Photosynthetic Responses of Canola and Wheat to Elevated Levels of CO2, O3 and Water Deficit in Open-Top Chambers
Abstract
1. Introduction
2. Results
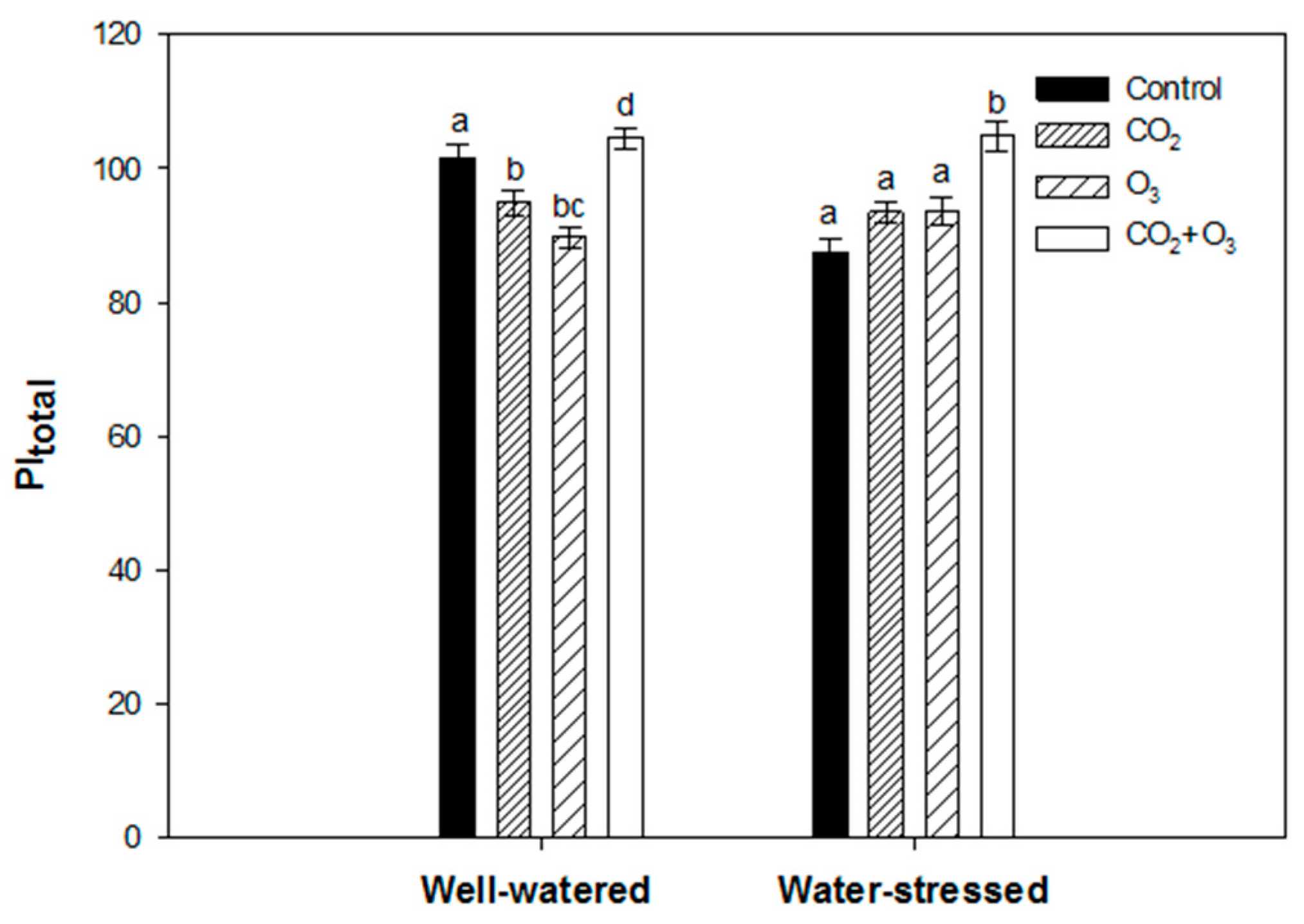
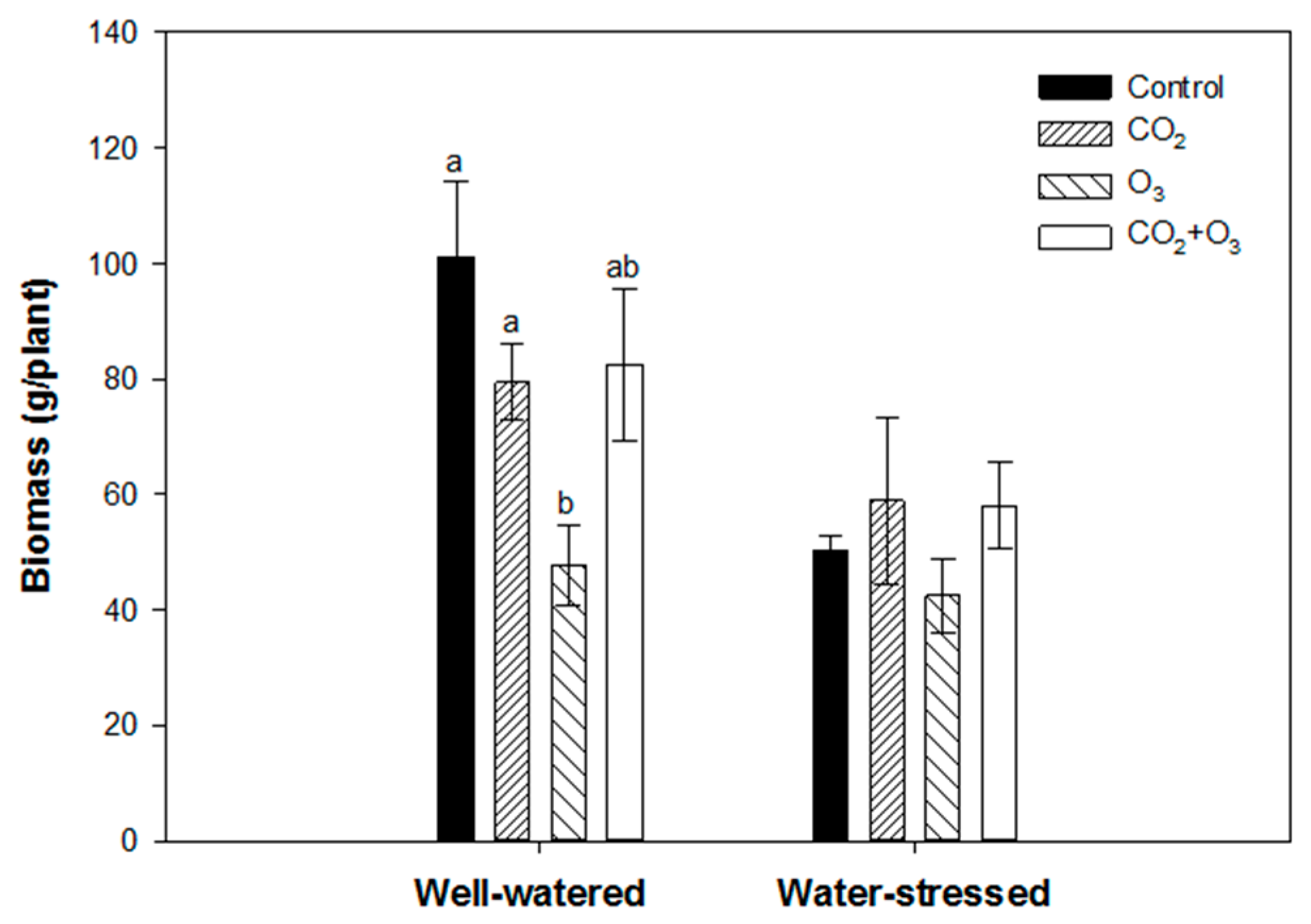
2.1. Photosynthetic Responses of Canola to Elevated Levels of CO2 and O3
2.2. Photosynthetic Responses of Wheat to Elevated Levels of CO2 and O3
3. Discussion
4. Materials and Methods
4.1. Experimental Site and Plant Materials
4.2. Fumigation and Water Treatment
4.3. Chlorophyll a Fluorescence
4.4. Biomass
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mulholland, B.J.; Craigon, J.; Black, C.R.; Colls, J.J.; Atherton, J.; Landon, G. Effects of elevated carbon dioxide and ozone on the growth and yield of spring wheat (Triticum aestivum L.). Environ. Exp. Bot. 1997, 48, 113–122. [Google Scholar] [CrossRef]
- Pleijel, H.; Gelang, J.; Sild, E.; Danielsson, H.; Younis, S.; Karlsson, P.E.; Wallin, G.; Skärby, L.; Selldén, G. Effects of elevated carbon dioxide, ozone and water availability on spring wheat growth and yield. Physiol. Plant 2000, 108, 61–70. [Google Scholar] [CrossRef]
- Jaggard, K.W.; Qi, A.; Ober, E.S. Possible changes to arable crop yields by 2050. Philos. Trans. R. Soc. B 2010, 365, 2835–2851. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Li, Y.; Ma, L.; Chen, Q.; Wang, L.L.; He, X. Effects of elevated carbon dioxide and/or ozone on endogenous plant hormones in the leaves of Ginkgo biloba. Acta Physiol. Plant 2011, 33, 129–136. [Google Scholar] [CrossRef]
- Porter, J.R.; Xie, L.; Challinor, A.J.; Cochrane, K.; Howden, S.M.; Iqbal, M.M.; Lobell, D.B.; Travasso, M.I. Food security and food production systems. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 485–533. [Google Scholar]
- Ainsworth, E.A.; Yendrek, C.R.; Sitch, S.; Collins, W.J.; Emberson, L.D. The effects of tropospheric ozone on net primary productivity and implications for climate change. Annu. Rev. Plant Biol. 2012, 63, 637–661. [Google Scholar] [CrossRef] [PubMed]
- Emberson, L.D.; Pleijel, H.; Ainsworth, E.A.; Van Den Berg, M.; Ren, W.; Osborne, S.; Mills, G.; Pandey, D.; Dentener, F.; Büker, P.; et al. Ozone effects on crops and consideration in crop models. Eur. J. Agron. 2018, 100, 19–34. [Google Scholar] [CrossRef]
- Krasensky, J.; Carmody, M.; Sierla, M.; Kangasjärvi, J. Ozone and reactive oxygen species. eLS. 2017. Available online: http://www.els.net (accessed on 26 October 2018). [CrossRef]
- Van Tienhoven, A.M.; Zunckel, M.; Emberson, L.; Koosailee, A.; Otter, L. Preliminary assessment of risk of ozone impacts to maize (Zea mays) in southern Africa. Environ. Pollut. 2006, 140, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Laakso, L.; Beukes, J.P.; Van Zyl, P.G.; Pienaar, J.J.; Josipovic, M.; Venter, A.D.; Jaars, K.; Vakkari, V.; Labuschagne, C.; Chiloane, K.; et al. Ozone concentrations and their potential impacts on vegetation in southern Africa. In Climate Change, Air Pollution and Global Challenges Understanding and Perspectives from Forest Research; Matyssek, R., Clarke, N., Cudlin, P., Mikkelsen, T.N., Tuovinen, J.-P., Wieser, G., Paoletti, E., Eds.; Elsevier: Oxford, UK, 2013; pp. 2–622. [Google Scholar]
- Zunckel, M.; Venjonoka, K.; Pienaar, J.J.; Brunke, E.G.; Pretorius, O.; Koosialee, A.; Raghunandan, A.; Van Tienhoven, A.M. Surface ozone over southern Africa: Synthesis of monitoring results during the Cross border Air Pollution Impact Assessment project. Atmos. Environ. 2004, 38, 6139–6147. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Boote, K.J.; Kimball, B.A.; Ziska, L.H.; Izaurralde, R.C.; Ort, D.; Thomson, A.M.; Wolfe, D. Climate impacts on agriculture: Implications for crop production. Agron. J. 2011, 103, 351–370. [Google Scholar] [CrossRef]
- Robredo, A.; Pérez-López, U.; de la Maza, H.S.; González-Moro, B.; Lacuesta, M.; Mena-Petite, A.; Munoz-Rueda, A. Elevated CO2 alleviates the impact of drought on barley improving water status by lowering stomatal conductance and delaying its effects on photosynthesis. Environ. Exp. Bot. 2007, 59, 252–263. [Google Scholar] [CrossRef]
- Van Der Kooi, C.J.; Reich, M.; Löw, M.; De Kok, L.J.; Tausz, M. Growth and yield stimulation under elevated CO2 and drought: A meta-analysis on crops. Environ. Exp. Bot. 2016, 122, 150–157. [Google Scholar] [CrossRef]
- Volin, J.C.; Reich, P.B.; Givnish, T.J. Elevated carbon dioxide ameliorates the effects of ozone on photosynthesis and growth: Species respond similarly regardless of photosynthetic pathway or plant functional group. New Phytol. 1998, 138, 315–325. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Rastogi, A.; Živčák, M.; Brestic, M.; Daszkowska-Golec, A.; Sitko, K.; Alsharafa, K.Y.; Lotfi, R.; Stypiński, P.; Samborska, I.A.; et al. Prompt chlorophyll fluorescence as a tool for crop phenotyping: An example of barley landraces exposed to various abiotic stress factors. Photosynthetica 2018, 56, 953–961. [Google Scholar] [CrossRef]
- Bhagat, K.P.; Kumar, R.A.; Ratnakumar, P.; Kumar, S.; Bal, S.K.; Agrawal, P.K. Photosynthesis and associated aspects under abiotic stresses environment. In Approaches to Plant Stress and Their Management; Springer: New Delhi, India, 2014; pp. 191–205. [Google Scholar]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and drought: Can we make metabolic connections from available data? Environ. Exp. Bot. 2010, 62, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Zivcak, M.; Brestic, M.; Balatova, Z.; Drevenakova, P.; Olsovska, K.; Kalaji, H.M.; Yang, X.; Allakhverdiev, S.I. Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynth. Res. 2013, 117, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Baghel, L.; Kataria, S.; Guruprasad, K.N. Effect of static magnetic field pretreatment on growth, photosynthetic performance and yield of soybean under water stress. Photosynthetica 2018, 56, 718–730. [Google Scholar] [CrossRef]
- Olechowicz, J.; Chomontowski, C.; Olechowicz, P.; Pietkiewicz, S.; Jajoo, A.; Kalaji, M.H. Impact of intraspecific competition on photosynthetic apparatus efficiency in potato (Solanum tuberosum) plants. Photosynthetica 2018, 56, 971–975. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M.; Strasser, R.J. Fingerprints for climate changes on the behaviour of the photosynthetic apparatus, monitored by the JIP-test. A case study on light and heat stress adaptation of the symbionts of temperate and coral reef foraminifers in hospite. In “Fingerprints” of Climate Changes–Adapted Behaviour and Shifting Species Ranges; Walther, G.-R., Burga, C.A., Edwards, P.J., Eds.; Kluwer Academic Publishers: New York, NY, USA, 2001; pp. 229–247. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll Fluorescence: A Signature of Photosynthesis; Papageorgiou, E., Govindjee, G.C., Eds.; Kluwer Academic Publishers: Rotterdam, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Bussotti, F.; Desotgiu, R.; Cascio, C.; Pollastrini, M.; Gravano, E.; Gerosa, G.; Marzuoli, R.; Nali, C.; Lorenzini, G.; Salvatori, E.; et al. Ozone stress in woody plants assessed with chlorophyll a fluorescence. A critical reassessment of existing data. Environ. Exp. Bot. 2011, 73, 19–30. [Google Scholar] [CrossRef]
- Perboni, A.; Cassol, D.; Silva, F.; Silva, D.; Bacarin, M. Chlorophyll a fluorescence study revealing effects of flooding in canola hybrids. Biologia 2012, 67, 38–346. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Oukarroum, A.; El Madidi, S.; Schansker, G.; Strasser, R.J. Probing the responses of barley cultivars (Hordeum vulgare L.) by chlorophyll a fluorescence OLKJIP under drought stress and re-watering. Environ. Exp. Bot. 2007, 60, 438–446. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Sarin, N.B. Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurements. Biochim. Biophys. Acta 2010, 1797, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Gururani, M.A.; Venkatesh, J.; Ganesan, M.; Strasser, R.J.; Han, Y.; Kim, J.I.; Lee, H.Y.; Song, P.S. In vivo assessment of cold tolerance through chlorophyll-a fluorescence in transgenic zoysiagrass expressing mutant phytochrome A. PLoS ONE 2015, 10, e0127200. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Seneweera, S.; Rodin, J.; Materne, M.; Burch, D.; Rothstein, S.J.; Spangenberg, G. Improving yield potential in crops under elevated CO2: Integrating the photosynthetic and nitrogen utilization efficiencies. Front Plant Sci. 2012, 3, 162. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Gamage, D.; Hirotsu, N.; Martin, A.; Seneweera, S. Effects of elevated carbon dioxide on photosynthesis and carbon partitioning: A perspective on root sugar sensing and hormonal crosstalk. Front Physiol. 2017, 8, 578. [Google Scholar] [CrossRef]
- Makino, A.; Mae, T. Photosynthesis and plant growth at elevated levels of CO2. Plant Cell Physiol. 1999, 40, 999–1006. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.; Schjoerring, J.K. Effects of elevated atmospheric CO2 on physiology and yield of wheat (Triticum aestivum L.): A meta-analytic test of current hypotheses. Agric. Ecosyst. Environ. 2013, 178, 57–63. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Kunderlikova, K.; Sytar, O.; Shao, H.; Kalaji, H.M.; Allakhverdiev, S.I. Low PSI content limits the photoprotection of PSI and PSII in early growth stages of chlorophyll b-deficient wheat mutant lines. Photosynth. Res. 2015, 125, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Zivcak, M.; Brestic, M.; Kunderlikova, K.; Sytar, O.; Allakhverdiev, S.I. Repetitive light pulse-induced photoinhibition of photosystem I severely affects CO2 assimilation and photoprotection in wheat leaves. Photosynth. Res. 2015, 126, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Brestic, M.; Tan, D.X.; Zivcak, M.; Zhu, X.; Liu, S.; Song, F.; Reiter, R.J.; Liu, F. Melatonin alleviates low PSI-limited carbon assimilation under elevated CO2 and enhances the cold tolerance of offspring in chlorophyll b-deficient mutant wheat. J. Pineal Res. 2018, 64, e12453. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.B.; Li, M.; Li, Q.M.; Cui, Q.Q.; Zhang, W.D.; Ai, X.Z.; Bi, H.G. Combined effects of elevated CO2 concentration and drought stress on photosynthetic performance and leaf structure of cucumber (Cucumis sativus L.) seedlings. Photosynthetica 2018, 56, 942–952. [Google Scholar] [CrossRef]
- Pšidová, E.; Živčák, M.; Stojnić, S.; Orlović, S.; Gömöry, D.; Kučerová, J.; Ditmarová, Ľ.; Střelcová, K.; Brestič, M.; Kalaji, H.M. Altitude of origin influences the responses of PSII photochemistry to heat waves in European beech (Fagus sylvatica L.). Environ. Exp. Bot. 2018, 152, 97–106. [Google Scholar] [CrossRef]
- Bender, J.; Hertstein, U.; Black, C.R. Growth and yield responses of spring wheat to increasing carbon dioxide, ozone and physiological stresses: A statistical analysis of ‘ESPACE-wheat’ results. Eur. J. Agron. 1999, 10, 185–195. [Google Scholar] [CrossRef]
- Clausen, S.K.; Frenck, G.; Linden, L.G.; Mikkelsen, T.N.; Lunde, C.; Jørgensen, R.B. Effects of single and multifactor treatments with elevated temperature, CO2 and ozone on oilseed rape and barley. J. Agron. Crop Sci. 2011, 197, 442–453. [Google Scholar] [CrossRef]
- Frenck, G.; Van Der Linden, L.; Mikkelsen, T.N.; Brix, H.; Jørgensen, R.B. Increased [CO2] does not compensate for negative effects on yield caused by higher temperature and [O3] in Brassica napus L. Eur. J. Agron. 2011, 35, 127–134. [Google Scholar] [CrossRef]
- Long, S.P.; Zhu, X.G.; Naidu, S.L.; Ort, D.R. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 2006, 29, 315–330. [Google Scholar] [CrossRef]
- Fitzgerald, G.J.; Tausz, M.; O’leary, G.; Mollah, M.R.; Tausz-Posch, S.; Seneweera, S.; Mock, I.; Löw, M.; Partington, D.L.; Mcneil, D.; et al. Elevated atmospheric [CO2] can dramatically increase wheat yields in semi-arid environments and buffer against heat waves. Glob. Change Biol. 2016, 22, 2269–2284. [Google Scholar] [CrossRef]
- Amthor, J.S. Effects of atmospheric CO2 concentration on wheat yield: Review of results from experiments using various approaches to control CO2 concentration. Field Crops Res. 2001, 73, 1–34. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Dangre, D.; Rai, M. Biophysical phenomics reveals functional building blocks of plants systems biology: A case study for the evaluation of the impact of mycorrhization with Piriformospora indica. In Advanced Techniques in Soil Microbiology; Varma, A., Oelmüller, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 319–342. [Google Scholar]
- Desotgiu, R.; Pollastrini, M.; Cascio, C.; Gerosa, G.; Marzuoli, R.; Bussotti, F. Chlorophyll a fluorescence analysis along a vertical gradient of the crown in a poplar (Oxford clone) subjected to ozone and water stress. Tree Physiol. 2012, 32, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Zivcak, M.; Tripathi, D.K.; Yadav, S.; Kalaji, H.M.; Brestic, M. Phytotoxic effect of silver nanoparticles in Triticum aestivum: Improper regulation of photosystem I activity as the reason for oxidative damage in the chloroplast. Photosynthetica 2019, 57, 209–216. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrova, S.; Piszcz, U.; Bielecki, K.; Karmowska, K.; et al. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Baghbani, F.; Lotfi, R.; Moharramnejad, S.; Bandehagh, A.; Roostaei, M.; Rastogi, A.; Kalaji, H.M. Impact of Fusarium verticillioides on chlorophyll fluorescence parameters of two maize lines. Eur. J. Plant Pathol. 2018, 1–10. [Google Scholar] [CrossRef]
- Maliba, B.G.; Inbaraj, P.M.; Berner, J.M. The effect of ozone and drought on the photosynthetic performance of canola. J. Integr. Agric. 2018, 17, 1137–1144. [Google Scholar] [CrossRef]
- Desotgiu, R.; Pollastrini, M.; Cascio, C.; Gerosa, G.; Marzuoli, R.I.; Bussotti, F. Responses to ozone on Populus “Oxford” clone in an open top chamber experiment assessed before sunrise and in full sunlight. Photosynthetica 2013, 51, 267–280. [Google Scholar] [CrossRef]
- Feng, Z.; Kobayashi, K.; Ainsworth, E.A. Impact of elevated ozone concentration on growth, physiology, and yield of wheat (Triticum aestivum L.): A meta-analysis. Glob. Change Biol. 2008, 14, 2696–2708. [Google Scholar]
- Ollerenshaw, J.H.; Lyons, T.; Barnes, J.D. Impacts of ozone on the growth and yield of field-grown winter oilseed rape. Environ. Pollut. 1999, 104, 53–59. [Google Scholar] [CrossRef]
- Monga, R.; Marzuoli, R.; Alonso, R.; Bermejo, V.; González-Fernández, I.; Faoro, F.; Gerosa, G. Varietal screening of ozone sensitivity in Mediterranean durum wheat (Triticum durum, Desf.). Atmos. Environ. 2015, 110, 18–26. [Google Scholar] [CrossRef]
- Clark, A.J.; Landolt, W.; Bucher, J.B.; Strasser, R.J. Beech (Fagus sylvatica) response to ozone exposure assessed with a chlorophyll a fluorescence performance index. Atmos. Environ. 2001, 109, 501–507. [Google Scholar] [CrossRef]
- Pollastrini, M.; Desotgiu, R.; Cascio, C.; Bussotti, F.; Cherubini, P.; Saurer, M.; Gerosa, G.; Marzuoli, R. Growth and physiological responses to ozone and mild drought stress of tree species with different ecological requirements. Trees 2010, 24, 695–704. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M.; Strasser, R.J. Biophysical Phenomics: Evaluation of the Impact of Mycorrhization with Piriformospora indica. In Piriformospora indica; Varma, A., Kost, G., Oelmüller, R., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2013; pp. 173–190. [Google Scholar]
- Heyneke, E.; Smit, P.R.; Van Rensburg, L.; Krüger, G.H.J. Open-top chambers to study air pollution impacts in South Africa. Part I: Microclimate in open-top chambers. S. Afr. J. Plant Soil 2012, 29, 1–2. [Google Scholar] [CrossRef]
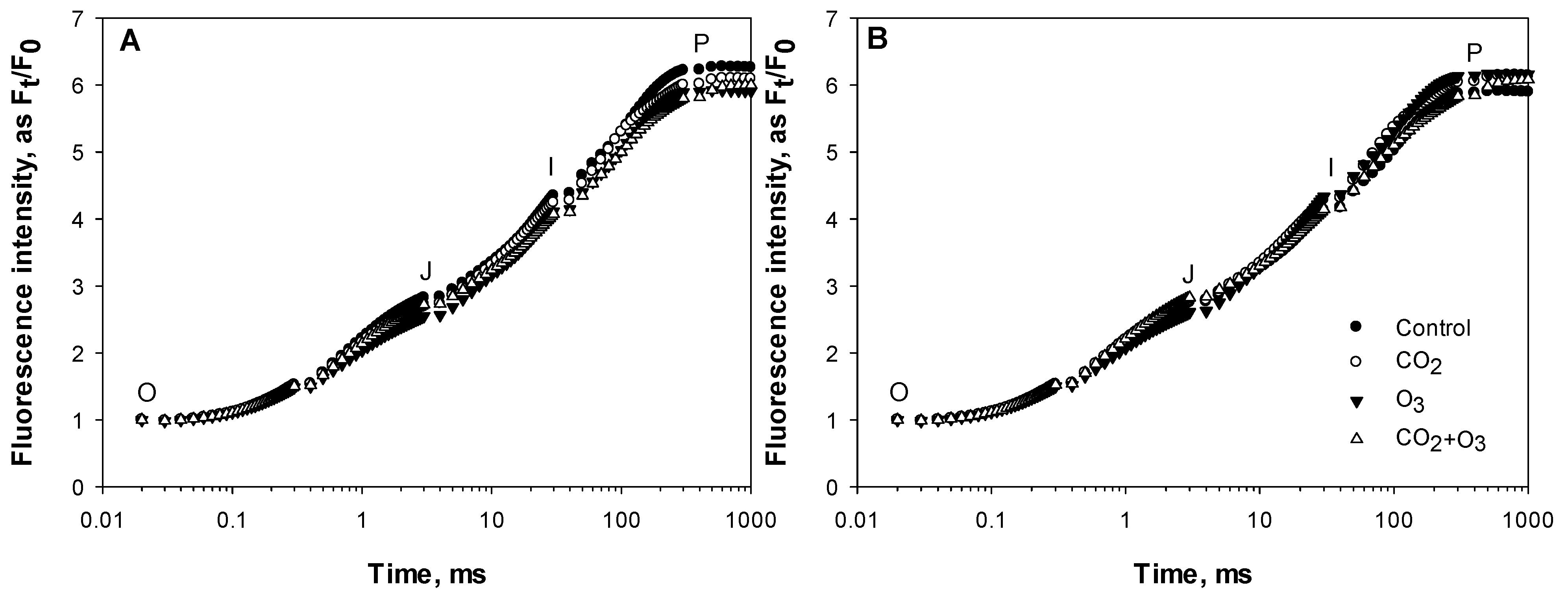
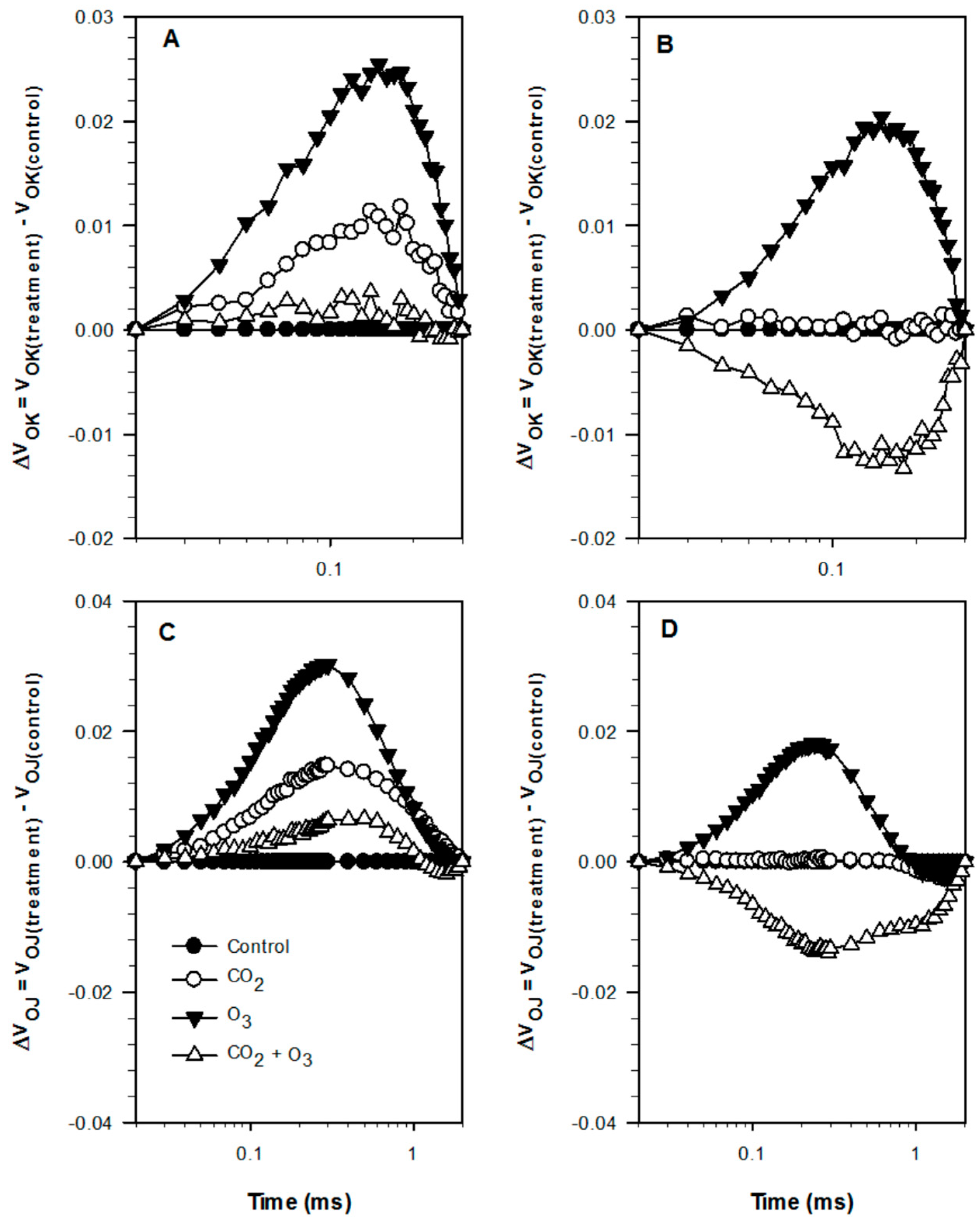
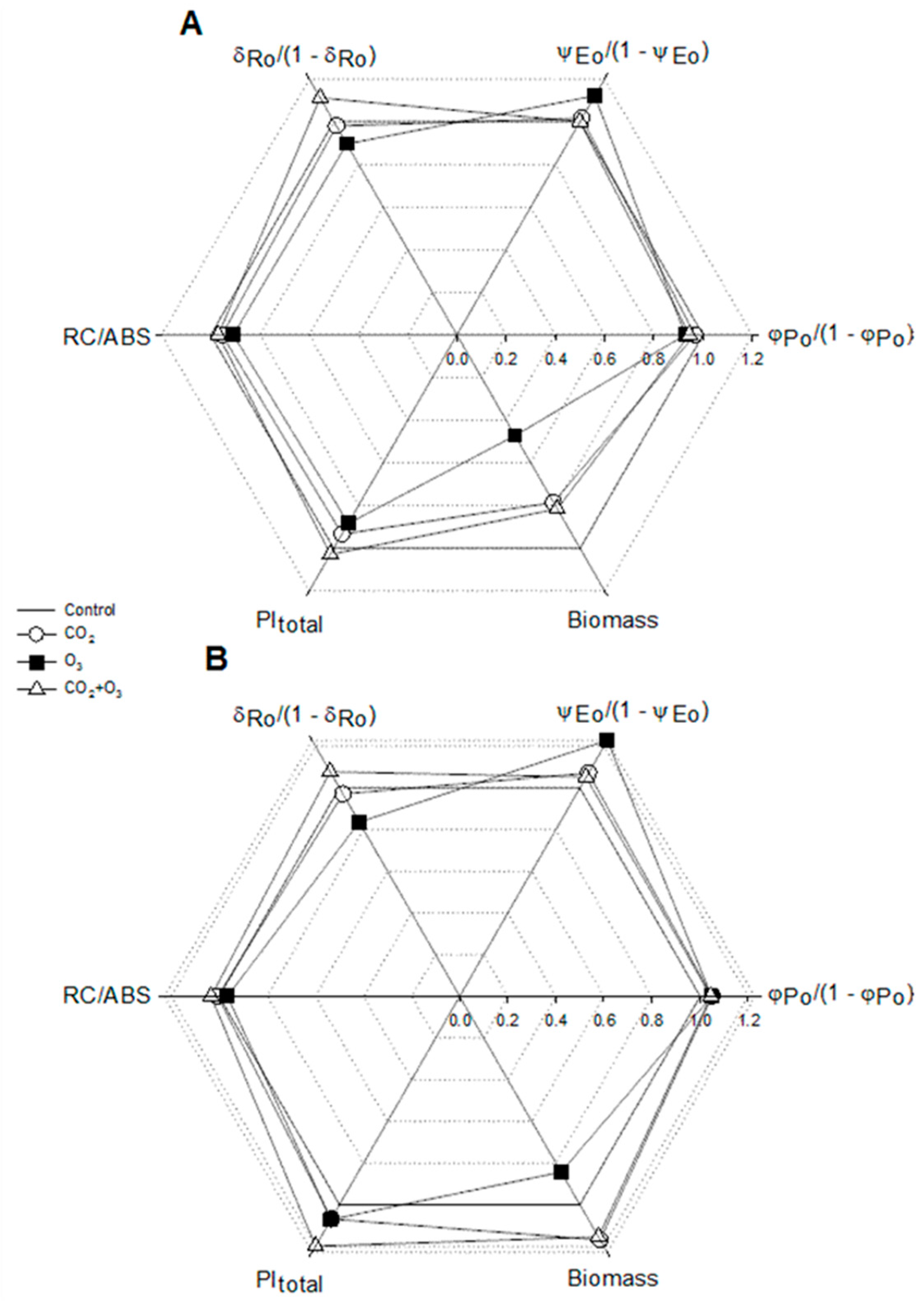
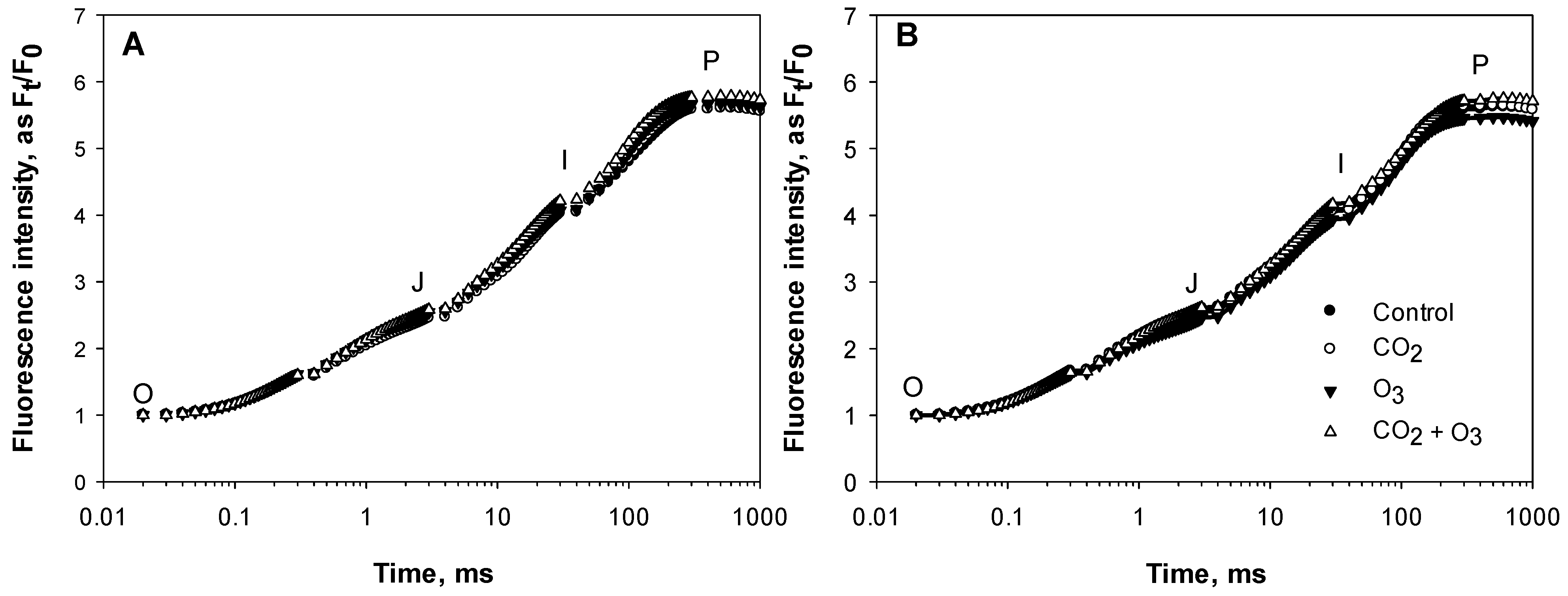
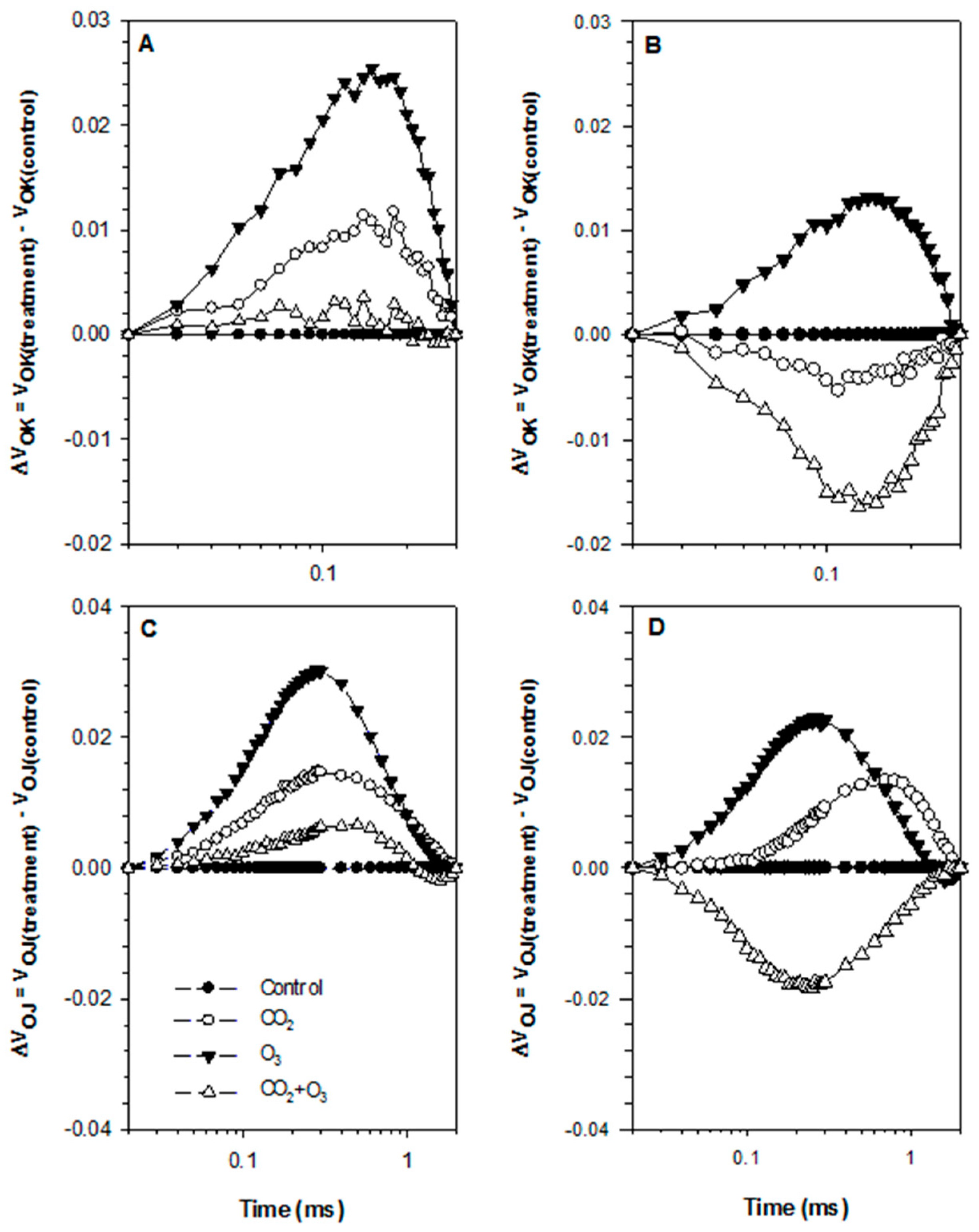
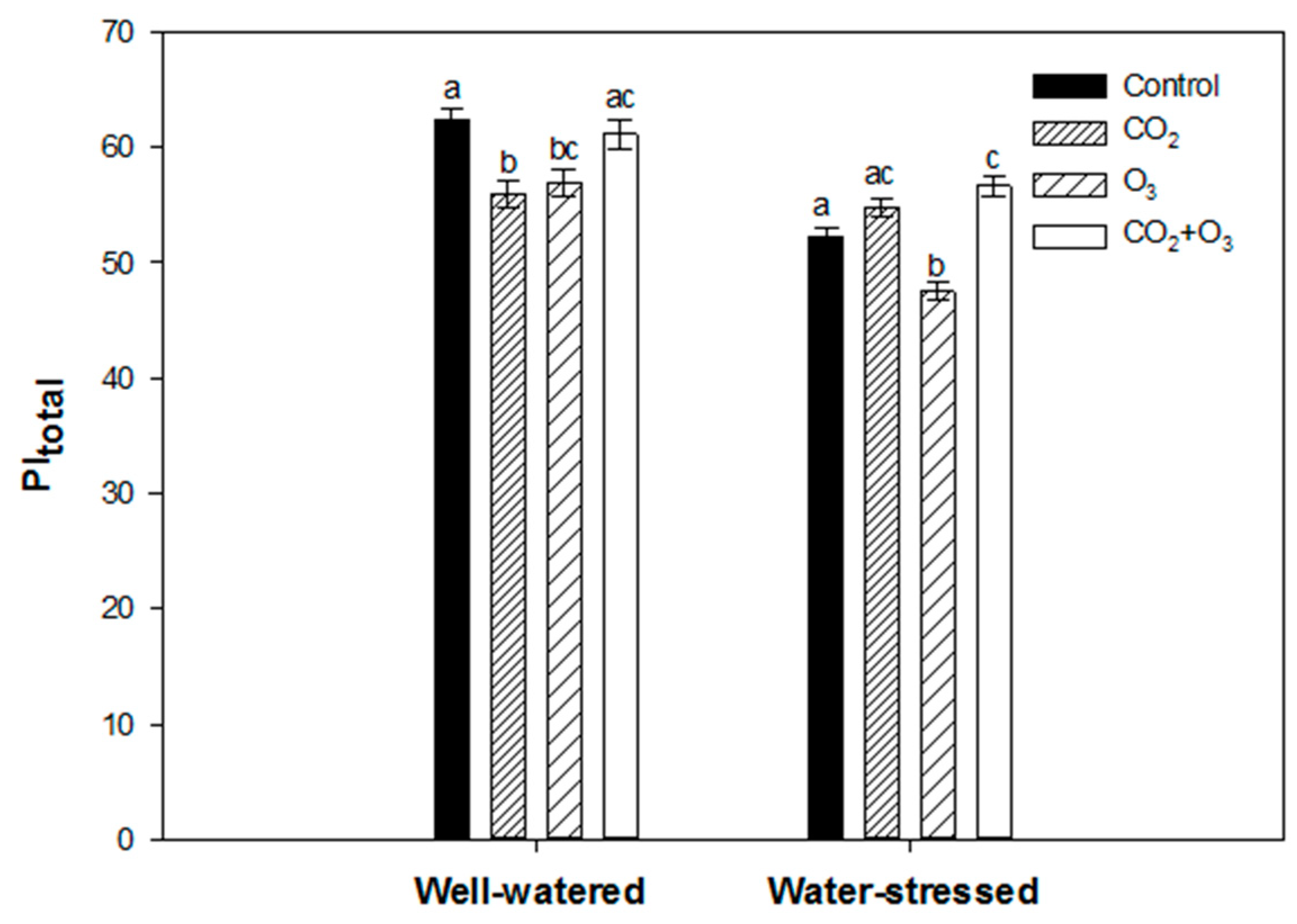
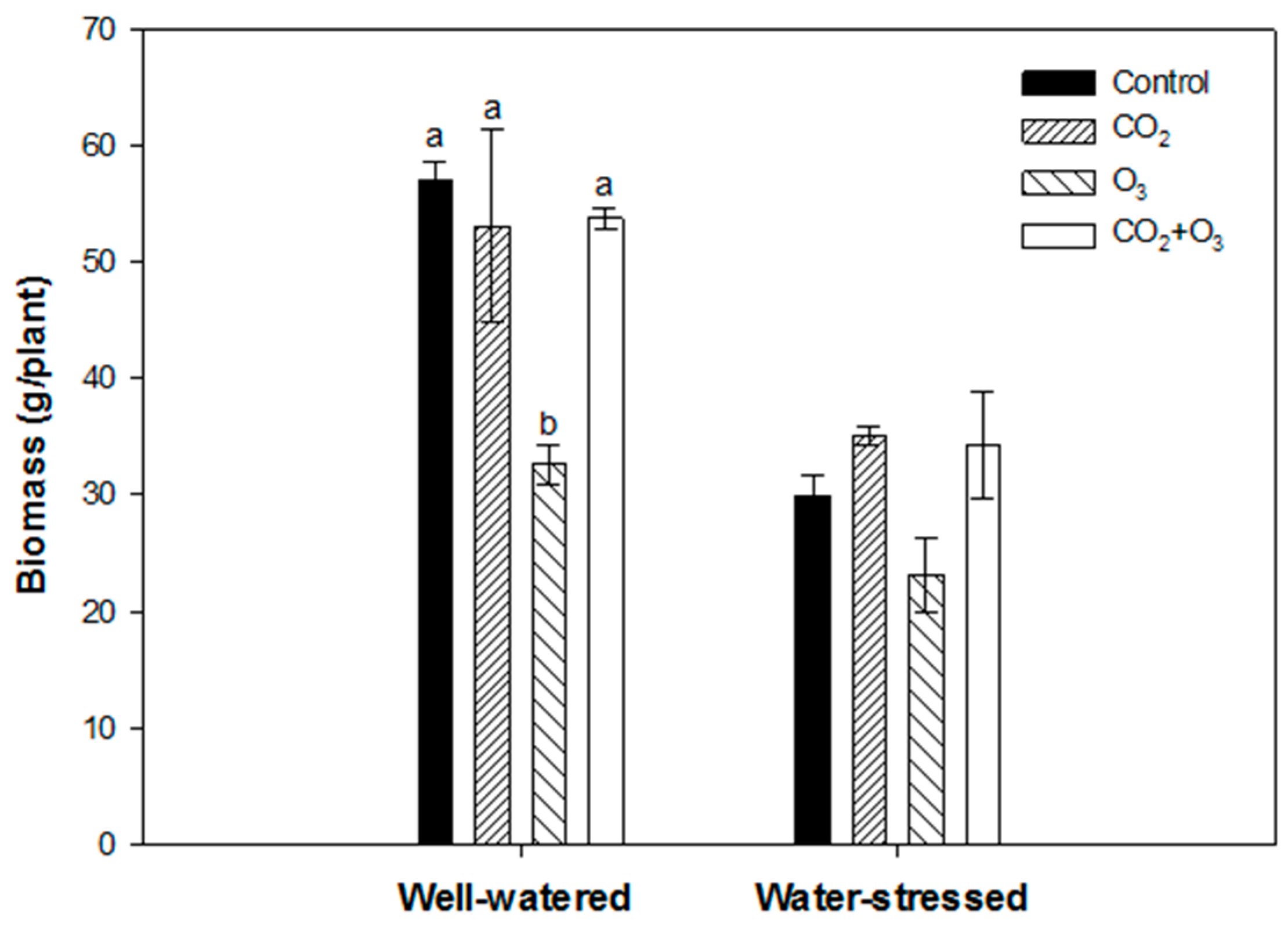
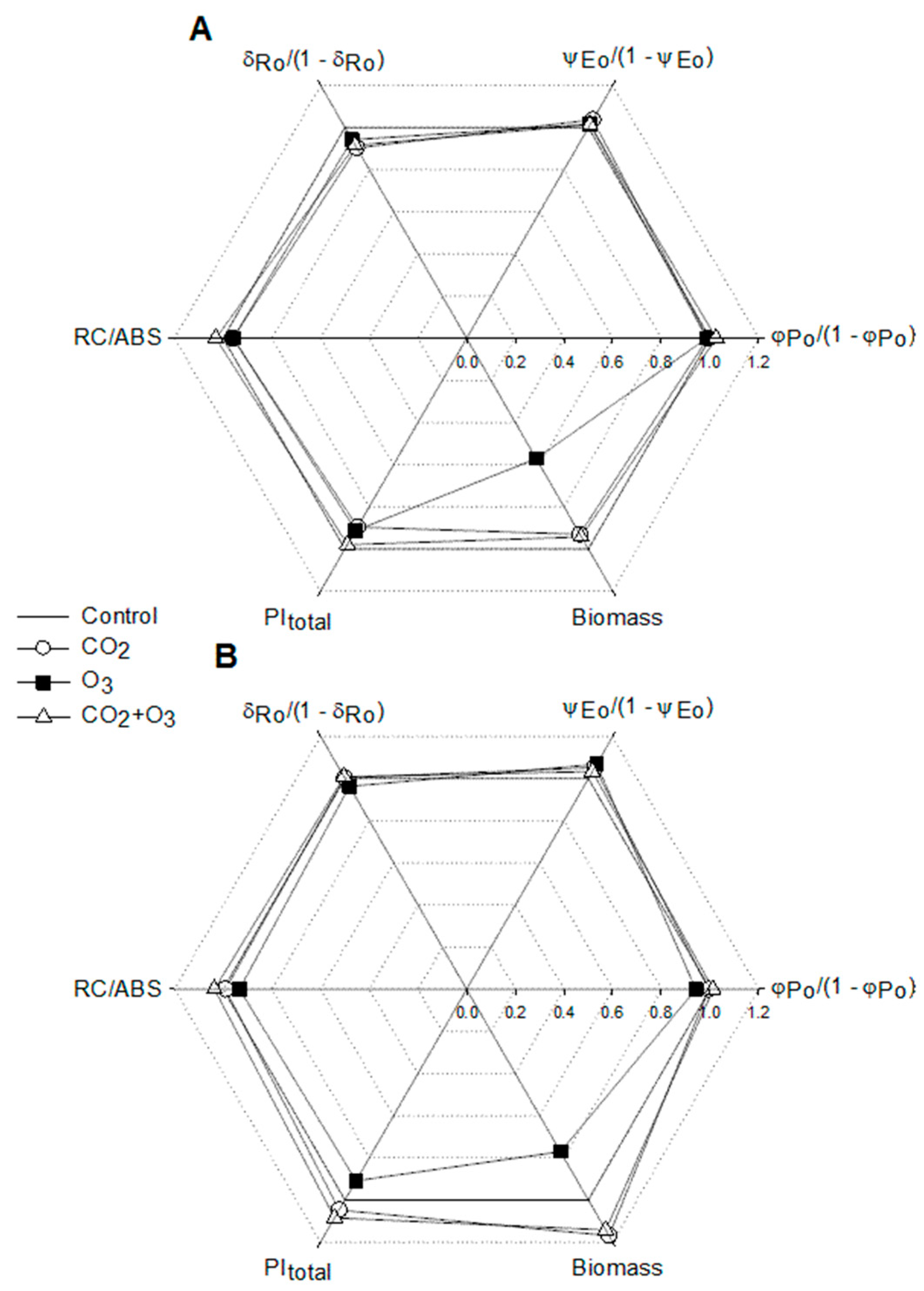
| Well-Watered | Control | CO2 | O3 | CO2 + O3 | p-level |
| φPo /(1 - φPo) | 5.775 ± 0.033a | 5.584 ± 0.029b | 5.359 ± 0.027c | 5.459 ± 0.031c | <0.001 |
| ψEo /(1 - ψEo) | 2.181 ± 0.043a | 2.214 ± 0.029a | 2.444 ± 0.031b | 2.180 ± 0.032a | <0.001 |
| δRo /(1 - δRo) | 1.164 ± 0.019a | 1.140 ± 0.023a | 1.042 ± 0.011b | 1.293 ± 0.020c | <0.001 |
| Density of reaction centers (RC/ABS) | 0.725 ± 0.005a | 0.692 ± 0.004b | 0.659 ± 0.004c | 0.704 ± 0.004b | <0.001 |
| Water-Stressed | |||||
| φPo /(1 - φPo) | 5.388 ± 0.038a | 5.652 ± 0.036b | 5.643 ± 0.025b | 5.624 ± 0.036b | <0.001 |
| ψEo /(1 - ψEo) | 2.020 ± 0.034a | 2.164 ± 0.033b | 2.480 ± 0.027c | 2.124 ± 0.035ab | <0.001 |
| δRo /(1 - δRo) | 1.154 ± 0.016a | 1.121 ± 0.016a | 0.964 ± 0.012b | 1.243 ± 0.021c | <0.001 |
| RC/ABS | 0.695 ± 0.004a | 0.699 ± 0.004a | 0.671 ± 0.005b | 0.718 ± 0.005c | <0.001 |
| Well-Watered | Control | CO2 | O3 | CO2 + O3 | p-level |
| φPo /(1 - φPo) | 5.034 ± 0.021a | 4.987 ± 0.030a | 4.965 ± 0.22a | 5.161 ± 0.033b | <0.001 |
| ψEo /(1 - ψEo) | 2.321 ± 0.036a | 2.406 ± 0.039a | 2.357 ± 0.033a | 2.354 ± 0.043a | N.S. |
| δRo /(1 - δRo) | 0.974 ± 0.010a | 0.880 ± 0.014b | 0.918 ± 0.015b | 0.892 ± 0.016b | <0.001 |
| RC/ABS | 0.555± 0.004a | 0.533 ± 0.003b | 0.532 ± 0.003b | 0.572 ± 0.004c | <0.001 |
| Water-Stressed | |||||
| φPo /(1 - φPo) | 5.039 ± 0.022a | 5.013 ± 0.021a | 4.745 ± 0.028b | 5.101 ± 0.028a | <0.001 |
| ψEo /(1 - ψEo) | 2.125 ± 0.030a | 2.236 ± 0.025b | 2.270 ± 0.031b | 2.196 ± 0.037ab | 0.006 |
| δRo /(1 - δRo) | 0.948 ± 0.011ab | 0.952 ± 0.009a | 0.913 ± 0.010b | 0.956 ± 0.015a | 0.017 |
| RC/ABS | 0.520 ± 0.003a | 0.515 ± 0.002a | 0.485 ± 0.003b | 0.539 ± 0.003c | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maliba, B.G.; Inbaraj, P.M.; Berner, J.M. Photosynthetic Responses of Canola and Wheat to Elevated Levels of CO2, O3 and Water Deficit in Open-Top Chambers. Plants 2019, 8, 171. https://doi.org/10.3390/plants8060171
Maliba BG, Inbaraj PM, Berner JM. Photosynthetic Responses of Canola and Wheat to Elevated Levels of CO2, O3 and Water Deficit in Open-Top Chambers. Plants. 2019; 8(6):171. https://doi.org/10.3390/plants8060171
Chicago/Turabian StyleMaliba, Bheki G., Prabhu M. Inbaraj, and Jacques M. Berner. 2019. "Photosynthetic Responses of Canola and Wheat to Elevated Levels of CO2, O3 and Water Deficit in Open-Top Chambers" Plants 8, no. 6: 171. https://doi.org/10.3390/plants8060171
APA StyleMaliba, B. G., Inbaraj, P. M., & Berner, J. M. (2019). Photosynthetic Responses of Canola and Wheat to Elevated Levels of CO2, O3 and Water Deficit in Open-Top Chambers. Plants, 8(6), 171. https://doi.org/10.3390/plants8060171





