Mutation of Inositol 1,3,4-trisphosphate 5/6-kinase6 Impairs Plant Growth and Phytic Acid Synthesis in Rice
Abstract
1. Introduction
2. Results
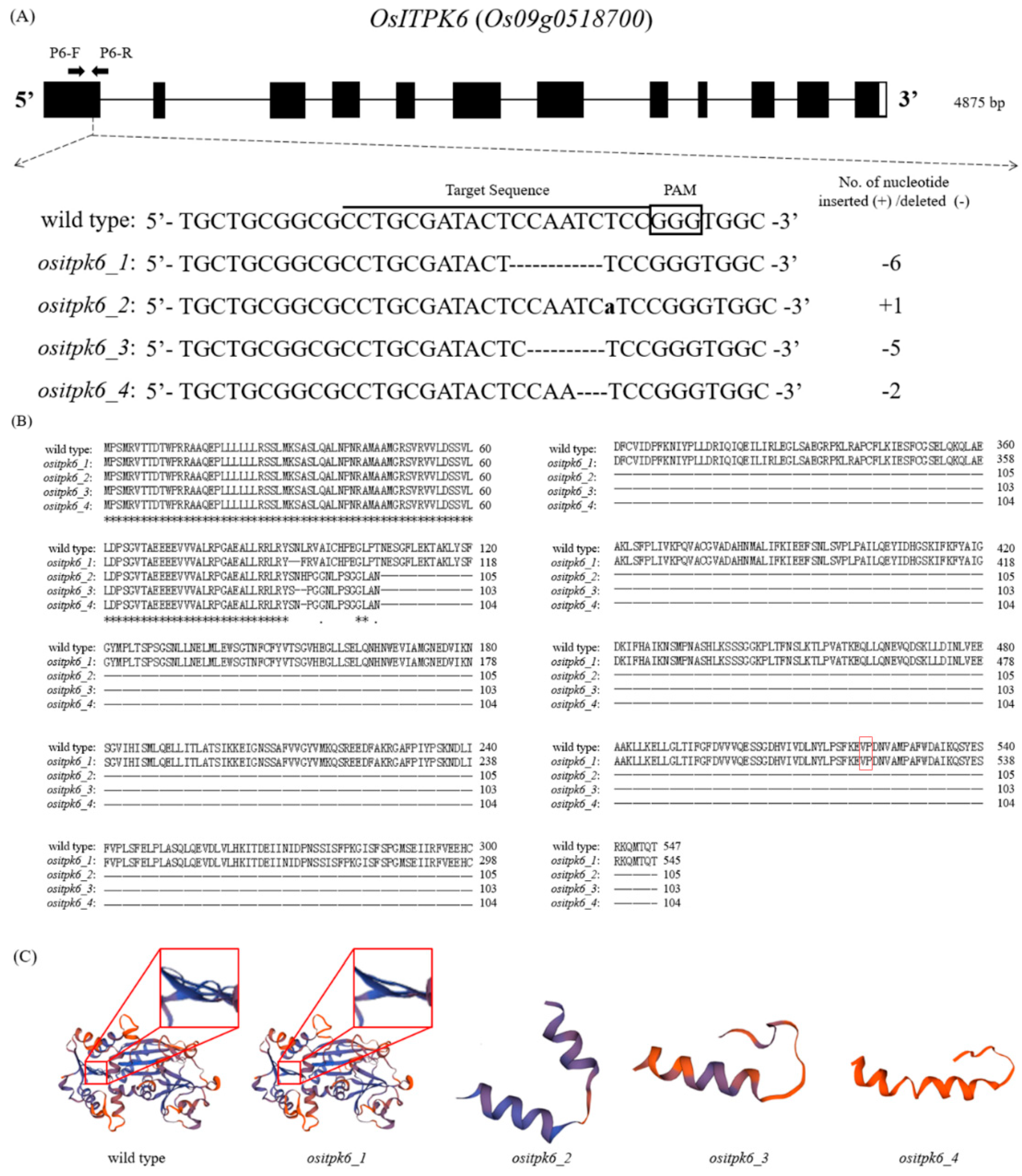
2.1. Mutations of OsITPK6 and Development of Homozygous Transgene-Free Mutant Lines
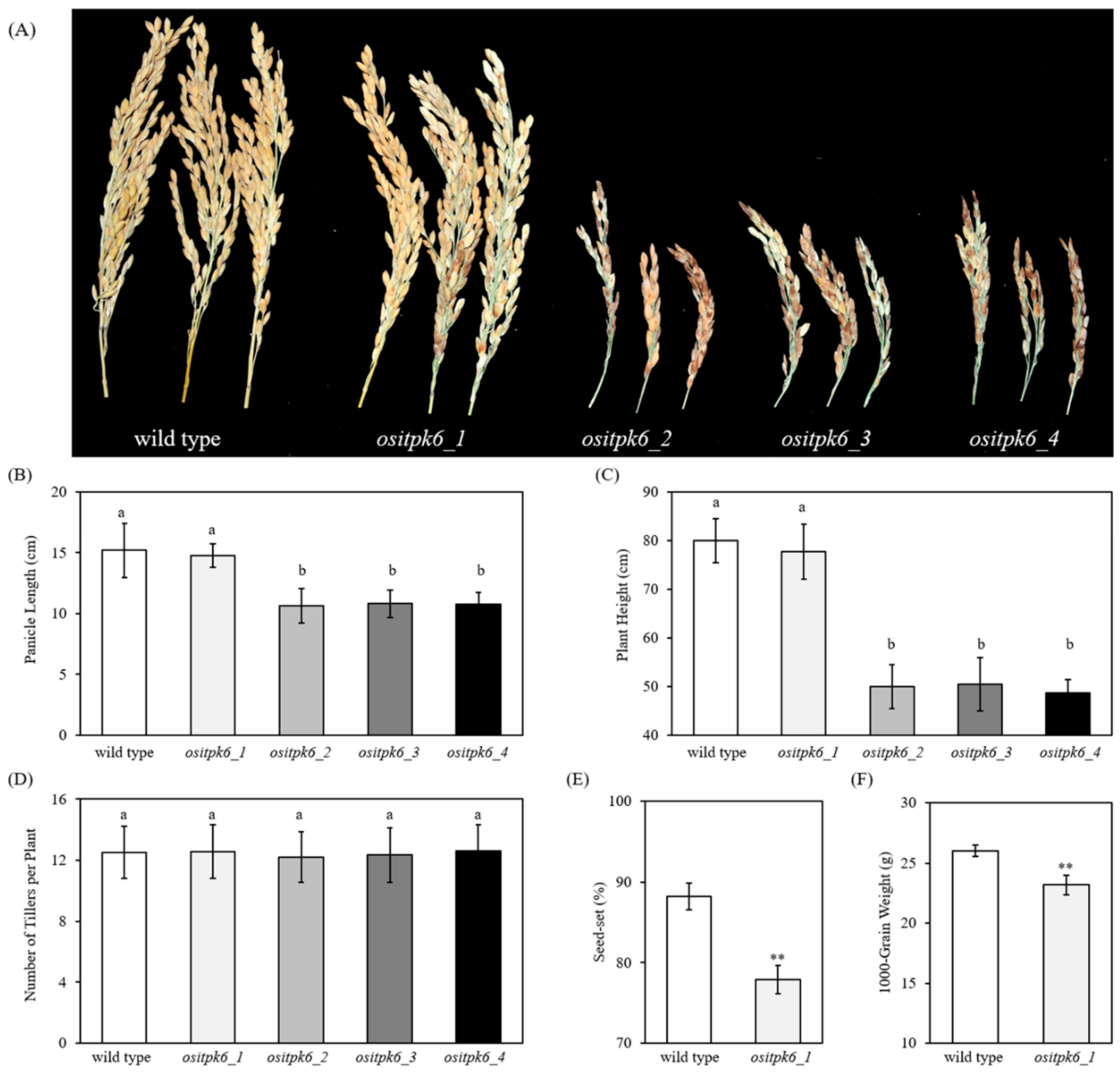
2.2. Impact of ositpk6 Mutations on Plant Growth and Seed Germination
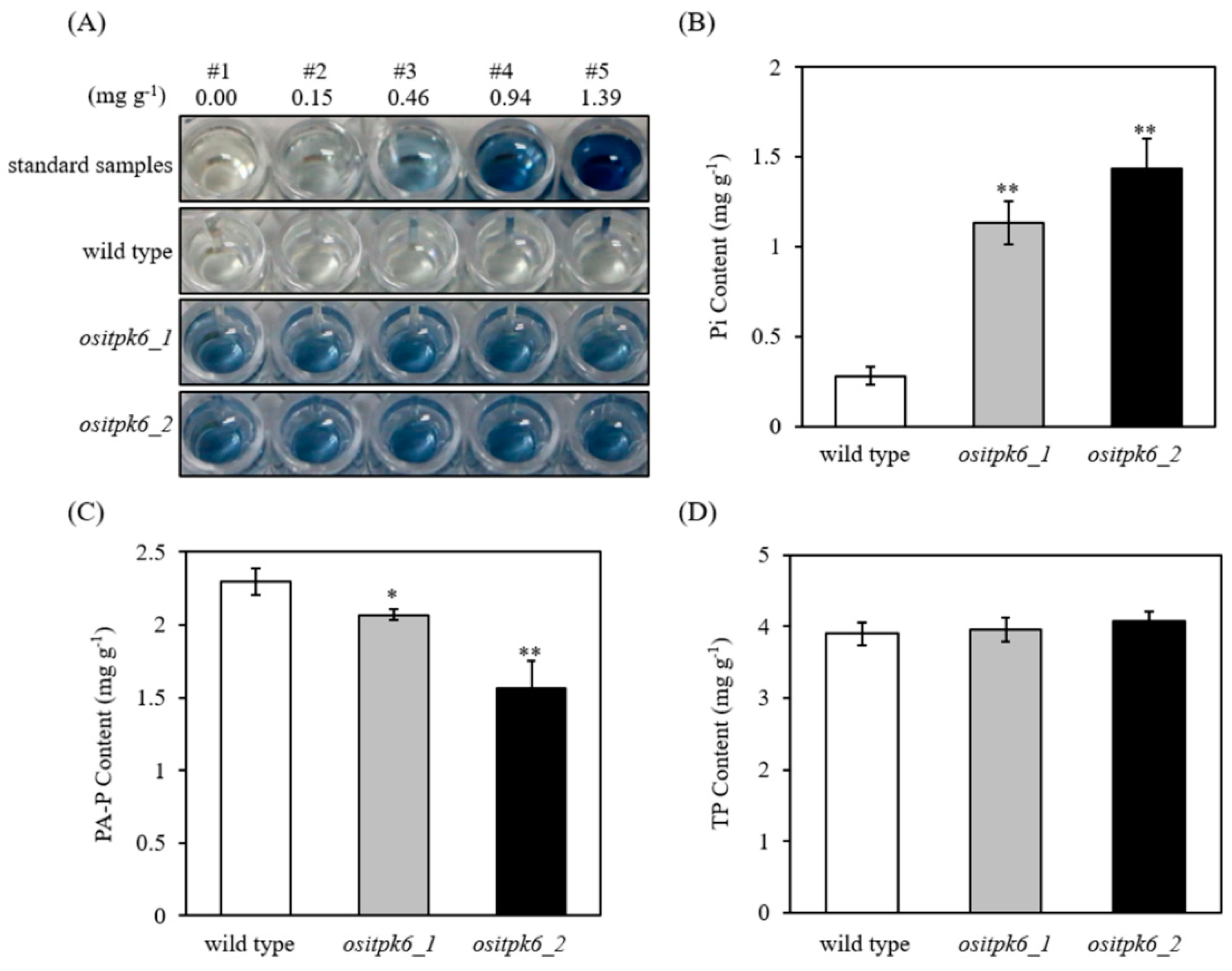
2.3. Effect of ositpk6 Mutations on Inorganic Phosphorus (Pi), Phytic Acid Phosphorus (PA-P), and Total Phosphorus (TP) in Brown Rice
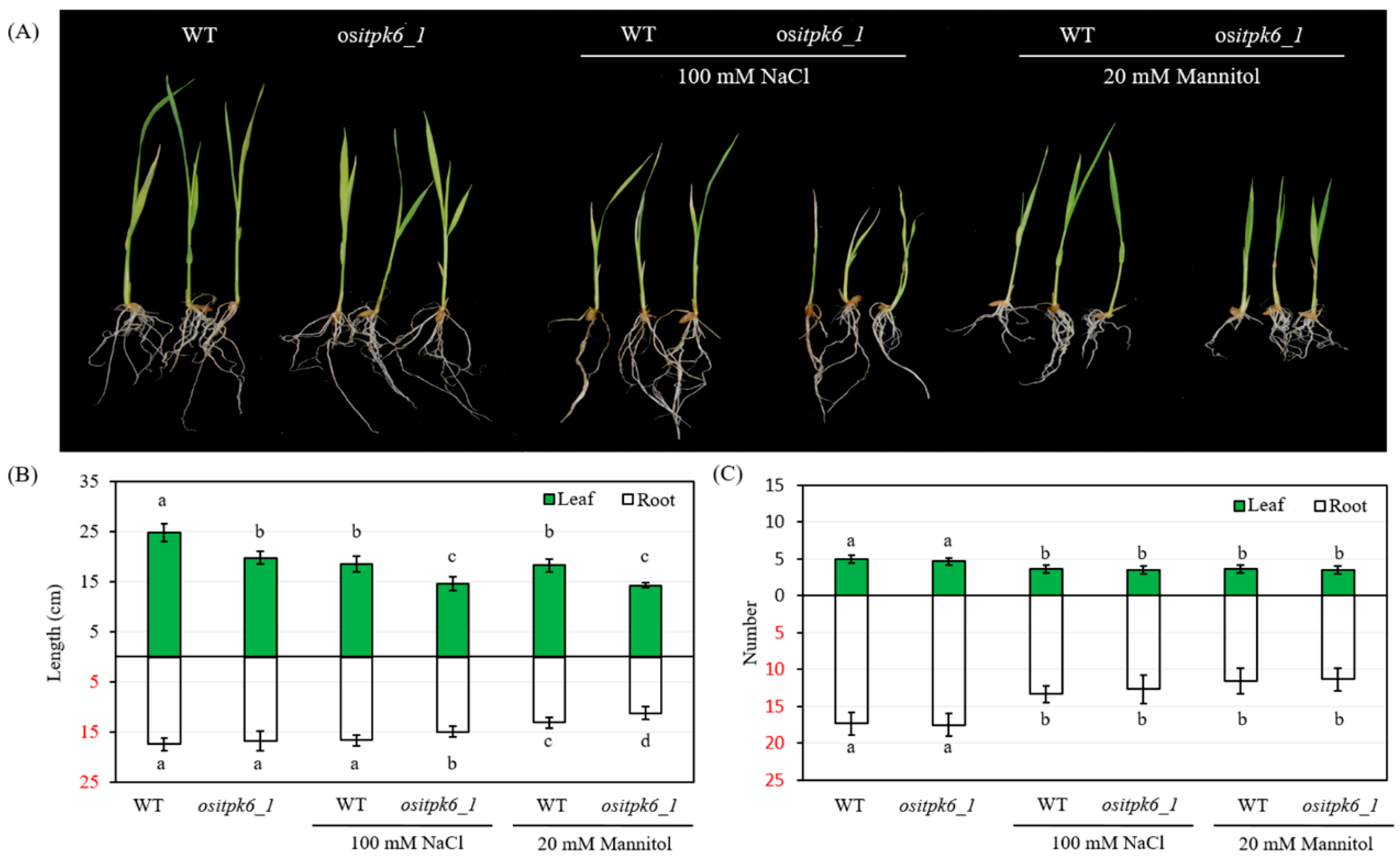
2.4. Effect of ositpk6 Mutation on Stress Tolerance
3. Discussion
4. Materials and Methods
4.1. CRISPR/Cas9 Vector Construction and Rice Transformation
4.2. Mutation Detection in T0 Plants
4.3. Development of Transgene-Free Mutant Lines
4.4. Agronomic Traits Assay
4.5. Seed Germination Assay
4.6. Seed Phosphorus Assay
4.7. Stress Treatment
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lott, J.N.A.; Ockenden, I.; Raboy, V.; Batten, G.D. Phytic acid and phosphorus in crops seeds and fruits: A global estimate. Seed Sci. Res. 2000, 10, 11–33. [Google Scholar]
- Raboy, V.; Young, K.A.; Dorsch, J.A.; Cook, A. Genetics and breeding of seed phosphorus and phytic acid. J. Plant Physiol. 2001, 158, 489–497. [Google Scholar] [CrossRef]
- Raboy, V.; Gerbasi, P.F.; Young, K.A.; Stoneberg, S.D.; Pickett, S.G.; Bauman, A.T.; Murthy, P.P.N.; Sheridan, W.F.; Ertlet, D.S. Origin and seed phenotype of maize low phytic acid 1-1 and low phytic acid 2-1. Plant Physiol. 2000, 124, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.R.; Rutger, J.N.; Young, K.A.; Raboy, V. Isolation and genetic mapping of a non-lethal rice (Oryza sativa L.) low phytic acid 1 mutation. Crop Sci. 2000, 40, 1397–1405. [Google Scholar] [CrossRef]
- Kim, S.I.; Andaya, C.B.; Newman, J.W.; Goyal, S.S.; Tai, T.H. Isolation and characterization of a low phytic acid rice mutant reveals a mutation in the rice orthologue of maize MIK. Theor. Appl. Genet. 2008, 117, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.L.; Xu, X.H.; Ren, X.L.; Fu, H.W.; Wu, D.X.; Shu, Q.Y. Generation and characterization of low phytic acid germplasm in rice (Oryza sativa L.). Theor. Appl. Genet. 2007, 114, 803–814. [Google Scholar] [CrossRef]
- Zhao, H.J.; Liu, Q.L.; Fu, H.W.; Xu, X.H.; Wu, D.X.; Shu, Q.Y. Effect of non-lethal low phytic acid mutations on grain yield and seed viability in rice. Field Crop. Res. 2008, 108, 206–211. [Google Scholar] [CrossRef]
- Raboy, V. Approaches and challenges to engineering seed phytate and total phosphorus. Plant Sci. 2009, 177, 281–296. [Google Scholar] [CrossRef]
- Xu, X.H.; Zhao, H.J.; Liu, Q.L.; Frank, T.; Engel, K.H.; An, G.; Shu, Q.Y. Mutations of the multi-drug resistance-associated protein ABC transporter gene 5 result in reduction of phytic acid in rice seeds. Theor. Appl. Genet. 2009, 119, 75–83. [Google Scholar] [CrossRef]
- Ali, N.; Paul, S.; Gayen, D.; Sarkar, S.N.; Datta, K.; Datta, S.K. Development of low phytate rice by RNAi mediated seed specific seed specific silencing of inositol 1,3,4,5,6-pentakisphosphate 2-kinase gene (IPK1). PLoS ONE 2013, 8, e68161. [Google Scholar] [CrossRef]
- Zhao, H.J.; Cui, H.R.; Xu, X.H.; Tan, Y.Y.; Fu, J.J.; Liu, G.Z.; Poirier, Y.; Shu, Q.Y. Characterization of OsMIK in a rice mutant with reduced phytate content reveals an insertion of a rearranged retrotransposon. Theor. Appl. Genet. 2013, 126, 3009–3020. [Google Scholar] [CrossRef]
- Li, W.X.; Huang, J.Z.; Zhao, H.J.; Tan, Y.Y.; Cui, H.R.; Poirier, Y.; Shu, Q.Y. Production of low phytic acid rice by hairpin RNA- and artificial microRNA-mediated silencing of OsMIK in seeds. Plant Cell Tiss. Org. 2014, 119, 15–25. [Google Scholar] [CrossRef]
- Li, W.X.; Zhao, H.J.; Pang, W.Q.; Cui, H.R.; Poirier, Y.; Shu, Q.Y. Seed-specific silencing of OsMRP5 reduces seed phytic acid and weight in rice. Transgenic Res. 2014, 23, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.J.; Frank, T.; Tan, Y.Y.; Zhou, C.G.; Jabnoune, M.; Arpat, A.B.; Cui, H.R.; Huang, J.Z.; He, Z.H.; Poirier, Y.; et al. Disruption of OsSULTR3;3 reduces phytate and phosphorus concentrations and alters the metabolite profile in rice grains. New Phytol. 2016, 211, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Guttieri, M.; Bowen, D.; Dorsch, J.A.; Raboy, V.; Souza, E. Identification and characterization of a low phytic acid wheat. Crop Sci. 2014, 44, 418–424. [Google Scholar] [CrossRef]
- Pilu, R.; Panzeri, D.; Gavazzi, G.; Rasmussen, S.K.; Consonni, G.; Nielsen, E. Phenotypic, genetic and molecular characterization of a maize low phytic acid mutant (lpa 241). Theor. Appl. Genet. 2003, 107, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, H.; Hazebroek, J.; Ertl, D.S.; Harp, T. The maize low-phytic acid 3 encodes a myo-inositol kinase that plays a role in phytic acid biosynthesis in developing seeds. Plant J. 2005, 42, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Tanaka, K.; Kuwano, M.; Yoshida, K.T. Expression pattern of inositol phosphate-related enzymes in rice (Oryza sativa L.): Implications for the phytic acid biosynthetic pathway. Gene 2007, 405, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Takazawa, K.; Perret, J.; Dumont, J.E.; Erneux, C. Molecular cloning and expression of a new putative inositol 1, 4, 5-trisphosphate 3-kinase isoenzyme. Biochem. J. 1991, 278, 883–886. [Google Scholar] [CrossRef]
- Wilson, M.P.; Majerus, P.W. Characterization of a cDNA encoding Arabidopsis thaliana inositol 1, 3, 4-trisphosphate 5/6-kinase. Biochem. Biophys. Res. Commun. 1997, 232, 678–681. [Google Scholar] [CrossRef]
- Sweetman, D.; Stavridou, I.; Johnson, S.; Green, P.; Caddick, S.E.; Brearley, C.A. Arabidopsis thaliana inositol 1, 3, 4-trisphosphate 5/6-kinase 4 (AtITPK4) is an outlier to a family of ATP-grasp fold proteins from Arabidopsis. FEBS Lett. 2007, 581, 4165–4171. [Google Scholar] [CrossRef]
- Field, J.; Wilson, M.P.; Mai, Z.; Majerus, P.W.; Samuelson, J. An Entamoeba histolytica inositol 1, 3, 4-trisphosphate 5/6-kinase has a novel 3-kinase activity. Mol. Biochem. Parasitol. 2000, 108, 119–123. [Google Scholar] [CrossRef]
- Qin, Z.X.; Chen, Q.J.; Tong, Z.; Wang, X.C. The Arabidopsis inositol 1, 3, 4-trisphosphate 5/6 kinase, AtItpk-1, is involved in plant photomorphogenesis under red light conditions, possibly via interaction with COP9 signalosome. Plant Physiol. Biochem. 2005, 43, 947–954. [Google Scholar] [CrossRef]
- Kuo, H.F.; Hsu, Y.Y.; Lin, W.C.; Chen, K.Y.; Munnik, T.; Brearley, C.A.; Chiou, T.J. Arabidopsis inositol phosphate kinases ipk1 and itpk1 constitute a metabolic pathway in maintaining phosphate homeostasis. Plant J. 2018, 95. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Tan, S.; Xue, H. Arabidopsis inositol 1,3,4-trisphosphate 5/6 kinase 2 is required for seed coat development. ActaBiochim. Biophys. Sin. 2013, 45, 549–560. [Google Scholar] [CrossRef]
- Krishnan, V.; Jain, P.; Vinutha, T.; Hada, A.; Manickavasagam, M.; Ganapathi, A.; Raj, D.R.; Archana, S. Molecular modeling and ‘in-silico’ characterization of ‘Glycine max’ inositol (1, 3, 4) tris 5/6 kinase-1(Gmitpk1)—A potential candidate gene for developing low phytate transgenics. Plant Omics. 2015, 8, 381–391. [Google Scholar]
- Marathe, A.; Krishnan, V.; Vinutha, T.; Dahuja, A.; Jolly, M.; Sachdev, A. Exploring the role of inositol 1, 3, 4- trisphosphate 5/6 kinase-2 (Gmitpk2) as a dehydration and salinity stress regulator in Glycine max (L.) merr through heterologous expression in E.coli. Plant Physiol. Biochem. 2017, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Chen, Q.; Wang, X. Ositl1 gene encoding an inositol 1,3,4-trisphosphate 5/6-kinase is a negative regulator of osmotic stress signaling. Biotechnol. Lett. 2008, 30, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, L.H.; You, L.; Yang, M.; He, Y.B.; Li, X.H.; Xiong, L.Z. Characterization of an inositol 1,3,4-trisphosphate 5/6-kinase gene that is essential for drought and salt stress responses in rice. Plant Mol. Biol. 2011, 77, 547–563. [Google Scholar] [CrossRef]
- Kim, S.I.; Tai, T.H. Identification of novel rice low phytic acid mutations via TILLING by sequencing. Mol. Breed. 2014, 34, 1717–1729. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Chen, K.; Liang, Z. Targeted genome modification of crop plants using a CRISPR/CAS system. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar]
- Cao, H.X.; Wang, W.; Le, H.T.T.; Vu, G.T.H. The power of CRISPR/Cas9-induced genome editing to speed up plant breeding. Int. J. Genom. 2016, 10, 5078796. [Google Scholar] [CrossRef] [PubMed]
- Kumlehn, J.; Pietralla, J.; Hensel, G.; Pacher, M.; Puchta, H. The CRISPR/Cas revolution continues: From efficient gene editing for crop breeding to plant synthetic biology. J. Integr. Plant Biol. 2018, 60, 12. [Google Scholar] [CrossRef]
- Jung, C.; Capistrano-Gossmann, G.; Braatz, J.; Sashidhar, N.; Melzer, S. Recent developments in genome editing and applications in plant breeding. Plant Breed. 2017, 137. [Google Scholar] [CrossRef]
- Lu, H.P.; Luo, T.; Fu, H.W.; Wang, L.; Tan, Y.Y.; Huang, J.Z.; Wang, Q.; Ye, G.Y.; Gatehouse, A.M.R.; Lou, Y.G.; et al. Resistance of rice to insect pests mediated by suppression of serotonin biosynthesis. Nat. Plants 2018, 4, 338–344. [Google Scholar] [CrossRef]
- Liu, S.M.; Jiang, J.; Liu, Y.; Meng, J.; Xu, S.L.; Tan, Y.Y.; Li, Y.F.; Shu, Q.Y.; Huang, J.Z. Characterization and Evaluation of OsLCT1 and OsNramp5 Mutants Generated Through CRISPR/Cas9-Mediated Mutagenesis for Breeding Low Cd Rice. Rice Sci. 2019, 26, 88–97. [Google Scholar]
- Lei, Y.; Lu, L.; Liu, H.Y.; Li, S.; Xing, F.; Chen, L.L. CRISPR-P: A web tool for synthetic single-guide RNA design of CRISPR-system in plants. Mol. Plant 2014, 7, 1494–1496. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.P.; Liu, S.M.; Xu, S.L.; Chen, W.Y.; Zhou, X.; Tan, Y.Y.; Huang, J.Z.; Shu, Q.Y. CRISPR-S: An active interference element for a rapid and inexpensive selection of genome-edited, transgene-free rice plants. Plant Biotechnol. J. 2017, 15, 1371–1373. [Google Scholar] [CrossRef]
- Xu, R.F.; Li, H.; Qin, R.Y.; Wang, L.; Li, L.; Wei, P.C.; Yang, J.B. Gene targeting using the Agrobacterium tumefaciens-mediated CRISPR-Cas system in rice. Rice 2014, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, W.X.; Wu, S.L.; Liu, Y.H.; Jin, G.L.; Zhao, H.J.; Fan, L.J.; Shu, Q.Y. Genome-wide profiling of genetic variation in Agrobacterium-transformed rice plants. J. Zhejiang Univ. Sci. B 2016, 17, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Huang, J.Z.; Chen, X.Y.; Tan, Y.Y.; Shu, Q.Y. Competitive amplification of differentially melting amplicons facilitates efficient genotyping of photoperiod-and temperature-sensitive genic male sterility in rice. Mol. Breed. 2014, 34, 1765–1776. [Google Scholar] [CrossRef]
- Li, W.L.; Xu, B.B.; Song, Q.J.; Liu, X.M.; Xu, J.M.; Brookes, P.C. The identification of ‘hotspots’ of heavy metal pollution in soil-rice systems at a regional scale in eastern China. Sci. Total Environ. 2014, 472, 407–420. [Google Scholar] [CrossRef]
- Li, S.; Liu, S.M.; Liu, Y.H.; Lu, H.P.; Tan, Y.Y.; Huang, J.Z.; Wei, P.C.; Shu, Q.Y. HRM-facilitated rapid identification and genotyping of mutations induced by CRISPR/Cas9 mutagenesis in rice. Crop Breed. Appl. Biotechnol. 2018, 18, 184–191. [Google Scholar] [CrossRef]
- Liu, W.Z.; Xie, X.R.; Ma, X.L.; Li, J.; Chen, J.H.; Liu, Y.G. DSDecode: Aweb-based tool for decoding of sequencing chromatograms for genotyping of targeted mutations. Mol. Plant 2015, 8, 1431–1433. [Google Scholar] [CrossRef]
- Campion, B.; Sparvoli, F.; Doria, E.; Tagliabue, G.; Galasso, I.; Fileppi, M.; Bollini, R.; Nielsen, E. Isolation and characterization of an lpa (low phytic acid) mutant in common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2009, 118, 1211–1221. [Google Scholar] [CrossRef]
- Chen, P.S.; Toribara, T.Y.; Warner, H. Micro determination of phosphorous. Anal. Chem. 1956, 28, 1756–1758. [Google Scholar] [CrossRef]
- Wilcox, J.R.; Premachandra, G.S.; Young, K.A.; Raboy, V. Isolation of high seed inorganic P, low-phytate soybean mutants. Crop Sci. 2000, 40, 1601–1605. [Google Scholar] [CrossRef]
- McKie, V.A.; McCleary, B.V.A. Novel and rapid colorimetric method for measuring total phosphorus and phytic acid in foods and animal feeds. J. AOAC Int. 2016, 99, 738–743. [Google Scholar] [CrossRef]
- Hu, L.F.; McBride, M.B.; Cheng, H.; Wu, J.J.; Shi, J.C.; Xu, J.M.; Wu, L.S. Root-induced changes to cadmium speciation in the rhizosphere of two rice (Oryza sativa L.) genotypes. Environ. Res. 2011, 111, 356–361. [Google Scholar] [CrossRef]
- Meng, J.; Zhong, L.B.; Wang, L.; Liu, X.M.; Tang, C.X.; Chen, H.L.; Xu, J.M. Contrasting effects of alkaline amendments on the bioavailability and uptake of Cd in rice plants in a Cd-contaminated acid paddy soil. Environ. Sci. Pollut. R. 2018, 25, 8827–8835. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plantarum 1962, 15, 473–497. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.; Liu, Y.; Liu, Y.; Tan, Y.; Huang, J.; Shu, Q. Mutation of Inositol 1,3,4-trisphosphate 5/6-kinase6 Impairs Plant Growth and Phytic Acid Synthesis in Rice. Plants 2019, 8, 114. https://doi.org/10.3390/plants8050114
Jiang M, Liu Y, Liu Y, Tan Y, Huang J, Shu Q. Mutation of Inositol 1,3,4-trisphosphate 5/6-kinase6 Impairs Plant Growth and Phytic Acid Synthesis in Rice. Plants. 2019; 8(5):114. https://doi.org/10.3390/plants8050114
Chicago/Turabian StyleJiang, Meng, Yang Liu, Yanhua Liu, Yuanyuan Tan, Jianzhong Huang, and Qingyao Shu. 2019. "Mutation of Inositol 1,3,4-trisphosphate 5/6-kinase6 Impairs Plant Growth and Phytic Acid Synthesis in Rice" Plants 8, no. 5: 114. https://doi.org/10.3390/plants8050114
APA StyleJiang, M., Liu, Y., Liu, Y., Tan, Y., Huang, J., & Shu, Q. (2019). Mutation of Inositol 1,3,4-trisphosphate 5/6-kinase6 Impairs Plant Growth and Phytic Acid Synthesis in Rice. Plants, 8(5), 114. https://doi.org/10.3390/plants8050114





