino Acids 2017, 49, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhou, W.W.; Zhou, K.J.; Liu, W.J.; Liang, X.; Chen, Y.; Sun, D.; Lin, X. Polyamines modulate aluminum-induced oxidative stress differently by inducing or reducing H2O2 production in wheat. Chemosphere 2018, 212, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Bagni, N.; Tassoni, A. Biosynthesis, oxidation and conjugation of aliphatic polyamines in higher plants. Amino Acids 2001, 20, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.M.; Fraser, A.V.; Hughes, A. A perspective of polyamine metabolism. Biochem. J. 2003, 376, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Federico, R.; Cona, A.; Angelini, R.; Schininà, M.E.; Giartosio, A. Characterization of maize polyamine oxidase. Phytochemistry 1990, 29, 2411–2414. [Google Scholar] [CrossRef]
- Federico, R.; Ercolini, L.; Laurenzi, M.; Angelini, R. Oxidation of cetylpolyamines by maize polyamine oxidase. Phytochemistry 1996, 43, 339–341. [Google Scholar] [CrossRef]
- Tavladoraki, P.; Shinina, M.E.; Cecconi, F.; Di Agostino, S.; Manera, F.; Rea, G.; Mariottini, P.; Federico, R.; Angelini, R. Maize polyamine oxidase, primary structure from protein and cDNA sequencing. FEBS Lett. 1998, 426, 62–66. [Google Scholar] [CrossRef]
- Tavladoraki, P.; Rossi, M.N.; Saccuti, G.; Perez-Amador, M.A.; Polticelli, F.; Angelini, R.; Federico, R. Heterologous expression and biochemical characterization of a polyamine oxidase from Arabidopsis involved in polyamine back conversion. Plant Physiol. 2006, 141, 1519–1532. [Google Scholar] [CrossRef]
- Tavladoraki, P.; Cona, A.; Angelini, R. Copper-containing Amine oxidases and FAD-dependent polyamine oxidases are key players in plant tissue differentiation and organ development. Front. Plant Sci. 2016, 7, 824. [Google Scholar] [CrossRef]
- Radova, A.; Sebela, M.; Galuszka, P.; Frebort, I.; Jacobsen, S.; Faulhammer, H.G.; Pec, P. Barley polyamine oxidase, characterisation and analysis of the cofactor and the N-terminal amino acid sequence. Phytochem. Anal. 2001, 12, 166–173. [Google Scholar] [CrossRef]
- Cervelli, M.; Cona, A.; Angelini, R.; Polticelli, F.; Federico, R.; Mariottini, P. A barley polyamine oxidase isoform with distinct structural features and subcellular localization. Eur. J. Biochem. 2001, 268, 3816–3830. [Google Scholar] [CrossRef]
- Cervelli, M.; Polticelli, F.; Federico, R.; Mariottini, P. Heterologous expression and characterization of mouse spermine oxidase. J. Biol. Chem. 2003, 278, 5271–5276. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, M.; Di Caro, O.; Di Penta, A.; Angelini, R.; Federico, R.; Vitale, A.; Mariottini, P. A novel C-terminal sequence from barley polyamine oxidase is a vacuolar sorting signal. Plant J. 2004, 40, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Cervelli, M.; Bianchi, M.; Cona, A.; Crosatti, C.; Stanca, M.; Angelini, R.; Federico, R.; Mariottini, P. Barley polyamine oxidase isoforms 1 and 2, a peculiar case of gene duplication. FEBS J. 2006, 273, 3990–4002. [Google Scholar] [CrossRef] [PubMed]
- Kusano, T.; Kim, D.; Liu, T.; Berberich, T. Polyamine Catabolism in Plants; Springer: Tokyo, Japan, 2015. [Google Scholar]
- Ono, Y.; Kim, D.W.; Watanabe, K.; Sasaki, A.; Niitsu, M.; Berberich, T.; Kusano, T.; Takahashi, Y. Constitutively and highly expressed Oryza sativa polyamine oxidases localize in peroxisomes and catalyze polyamine back conversion. Amino Acids 2012, 42, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Kim, D.W.; Niitsu, M.; Berberich, T.; Kusano, T. Oryza sativa polyamine oxidase 1 back-converts tetraamines, spermine and thermospermine, to spermidine. Plant Cell Rep. 2014, 33, 143–151. [Google Scholar] [CrossRef]
- Liu, T.B.; Kim, D.W.; Niitsu, M.; Maeda, S.; Watanabe, M.; Kamio, Y.; Berberich, T.; Kusano, T. Polyamine oxidase 7 is a terminal catabolism-type enzyme in Oryza sativa and is specifically expressed in anthers. Plant Cell Physiol. 2014, 55, 1110–1122. [Google Scholar] [CrossRef]
- Liu, T.B.; Dobashi, H.; Kim, D.W.; Sagor, G.H.M.; Niitsu, M.; Berberich, T.; Kusano, T. Differential sensitivity to exogenous cadaverine of Arabidopsis mutants with defect in polyamine metabolic genes may be explained by spermine content in their plants. Physiol. Mol. Biol. Plants 2014, 20, 151–159. [Google Scholar] [CrossRef]
- Kim, D.W.; Watanabe, K.; Murayama, C.; Izawa, S.; Niitsu, M.; Michael, A.J.; Berberich, T.; Kusano, T. Polyamine oxidase 5 regulates Arabidopsis growth through thermospermine oxidase activity. Plant Physiol. 2014, 165, 1575–1590. [Google Scholar] [CrossRef]
- Mo, H.J.; Wang, X.F.; Zhang, Y.; Zhang, G.Y.; Zhang, J.F.; Ma, Z.Y. Cotton polyamine oxidase is required for spermine and camalexin signalling in the defence response to Verticillium dahlia. Plant J. 2015, 83, 962–975. [Google Scholar] [CrossRef]
- Sagor, G.H.M.; Inoue, M.; Kim, D.W.; Kojima, S.; Niitsu, M.; Berberich, T.; Kusano, T. The polyamine oxidase from lycophyte Selaginella lepidophylla (SelPAO5), unlike that of angiosperms, back-converts thermospermine to norspermidine. FEBS Lett. 2015, 589, 3071–3078. [Google Scholar] [CrossRef]
- Sagor, G.H.M.; Berberich, T.; Kojima, S.; Niitsu, M.; Kusano, T. Spermine modulates the expression of two probable polyamine transporter genes and determines growth responses to cadaverine in Arabidopsis. Plant Cell Rep. 2016, 35, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, J.-H. CsPAO4 of Citrus sinensis functions in polyamine terminal catabolism and inhibits plant growth under salt stress. Sci. Rep. 2016, 6, 31384. [Google Scholar] [CrossRef] [PubMed]
- Bordenave, C.D.; Mendoza, C.G.; Bremont, J.F.J.; Garriz, A.; Rodriguez, A.A. Defining novel plant polyamine oxidase subfamilies through molecular modeling and sequence analysis. BMC Evol. Biol. 2019, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.J.; Furze, J.M.; Rhodes, M.J.; Burtin, D. Molecular cloning and functional identification of a plant ornithine decarboxylase cDNA. Biochem. J. 1996, 314, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Hanzawa, Y.; Imai, A.; Michael, A.J.; Komeda, Y.; Takahashi, T. Characterization of the spermidine synthase-related gene family in Arabidopsis thaliana. FEBS Lett. 2002, 527, 176–180. [Google Scholar] [CrossRef]
- Sagor, G.H.M.; Liu, T.B.; Takahashi, H.; Niitsu, M.; Berberich, T.; Kusano, T. Longer uncommon polyamines have a stronger defense gene-induction activity and a higher suppressing activity of Cucumber mosaic virus multiplication compared to that of spermine in Arabidopsis thaliana. Plant Cell Rep. 2013, 32, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Sagor, G.H.M.; Kusano, T.; Berberich, T. Identification of the actual coding region for polyamine oxidase 6 from rice (OsPAO6) and its partial characterization. Plant Signal. Behav. 2017, 12, e1359456. [Google Scholar] [CrossRef] [PubMed]
- Fincato, P.; Moschou, P.N.; Spedaletti, V.; Tavazza, R.; Angelini, R.; Federico, R.; Roubelakis-Angelakis, K.A.; Tavladoraki, P. Functional diversity inside the Arabidopsis polyamine oxidase gene family. J. Exp. Bot. 2011, 62, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kakehi, J.I. Polyamines, ubiquitous polycations with unique roles in growth and stress responses. Ann. Bot Lond. 2010, 105, 1–6. [Google Scholar] [CrossRef]
- Hao, Y.; Huang, B.; Jia, D.; Mann, T.; Jiang, X.; Qiu, Y.; Niitsu, M.; Berberich, T.; Kusano, T.; Liu, T. Identification of seven polyamine oxidase genes in tomato (Solanum lycopersicum L.) and their expression profiles under physiological and various stress conditions. J. Plant Physiol. 2018, 228, 1–11. [Google Scholar] [CrossRef]
- Kamada-Nobusada, T.; Hayashi, M.; Fukazawa, M.; Sakakibara, H.; Nishimura, M. A putative peroxisomal polyamine oxidase, AtPAO4, is involved in polyamine catabolism in Arabidopsis thaliana. Plant Cell Physiol. 2008, 49, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Fincato, P.; Moschou, P.N.; Ahou, A.; Angelini, R.; Roubelakis-Angelakis, K.A.; Federico, R.; Tavladoraki, P. The members of Arabidopsis thaliana PAO gene family exhibit distinct tissue and organ-specific expression pattern during seedling growth and flower development. Amino Acids 2012, 42, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Sequera-Mutiozabal, M.I.; Erban, A.; Kopka, J.; Atanasov, K.E.; Bastida, J.; Fotopoulos, V.; Alcázar, R.; Tiburcio, A.F. Global metabolic profiling of Arabidopsis polyamine oxidase 4 (AtPAO4) loss-of-function mutants exhibiting delayed dark-induced senescence. Front. Plant Sci. 2016, 7, 173. [Google Scholar] [CrossRef] [PubMed]
- Zarza, X.; Atanasov, K.E.; Marco, F.; Arbona, V.; Carrasco, P.; Kopka, J.; Fotopoulos, V.; Munnik, T.; Gómez-Cadenas, A.; Tiburcio, A.F.; et al. Polyamine oxidase 5 loss-of-function mutations in Arabidopsis thaliana trigger metabolic and transcriptional reprogramming and promote salt stress tolerance. Plant Cell Environ. 2017, 40, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Alabdallah, O.; Ahou, A.; Mancuso, N.; Pompili, V.; Macone, A.; Pashkoulov, D.; Stano, P.; Cona, A.; Angelini, R.; Tavladoraki, P. The Arabidopsis polyamine oxidase/dehydrogenase 5 interferes with cytokinin and auxin signaling pathways to control xylem differentiation. J. Exp. Bot. 2017, 68, 997–1012. [Google Scholar] [CrossRef] [PubMed]
- Ahou, A.; Martignago, D.; Alabdallah, O.; Tavazza, R.; Stano, P.; Macone, A.; Rambla, J.L.; Vera-Sirera, F.; Angelini, R.; Federico, R.; et al. A plant spermine oxidase/dehydrogenase regulated by the proteasome and polyamines. J. Exp. Bot. 2014, 65, 1585–1603. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Ono, K.; Akamine, Y.; Asano, T.; Ezaki, M.; Mouri, I. Highly-expressed polyamine oxidases catalyze polyamine back conversion in Brachypodium distachyon. J. Plant Res. 2018, 131, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, J.H. Genome-wide identification and expression analysis of the polyamine oxidase gene family in sweet orange (Citrus sinensis). Gene 2015, 555, 421–429. [Google Scholar] [CrossRef]
- Clay, N.K.; Nelson, T. Arabidopsis thickvein mutation affects vein thickness and organ vascularization, and resides in a provascular cell-specific spermine synthase involved in vein definition and in polar auxin transport. Plant Physiol. 2005, 138, 767–777. [Google Scholar] [CrossRef]
- Kakehi, J.; Kuwashiro, Y.; Motose, H.; Igarashi, K.; Takahashi, T. Norspermine substitutes for thermospermine in the control of stem elongation in Arabidopsis thaliana. FEBS Lett. 2010, 584, 3042–3046. [Google Scholar] [CrossRef]
- Tsaniklidis, G.; Kotsiras, A.; Tsafouros, A.; Roussos, P.A.; Aivalakis, G.; Katinakis, P.; Delis, C. Spatial and temporal distribution of genes involved in polyamine metabolism during tomato fruit development. Plant Physiol. Biochem. 2016, 100, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Tan, J.; Zhuo, C.; Wang, C.; Xiang, B.; Wang, Z. Abscisic acid, H2O2 and nitric oxide interactions mediated cold-induced S-adenosylmethionine synthetase in Medicgo sativa subsp. falcata that confers cold tolerance through up-regulating polyamine oxidation. Plant Biotechnol. J. 2014, 12, 601–612. [Google Scholar] [PubMed]
- Zhuo, C.; Liang, L.; Zhao, Y.; Guo, Z.; Lu, S. A cold responsive ethylene responsive factor from Medicago falcata confers cold tolerance by up-regulation of polyamine turnover, antioxidant protection, and proline accumulation. Plant Cell Environ. 2018, 41, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Diao, Q.; Song, Y.; Shi, D.; Qi, H. Interaction of polyamines, abscisic acid, nitric oxide, and hydrogen peroxide under chilling stress in tomato (Lycopersicon esculentum Mill.) seedlings. Front. Plant Sci. 2017, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Parvin, S.; Lee, O.R.; Sathiyaraj, G.; Khorolragchaa, A.; Kim, Y.J.; Yang, D.C. Spermidine alleviates the growth of saline-stressed ginseng seedlings through antioxidative defense system. Gene 2014, 537, 70–78. [Google Scholar] [CrossRef]
- Recalde, L.; Vázquez, A.; Groppa, M.D.; Benavides, M.P. Reactive oxygen species and nitric oxide are involved in polyamine-induced growth inhibition in wheat plants. Protoplasma 2018, 255, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Takács, Z.; Poór, P.; Tari, I. Comparison of polyamine metabolism in tomato plants exposed to different concentrations of salicylic acid under light or dark conditions. Plant Physiol. Biochem. 2016, 108, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, R.; Bertea, C.M.; Foti, M.; Narayana, R.; Arimura, G.; Muroi, A.; Horiuchi, J.; Nishioka, T.; Maffei, M.E.; Takabayashi, J. Exogenous polyamines elicit herbivore-induced volatiles in lima bean leaves: Involvement of calcium, H2O2 and jasmonic acid. Plant Cell Physiol. 2009, 50, 2183–2199. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, X.; Yang, K.; Shi, Z.; Wang, N.; Yang, L.; Chen, J. Characterization, expression, and functional analysis of polyamine oxidases and their role in selenium-induced hydrogen peroxide production in Brassica rapa. J. Sci. Food Agric. 2019, 99, 4082–4093. [Google Scholar] [CrossRef]
- Dong, Q.; Magwanga, R.O.; Cai, X.; Lu, P.; Nyangasi Kirungu, J.; Zhou, Z.; Wang, X.; Wang, X.; Xu, Y.; Hou, Y.; et al. RNA-sequencing, physiological and RNAi analyses provide insights into the response mechanism of the ABC-mediated resistance to Verticillium dahliae infection in cotton. Genes 2019, 10, 110. [Google Scholar]
- Jasso-Robles, F.I.; Jiménez-Bremont, J.F.; Becerra-Flora, A.; Juárez-Montiel, M.; Gonzalez, M.E.; Pieckenstain, F.L.; García de la Cruz, R.F.; Rodríguez-Kessler, M. Inhibition of polyamine oxidase activity affects tumor development during the maize-Ustilago maydis interaction. Plant Physiol. Biochem. 2016, 102, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Lulai, E.C.; Neubauer, J.D.; Olson, L.L.; Suttle, J.C. Wounding induces changes in tuber polyamine content, polyamine metabolic gene expression, and enzyme activity during closing layer formation and initiation of wound periderm formation. J. Plant Physiol. 2015, 176, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, C.; Veeranagamallaiah, G.; Nareshkumar, A.; Sudhakarbabu, O.; Sivakumar, M.; Pandurangaiah, M.; Kiranmai, K.; Lokesh, U. Polyamine metabolism influences antioxidant defense mechanism in foxtail millet (Setaria italica L.) cultivars with different salinity tolerance. Plant Cell Rep. 2015, 34, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xu, Y.; Wang, J.; Wang, Z.; Yang, J.; Zhang, J. Polyamines and ethylene interact in rice grains in response to soil drying during grain filling. J. Exp. Bot. 2013, 64, 2523–2538. [Google Scholar] [CrossRef] [PubMed]
- Polticelli, F.; Salvi, D.; Mariottini, P.; Amendola, R.; Cervelli, M. Molecular evolution of the polyamine oxidase gene family in Metazoa. BMC Evol. Biol. 2012, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.Z.; Chen, C.W.; Wang, Y.; Liu, J.H.; Moriguchi, T. Ectopic expression of MdSPDS1 in sweet orange (Citrus sinensis Osbeck) reduces canker susceptibility: Involvement of H₂O₂ production and transcriptional alteration. BMC Plant Biol. 2011, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Angelini, R.; Cona, A.; Federico, R.; Fincato, P.; Tavladoraki, P.; Tisi, A. Plant amine oxidases “on the move”: An update. Plant Physiol. Biochem. 2010, 48, 560–564. [Google Scholar] [CrossRef]
- Xue, B.; Zhang, A.; Jiang, M. Involvement of polyamine oxidase in abscisic acid-induced cytosolic antioxidant defense in leaves of maize. J. Integr. Plant Biol. 2009, 51, 225–234. [Google Scholar] [CrossRef]
- Moschou, P.N.; Sarris, P.F.; Skandalis, N.; Andriopoulou, A.H.; Paschalidis, K.A.; Panopoulos, N.J.; Roubelakis-Angelakis, K.A. Engineered polyamine catabolism preinduces tolerance of tobacco to bacteria and oomycetes. Plant Physiol. 2009, 149, 1970–1981. [Google Scholar] [CrossRef]
- Moschou, P.N.; Delis, I.D.; Paschalidis, K.A.; Roubelakis-Angelakis, K.A. Transgenic tobacco plants overexpressing polyamine oxidase are not able to cope with oxidative burst generated by abiotic factors. Physiol. Plant 2008, 133, 140–156. [Google Scholar] [CrossRef]
- Yoda, H.; Hiroi, Y.; Sano, H. Polyamine oxidase is one of the key elements for oxidative burst to induce programmed cell death in tobacco cultured cells. Plant Physiol. 2006, 142, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Yoda, H.; Yamaguchi, Y.; Sano, H. Induction of hypersensitive cell death by hydrogen peroxide produced through polyamine degradation in tobacco plants. Plant Physiol. 2003, 132, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- Cona, A.; Cenci, F.; Cervelli, M.; Federico, R.; Mariottini, P.; Moreno, S.; Angelini, R. Polyamine oxidase, a hydrogen peroxide-producing enzyme, is up-regulated by light and down-regulated by auxin in the outer tissues of the maize mesocotyl. Plant Physiol. 2003, 131, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Hatmi, S.; Trotel-Aziz, P.; Villaume, S.; Couderchet, M.; Clément, C.; Aziz, A. Osmotic stress-induced polyamine oxidation mediates defence responses and reduces stress-enhanced grapevine susceptibility to Botrytis cinerea. J. Exp. Bot. 2014, 65, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, I.; Cai, G.; Serafini-Fracassini, D.; Del Duca, S. Polyamines in pollen: From microsporogenesis to fertilization. Front. Plant Sci. 2016, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Cvikrová, M.; Gemperlová, L.; Martincová, O.; Vanková, R. Effect of drought and combined drought and heat stress on polyamine metabolism in proline-over-producing tobacco plants. Plant Physiol. Biochem. 2013, 73, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Cvikrová, M.; Gemperlová, L.; Dobrá, J.; Martincová, O.; Prásil, I.T.; Gubis, J.; Vanková, R. Effect of heat stress on polyamine metabolism in proline-over-producing tobacco plants. Plant Sci. 2012, 182, 49–58. [Google Scholar] [CrossRef]
- Filippou, P.; Antoniou, C.; Fotopoulos, V. The nitric oxide donor sodium nitroprusside regulates polyamine and proline metabolism in leaves of Medicago truncatula plants. Free Radic. Biol. Med. 2013, 56, 172–183. [Google Scholar] [CrossRef]
- Xiong, H.; Guo, H.; Xie, Y.; Zhao, L.; Gu, J.; Zhao, S.; Li, J.; Liu, L. RNAseq analysis reveals pathways and candidate genes associated with salinity tolerance in a spaceflight-induced wheat mutant. Sci. Rep. 2017, 7, 2731. [Google Scholar] [CrossRef]
- Sagor, G.H.M.; Kusano, T.; Berberich, T. Polyamine oxidase from Selaginella lepidophylla (SelPAO5) can replace AtPAO5 in Arabidopsis through converting thermospermine to norspermidine instead to spermidine. Plants 2019, 8, 99. [Google Scholar] [CrossRef]
- Brikis, C.J.; Zarei, A.; Chiu, G.Z.; Deyman, K.L.; Liu, J.; Trobacher, C.P.; Hoover, G.J.; Subedi, S.; DeEll, J.R.; Bozzo, G.G.; et al. Targeted quantitative profiling of metabolites and gene transcripts associated with 4-aminobutyrate (GABA) in apple fruit stored under multiple abiotic stresses. Hortic. Res. 2018, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shi, G.; Jia, R. Changes of polyamine levels in roots of Sagittaria sagittifolia L. under copper stress. Environ. Sci. Pollut. Res. Int. 2012, 19, 2973–2982. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shi, G.; Wang, H.; Xu, Q. Involvement of polyamines in adaptation of Potamogeton crispus L. to cadmium stress. Aquat. Toxicol. 2010, 100, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Cona, A.; Moreno, S.; Cenci, F.; Federico, R.; Angelini, R. Cellular re-distribution of flavin-containing polyamine oxidase in differentiating root and mesocotyl of Zea mays L. seedlings. Planta 2005, 221, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Rea, G.; de Pinto, M.C.; Tavazza, R.; Biondi, S.; Gobbi, V.; Ferrante, P.; De Gara, L.; Federico, R.; Angelini, R.; Tavladoraki, P. Ectopic expression of maize polyamine oxidase and pea copper amine oxidase in the cell wall of tobacco plants. Plant Physiol. 2004, 134, 1414–1426. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Jimenez, M.C.; Paredes, M.A.; Gallardo, M.; Sanchez-Calle, I.M. Mature fruit abscission is associated with up-regulation of polyamine metabolism in the olive abscission zone. J. Plant Physiol. 2010, 167, 1432–1441. [Google Scholar] [CrossRef]
- Paschalidis, K.A.; Roubelakis-Angelakis, K.A. Sites and regulation of polyamine catabolism in the tobacco plant. Correlations with cell division/expansion, cell cycle progression, and vascular development. Plant Physiol. 2005, 138, 2174–2184. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shang, Z.; Wu, J.; Jiang, X.; Moschou, P.N.; Sun, W.; Roubelakis-Angelakis, K.A.; Zhang, S. Spermidine oxidase-derived H₂O₂ regulates pollen plasma membrane hyperpolarization-activated Ca2+ -permeable channels and pollen tube growth. Plant J. 2010, 63, 1042–1053. [Google Scholar] [CrossRef]
- Agudelo-Romero, P.; Bortolloti, C.; Pais, M.S.; Tiburcio, A.F.; Fortes, A.M. Study of polyamines during grape ripening indicate an important role of polyamine catabolism. Plant Physiol. Biochem. 2013, 67, 105–119. [Google Scholar] [CrossRef]
- Planas-Portell, J.; Gallart, M.; Tiburcio, A.F.; Altabella, T. Copper-containing amine oxidases contribute to terminal polyamine oxidation in peroxisomes and apoplast of Arabidopsis thaliana. BMC Plant Biol. 2013, 13, 109. [Google Scholar] [CrossRef]

| Gene Name | Accession No. | Gene Name | Accession No. | Gene Name | Accession No. | Gene Name | Accession No. |
|---|---|---|---|---|---|---|---|
| OsPAO1 | NM_001050573 | BdPAO1 | XM_003573843 | SmPAO3 | XP_002968082.1 | PpPAO2 | XM_001776435 |
| OsPAO2 | NM_001055782 | BdPAO2 | XM_010242147 | SmPAO4 | XP_002969966.1 | RcPAO | XM_002521542 |
| OsPAO3 | NM_001060458 | BdPAO3 | XM_003580746 | SmPAO5 | XP_002981437.1 | PtPAO | XM_002306729 |
| OsPAO4 | NM_001060753 | BdPAO4 | XM_003580747 | SmPAO6 | XP_002984796.1 | SbPAO | XM_002448510 |
| OsPAO5 | NM_001060754 | BdPAO5 | XM_003566997 | SmPAO7 | XP_002985859.1 | GmPAO1 | XP_003535841.1 |
| OsPAO6 | XM_015755533 | BrPAO1 | Bra006210 | SmPAO8 | XP_002986593.1 | MtPAO | XP_003599417.1 |
| OsPAO7 | NM_001069546 | BrPAO2 | Bra037741 | VvPAO1 | VIT_01s0127g00750 | SynPAO | WP_011153630.1 |
| AtPAO1 | NM_121373 | BrPAO3 | Bra003362 | VvPAO2 | VIT_01s0127g00800 | PpPAO1 | XM_001756812 |
| AtPAO2 | AF364952 | BrPAO4 | Bra039742 | VvPAO3 | VIT_03s0017g01000 | ZmPAO1 | NM_001111636 |
| AtPAO3 | AY143905 | BrPAO5 | Bra011132 | VvPAO4 | VIT_04s0043g00220 | AhPAO | AAM43922.1 |
| AtPAO4 | AF364953 | BrPAO6 | Bra024137 | VvPAO5 | VIT_12s0028g01120 | GhPAO | KC762210.1 |
| AtPAO5 | AK118203 | CsPAO1 | Cs7g02060.1 | VvPAO6 | VIT_12s0055g00480 | HvPAO1 | AJ298131 |
| SlPAO1 | XP_004229651 | CsPAO2 | Cs7g18840.2 | VvPAO7 | VIT_13s0019g04820 | HvPAO2 | AJ298132 |
| SlPAO2 | XP_004243630 | CsPAO3 | Cs6g15870.1 | MdPAO1 | ANJ77637.1 | SelPAO5 | LC036642 |
| SlPAO3 | XP_004251556 | CsPAO4 | Cs4g14150.1 | MdPAO2 | ANJ77639.1 | NtPAO | AB200262 |
| SlPAO4 | XP_004232664 | CsPAO5 | Cs7g23790.1 | MdPAO3 | ANJ77642.1 | BjPAO | AY188087 |
| SlPAO5 | XP_004234492 | CsPAO6 | Cs7g23760.1 | MdPAO4 | ANJ77638.1 | ||
| SlPAO6 | XP_004243758 | SmPAO1 | XP_002965265.1 | MdPAO5 | ANJ77640.1 | ||
| SlPAO7 | XP_004239292 | SmPAO2 | XP_002965599.1 | MdPAO6 | ANJ77641.1 |
| Gene Name | Gene ID | Subcellular Localization | Substrate Specificity | Mode of Reaction | Tissue Expression | Functions (or Potential Functions) | Reference |
|---|---|---|---|---|---|---|---|
| Oryza sativa | |||||||
| OsPAO1 | Os01g0710200 | cytoplasm | Spm, T-Spm | BC | rachis | rachis development, tolerances, seed germination | [31,46,47,48] |
| OsPAO2 | Os03g0193400 | n.d. | n.d. | n.d. | root (with very low expression levels) | tolerances, seed germination | [31,46,49] |
| OsPAO3 | Os04g0623300 | peroxisome | Spd, Spm, T-Spm | BC | All stages. Strongest expressed in leaf, rachis, node, lower leaf blade, mature floral organ | leaf and node development, floral development, fertility, seed germination | [31,46,47] |
| OsPAO4 | Os04g0671200 | peroxisome | Spm, T-Spm | BC | rachis, mature floral organ | rachis and floral development, fertility, seed germination | [31,46,47] |
| OsPAO5 | Os04g0671300 | peroxisome | Spm, T-Spm | BC | flag leaf, lower leaf blade, leaf sheath, mature floral organ | development of leaf and flower, fertility, seed germination | [31,46,47] |
| OsPAO6 | Os09g0368200 | apoplast | n.d. | TC (?) | expressed at negligible levels | tolerances, seed germination | [31,46,60] |
| OsPAO7 | Os09g0368500 | apoplast | Spm, Spd | TC | anther, pollen | floral development, fertility, seed germination | [31,46,49] |
| Arabidopsis thaliana | |||||||
| AtPAO1 | At5g13700 | cytoplasm | Spm, T-Spm | BC | root transition region, anther | stress tolerance, root development, fertility | [39,46,61,62,65] |
| AtPAO2 | At2g43020 | peroxisome | Spd, Spm, T-Spm | BC | root meristem, anther, main vein of rosette leaf | root development, fertility, vein development of leaf | [46,61,62,64,65] |
| AtPAO3 | At3g59050 | peroxisome | Spd, Spm, T-Spm | BC | All stages. Strongest expressed in root tip, flower, guard cell | root and leaf development, fertility | [12,46,61,62,65] |
| AtPAO4 | At1g65840 | peroxisome | Spm, T-Spm | BC | All stages. Strongest expressed in root and floral organ | Delay dark-induced senescence. Root development, fertility | [46,61,62,64,65,66] |
| AtPAO5 | At4g29720 | cytoplasm | Spm, T-Spm | BC | All stages. Strongest expressed in mature leaf, vascular tissue, flower, stem | xylem differentiation, stem elongation, development of rosette leaves and vein, tolerance | [46,51,61,62,65,67,68,69] |
| Solanum lycopersicum | |||||||
| SlPAO1 | Solyc01g087590 | n.d. | n.d. | n.d. | root, stem, leaf of seedling stage | vegetative growth | [63] |
| SlPAO2 | Solyc07g043590 | peroxisome (?) | n.d. | n.d. | All stages. Strongest expressed in anther, Br, Br+2, stem | floral development, fruit maturity | [63] |
| SlPAO3 | Solyc12g006370 | peroxisome (?) | n.d. | n.d. | All stages. Strongest expressed in anther, Br, Br+2, leaf | floral development, fruit maturity | [63] |
| SlPAO4 | Solyc02g081390 | peroxisome (?) | n.d. | n.d. | All stages. Strongest expressed in anther, Br, Br+2, Br+7, root, leaf | floral development, fruit maturity | [63] |
| SlPAO5 | Solyc03g031880 | peroxisome (?) | n.d. | n.d. | All stages. Strongest expressed in anther, leaf, stem | floral development | [63] |
| SlPAO6 | Solyc07g039310 | n.d. | n.d. | n.d. | root, stem of seedling stage | vegetative growth | [63] |
| SlPAO7 | Solyc05g018880 | n.d. | n.d. | n.d. | root, stem of seedling stage | vegetative growth | [63] |
| Brachypodium distachyon | |||||||
| BdPAO1 | XM_003573843 | n.d. | n.d. | n.d. | expressed at very low levels | unknown | [70] |
| BdPAO2 | XM_010242147 | peroxisome (?) | Spd, Spm, T-Spm, Nor-Spm, Nor-Spd | BC | All stages. Highly expressed in leaf, stem, and inflorescence | development of stem and inflorescence | [70] |
| BdPAO3 | XM_003580746 | n.d. | Spm, | BC | leaf, stem, and inflorescence | development of stem and inflorescence | [70] |
| BdPAO4 | XM_003580747 | peroxisome (?) | n.d. | n.d. | leaf, stem, and inflorescence | development of stem and inflorescence | [70] |
| BdPAO5 | XM_003566997 | n.d. | n.d. | n.d. | expressed at very low levels | unknown | [70] |
| Citrus sinensis | |||||||
| CsPAO1 | Cs7g02060.1 | n.d. | n.d. | BC (?) | leaf, stem, root, cotyledon | root growth, vegetative growth | [55,71] |
| CsPAO2 | Cs7g18840.2 | peroxisome (?) | n.d. | BC (?) | leaf, stem, root, cotyledon | root growth, vegetative growth | [55,71] |
| CsPAO3 | Cs6g15870.1 | peroxisome (?) | n.d. | BC (?) | leaf, stem, root, cotyledon | root growth, vegetative growth | [55,71] |
| CsPAO4 | Cs4g14150.1 | apoplast | Spd, Spm | TC | leaf, stem, root | seed germination, the growth of root and vegetative, salt tolerance | [55,71] |
| CsPAO5 | Cs7g23790.1 | n.d. | n.d. | BC (?) | leaf, stem, root, cotyledon | root growth, vegetative growth | [55,71] |
| CsPAO6 | Cs7g23760.1 | n.d. | n.d. | BC (?) | stem, root, cotyledon | root growth, vegetative growth | [55,71] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Jia, D.; Liu, T. Polyamine Oxidases Play Various Roles in Plant Development and Abiotic Stress Tolerance. Plants 2019, 8, 184. https://doi.org/10.3390/plants8060184
Yu Z, Jia D, Liu T. Polyamine Oxidases Play Various Roles in Plant Development and Abiotic Stress Tolerance. Plants. 2019; 8(6):184. https://doi.org/10.3390/plants8060184
Chicago/Turabian StyleYu, Zhen, Dongyu Jia, and Taibo Liu. 2019. "Polyamine Oxidases Play Various Roles in Plant Development and Abiotic Stress Tolerance" Plants 8, no. 6: 184. https://doi.org/10.3390/plants8060184
APA StyleYu, Z., Jia, D., & Liu, T. (2019). Polyamine Oxidases Play Various Roles in Plant Development and Abiotic Stress Tolerance. Plants, 8(6), 184. https://doi.org/10.3390/plants8060184




