Flowering and Morphogenesis of Kalanchoe in Response to Quality and Intensity of Night Interruption Light
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Photoperiodic Light Treatments
2.3. Data Collection and Statistical Analysis
2.3.1. Growth Characteristics
2.3.2. SPAD Value and Chlorophyll Estimation
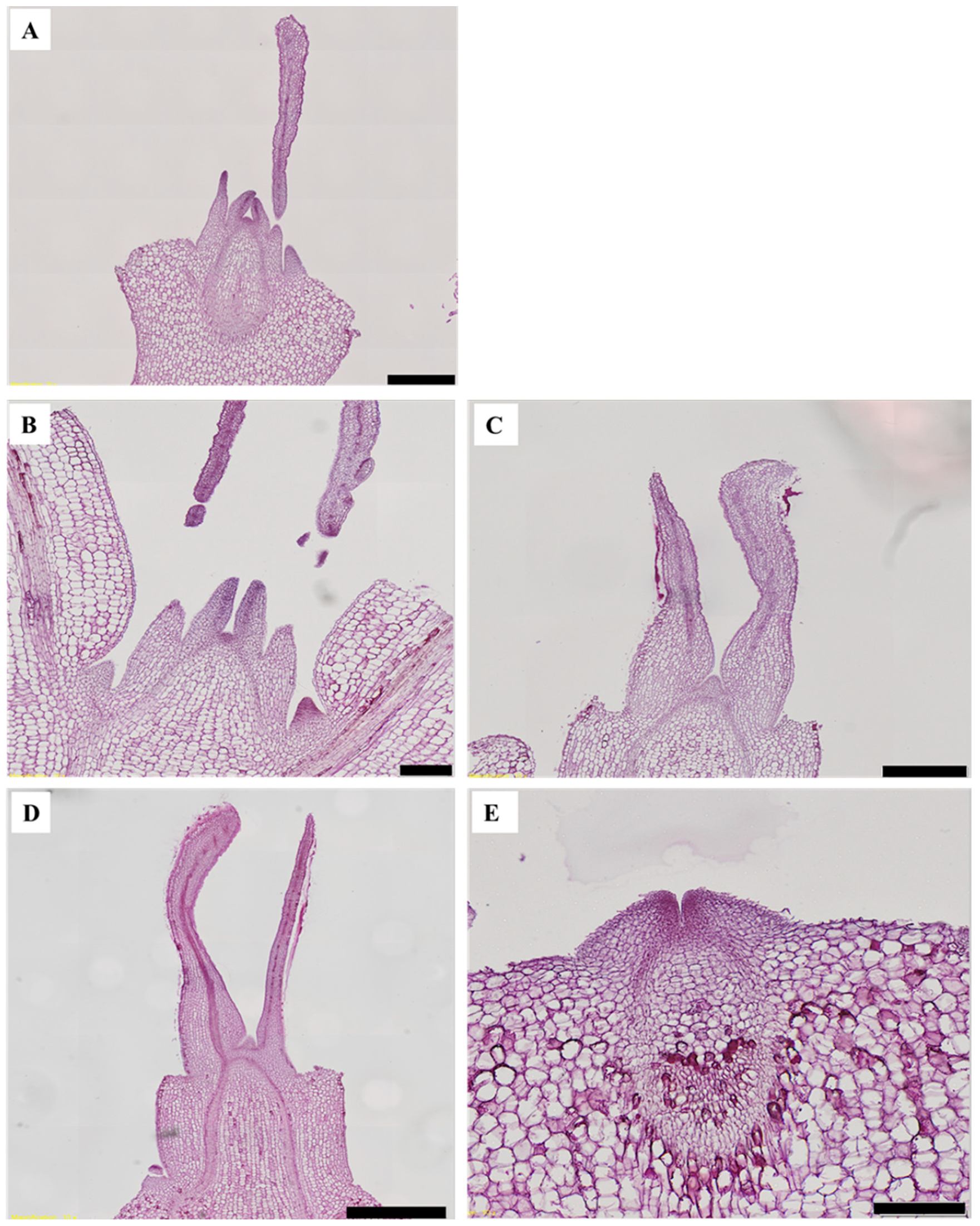
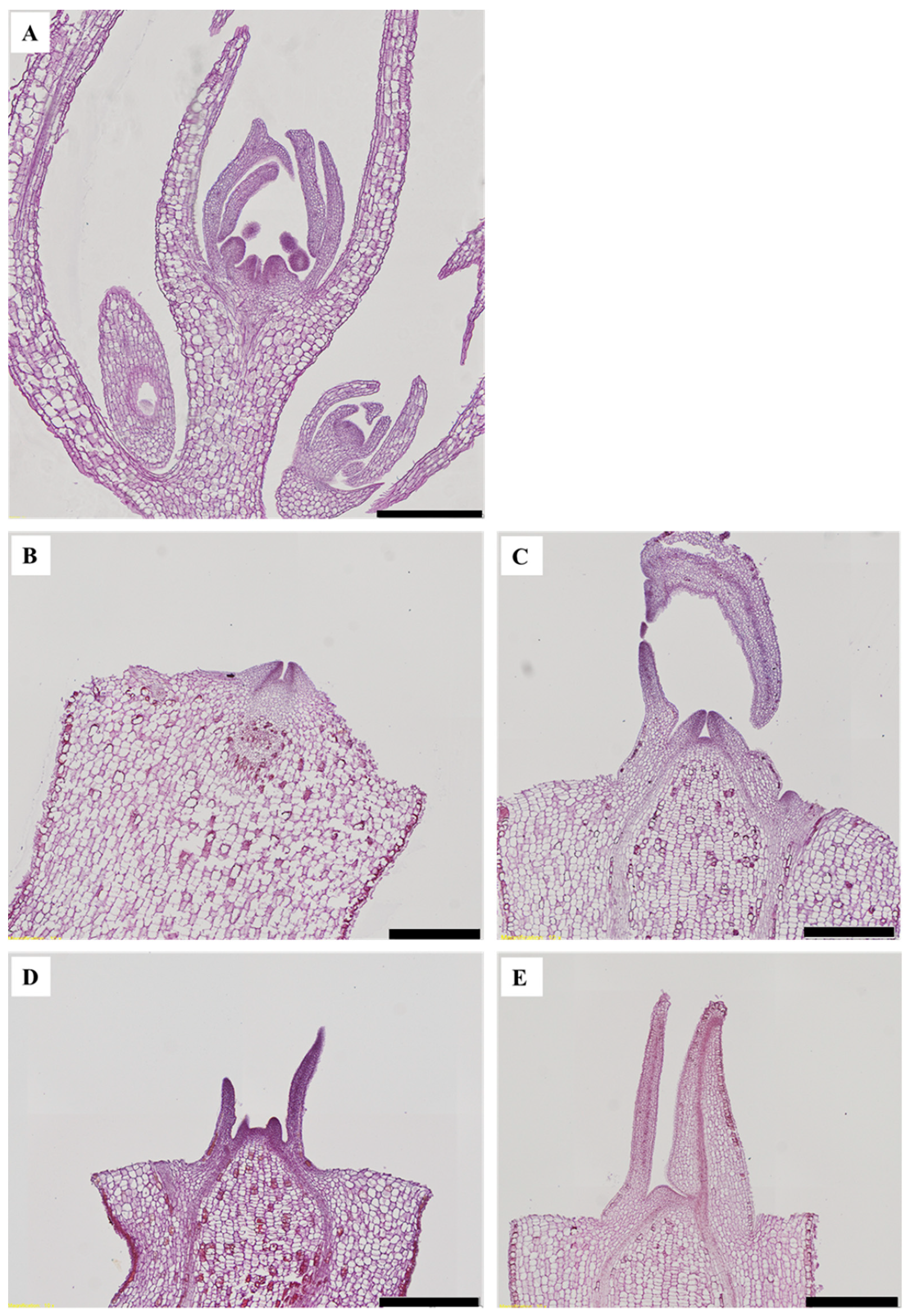
2.3.3. Harvesting, Microtomization, and Staining
2.3.4. Statistical Analysis
3. Results
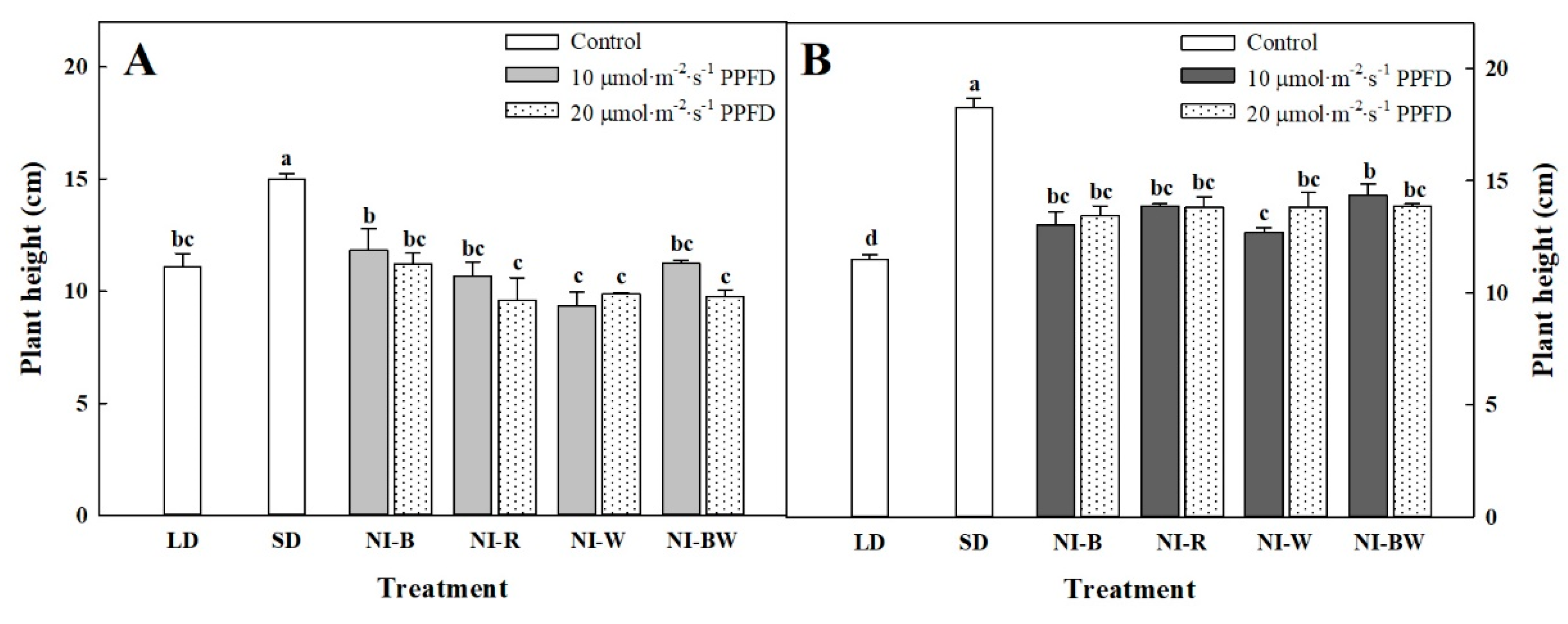
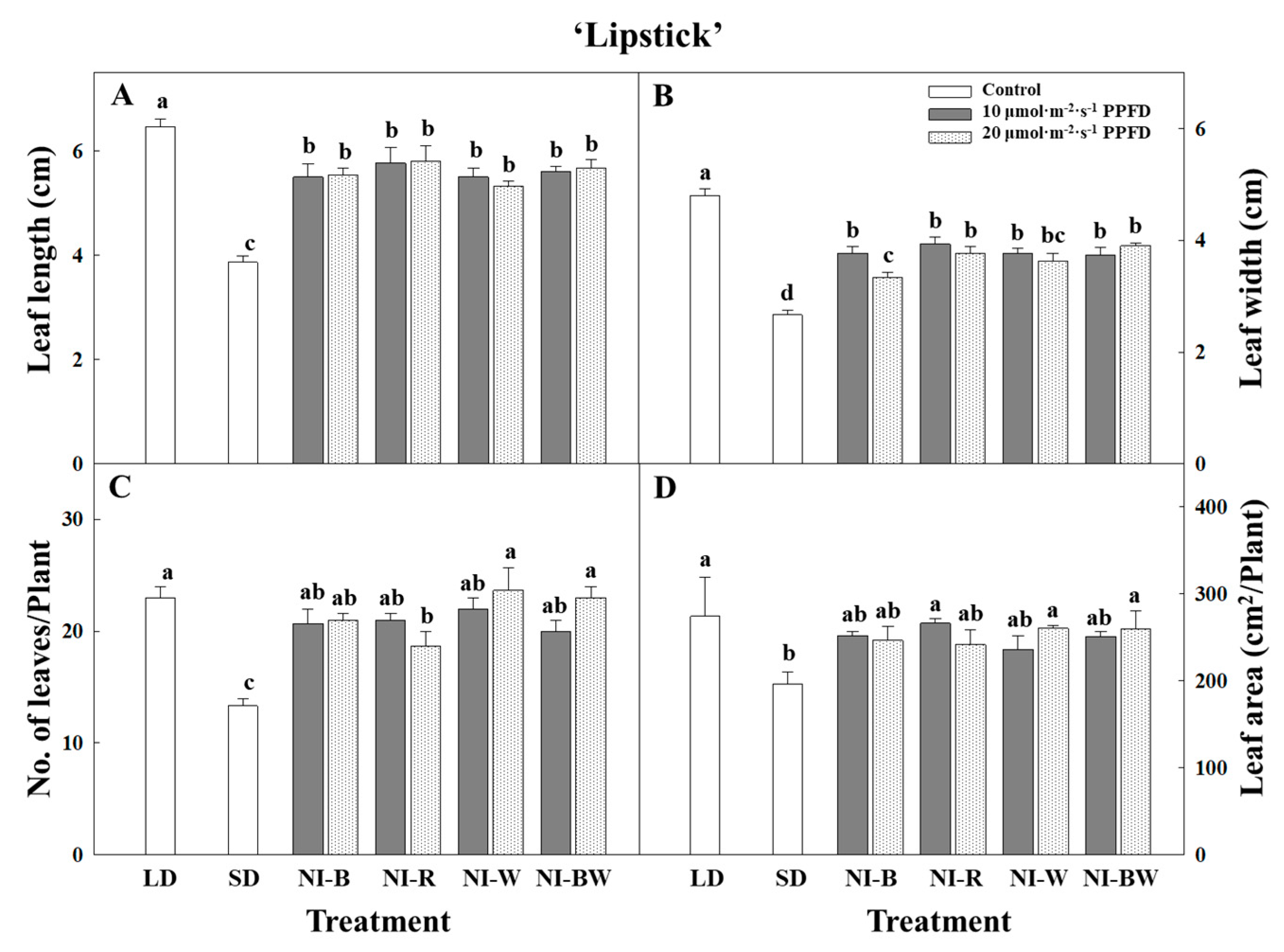
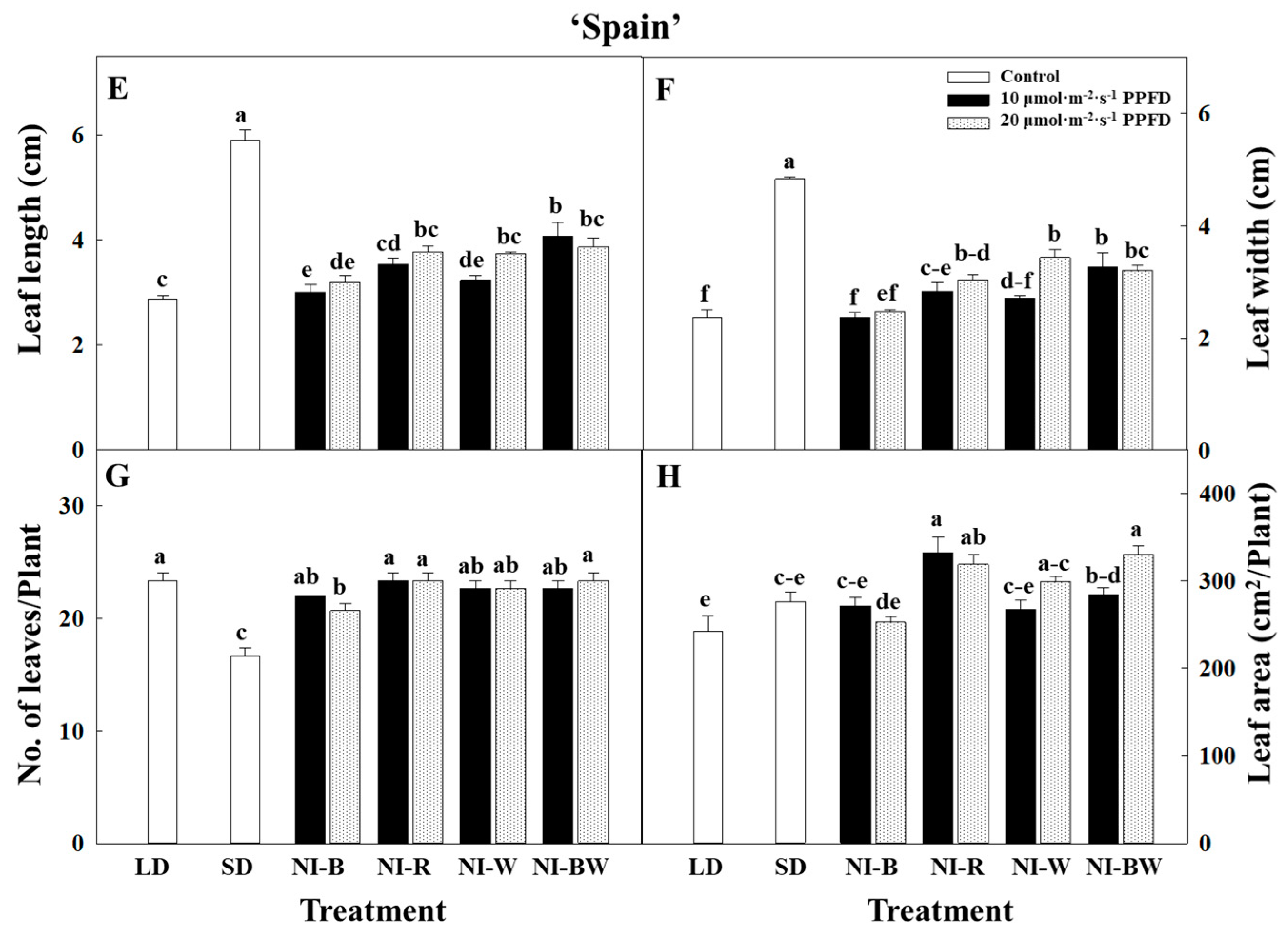
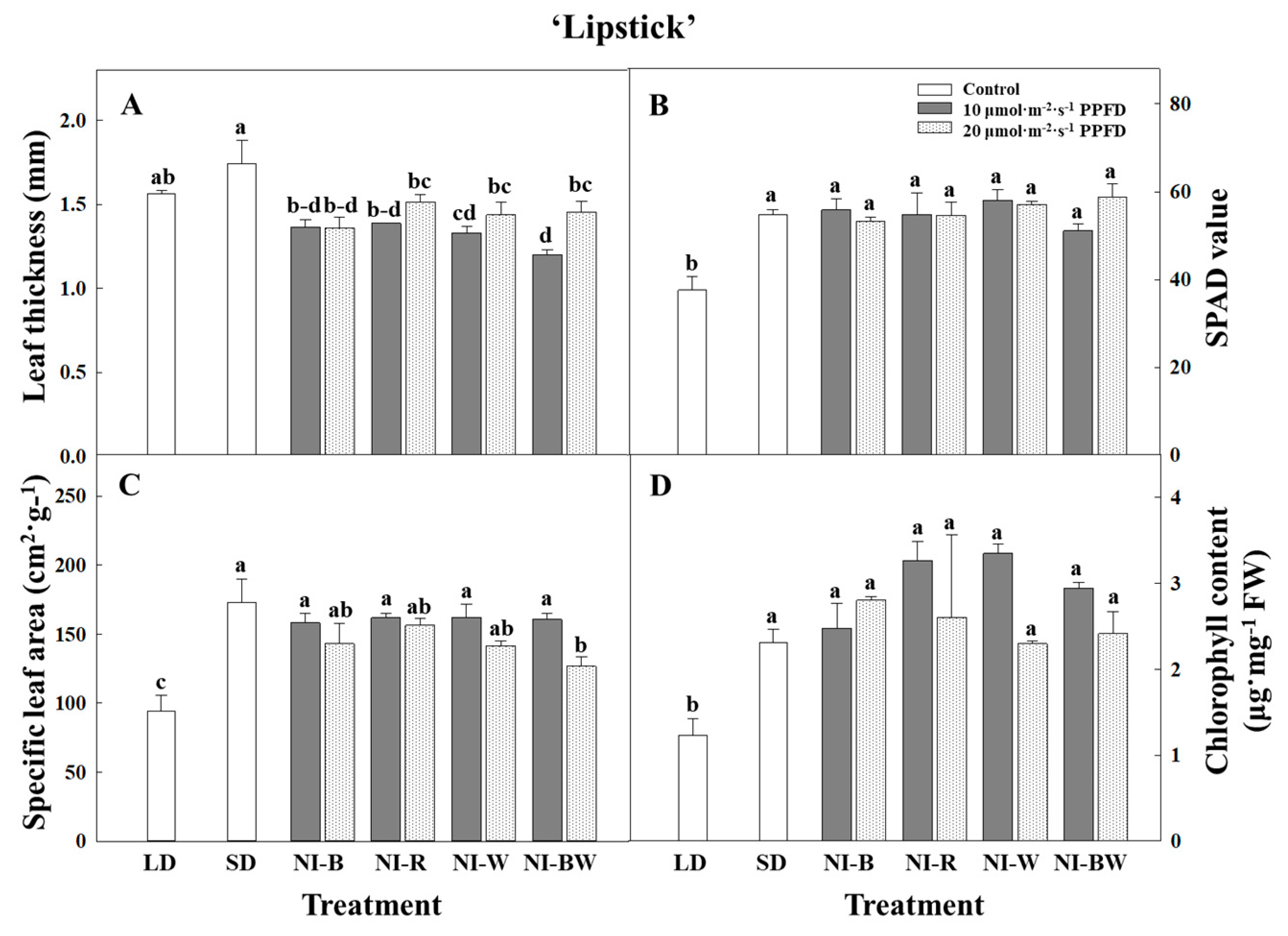
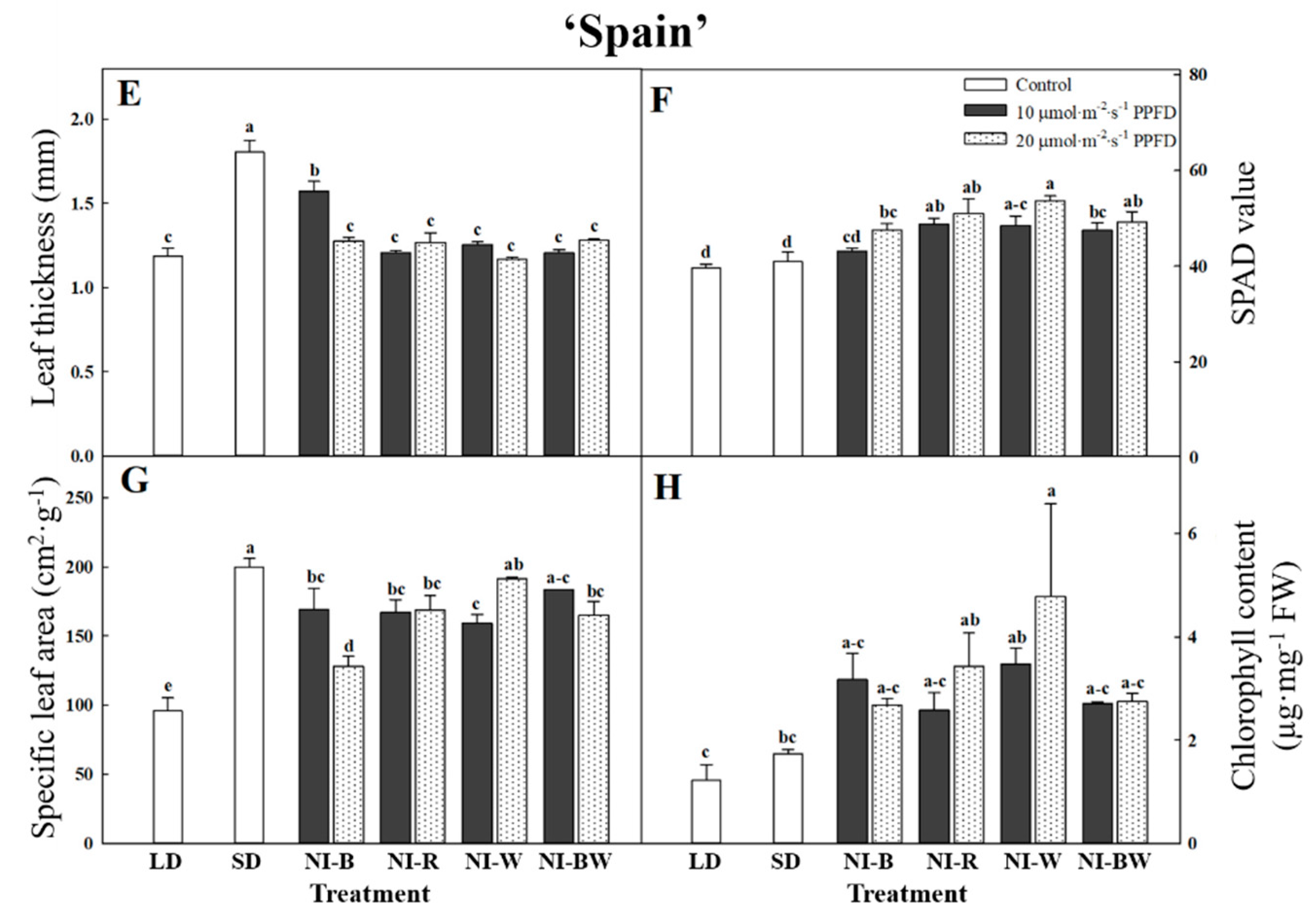
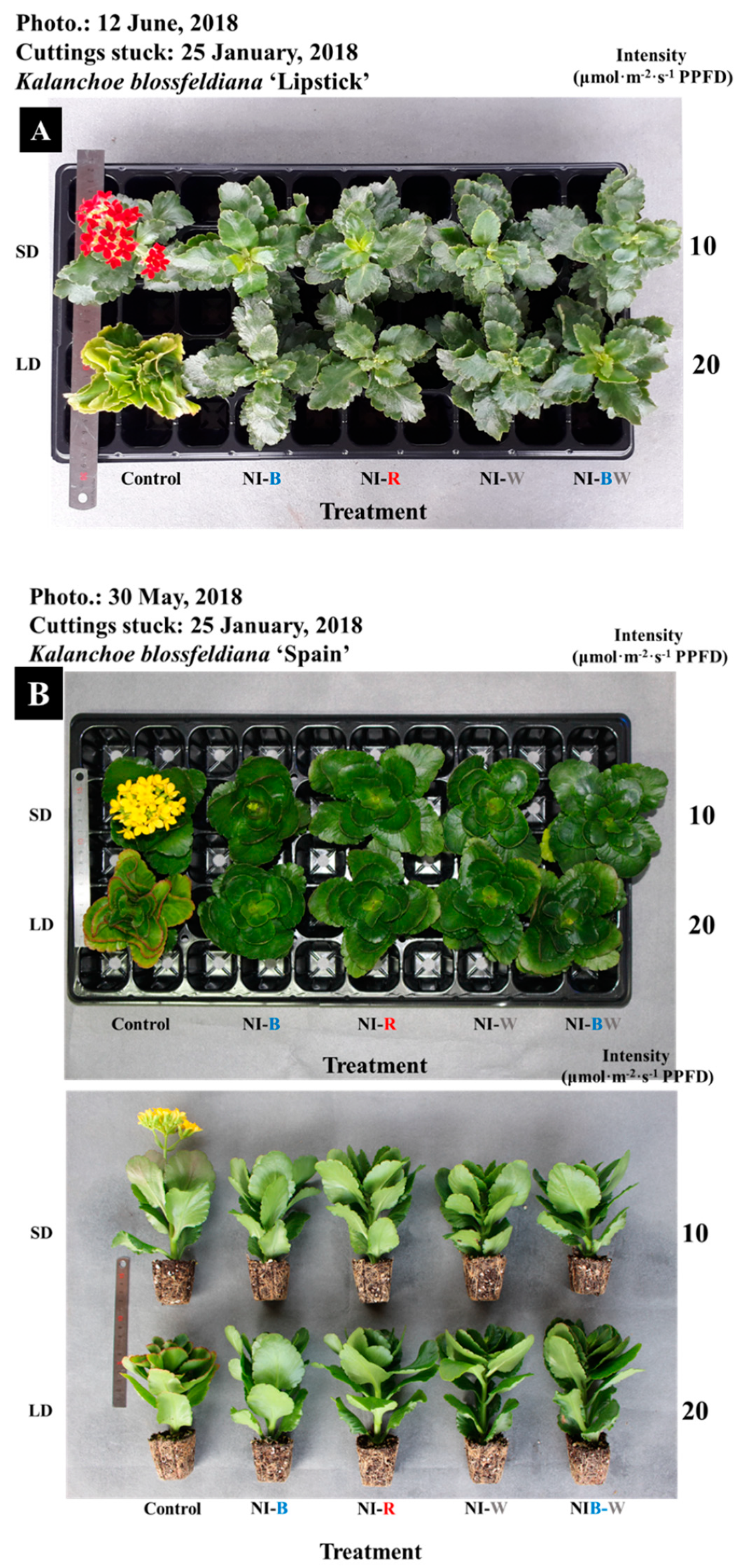
3.1. Morphogenesis
3.2. Flowering
4. Discussion
4.1. Morphogenesis
4.2. Flowering
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zheng, L.; Labeke, M. Effects of different irradiation levels of light quality on chrysanthemum. Sci. Hortic. 2018, 233, 124–131. [Google Scholar] [CrossRef]
- Folta, K.; Carvalho, S. Photoreceptors and control of horticultural plant traits. HortScience 2015, 50, 1274–1280. [Google Scholar] [CrossRef]
- Galvão, V.; Fankhauser, C. Sensing the light environment in plants: Photo receptors and early signaling steps. Curr. Opin. Neurobiol. 2015, 34, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, H.; Hideo, S.; Tadahisa, H. Budding response of horticultural crops to night break with red light on alternate days. Environ. Control Biol. 2005, 43, 21–27. [Google Scholar] [CrossRef]
- Park, Y.J.; Kim, Y.J.; Kim, K.S. Vegetative growth and flowering of Dianthus, Zinnia, and Pelargonium as affected by night interruption at different timings. Hortic. Environ. Biotechnol. 2013, 54, 236–242. [Google Scholar] [CrossRef]
- Adams, S.R.; Valdes, V.M.; Langton, F.A. Why does low intensity, long-day lighting promote growth in petunia, impatiens, and tomato? J. Hortic. Sci. Biotechnol. 2008, 83, 609–615. [Google Scholar] [CrossRef]
- Damann, M.P.; Lyons, R.E. Natural chilling and limited inductive photoperiod affect flowering in two Asteraceae genera. J. Am. Soc. Hortic. Sci. 1996, 121, 694–698. [Google Scholar] [CrossRef]
- Runkle, E.S.; Heins, R.D.; Cameron, A.C.; Carlson, W.H. Flowering of herbaceous perennials under various night interruption and cyclic lighting treatments. HortScience 1998, 33, 672–677. [Google Scholar]
- Kang, K.J.; Oh, W.; Shin, J.H.; Kim, K.S. Night interruption and cyclic lighting promote flowering of Cyclamen persicum under low temperature regime. Hortic. Environ. Biotechnol. 2008, 49, 72–77. [Google Scholar]
- Meng, Q.; Runkle, E.S. Low-intensity blue light in night-interruption lighting does not influence flowering of herbaceous ornamentals. Sci. Hortic. 2015, 186, 230–238. [Google Scholar] [CrossRef]
- Blanchard, M.G.; Runkle, E.S. Use of a cyclic high-pressure sodium lamp to inhibit flowering of chrysanthemum and velvet sage. Sci. Hortic. 2009, 122, 448–454. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, H.J.; Kim, K.S. Night interruption promotes vegetative growth and flowering of Cymbidium. Sci. Hortic. 2011, 130, 887–893. [Google Scholar] [CrossRef]
- Thomas, B.; Vince-Prue, D. Some general principle. In Photoperiodism in Plants, 2nd ed.; Thomas, B., Vince-Prue, D., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 3–26. ISBN 9780126884906. [Google Scholar]
- Craig, D.S.; Runkle, E.S. Using LEDs to quantify the effect of the red to far-red ratio of night-interruption lighting on flowering of photoperiodic crops. Acta Hortic. 2012, 956, 179–185. [Google Scholar] [CrossRef]
- Park, Y.G.; Muneer, S.; Soundararajan, P.; Manivanan, A.; Jeong, B.R. Light quality during night interruption affects morphogenesis and flowering in geranium. Hortic. Environ. Biotechnol. 2017, 58, 212–217. [Google Scholar] [CrossRef]
- Shin, J.H.; Jung, H.H.; Kim, K.S. Night interruption using light emitting diodes (LEDs) promotes flowering of Cyclamen persicum in winter cultivation. Hortic. Environ. Biotechnol. 2010, 51, 391–395. [Google Scholar]
- Higuchi, Y.; Sumitomo, K.; Oda, A.; Shimizu, H.; Hisamatsu, T. Days light quality affects the night-break response in the short day plant chrysanthemum, suggesting differential phytochrome-mediated regulation of flowering. J. Plant. Physiol. 2012, 169, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.H.; Yang, C.M.; Hsiao, C.L. Effects of nighttime lighting with specific wavebands on flowering and flower quality of chrysanthemum. Crop Environ. Bioinform. 2012, 9, 265–277. [Google Scholar]
- Park, Y.G.; Muneer, S.; Jeong, B.R. Morphogenesis, flowering, and gene expression of Dendranthema grandiflorum in response to shift in light quality of night interruption. Int. J. Mol. Sci. 2015, 16, 16497–16513. [Google Scholar] [CrossRef]
- Dere, S.; Gunes, T.; Sivaci, R. Spectrophotometric determination of chlorophyll-a, b and total carotenoid contents of some algae species using different solvents. Turk J. Bot. 1998, 22, 13–17. [Google Scholar]
- Karnovsky, M.J. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 137–138. [Google Scholar]
- Winter, K.; Holtum, J.A.M. Facultative crassulacean acid metabolism (CAM) plants: Powerful tools for unravelling the functional elements of CAM photosynthesis. J. Exp. Bot. 2014, 65, 3425–3441. [Google Scholar] [CrossRef] [PubMed]
- Negin, B.; Moshelion, M. The evolution of the role of ABA in the regulation of water-use efficiency: From biochemical mechanisms to stomatal conductance. Plant Sci. 2016, 251, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Noh, E.H.; Jun, H.J.; Son, J.E. Growth characteristics and nutrient uptake of kalanchoe plants (Kalanchoe blossfeldiana ‘Marlene’) at different light intensities and nutrient strengths in ebb and flow subirrigation systems. Korean J. Hortic. Sci. Technol. 2011, 29, 187–194. [Google Scholar]
- Rustin, P.; Queiroz-Clare, C. Changes in oxidative properties of Kalanchoe blossfeldiana leaf mitochondria during development of Crassulacean acid metabolism. Planta 1985, 164, 415–422. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Shimizu, H.; Saito, Y.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. Light environment optimization for lettuce growth in plant factory. IFAC Proc. 2011, 44, 605–609. [Google Scholar] [CrossRef]
- Wu, M.C.; Hou, C.Y.; Jiang, C.M.; Wang, Y.T.; Wang, C.Y.; Chen, H.H.; Chang, H.M. A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem. 2007, 101, 1753–1758. [Google Scholar] [CrossRef]
- Nishimura, T.; Ohyama, K.; Goto, E.; Inagaki, N. Concentrations of perillaldehyde, limonene, and anthocyanin of Perilla plants as affected by light quality under controlled environments. Sci. Hortic. 2009, 122, 134–137. [Google Scholar] [CrossRef]
- Kim, H.R.; You, T.H. Effects of red, blue, white, and far-red LED source on growth responses of Wasabia japonica seedlings in plant factory. Korean J. Hortic. Sci. Technol. 2013, 31, 415–422. [Google Scholar] [CrossRef]
- Brulfert, J.; Müller, D.; Kluge, M.; Queiroz, O. Photoperiodism and crassulacean acid metabolism. I. Immunological and kinetic evidences for different patterns of phosphoenolpyruvate carboxylase isoforms in photoperiodically inducible and non-inducible crassulacean acid metabolism plants. Planta 1982, 154, 326–331. [Google Scholar] [CrossRef]
- Pons, T.L.; Pearcy, R.W. Nitrogen reallocation and photosynthetic acclimation in response to partial shading in soybean plants. Physiol. Plant 1994, 92, 636–644. [Google Scholar] [CrossRef]
- Mitchell, K.A.; Bolstad, P.V.; Vose, J.M. Interspecific and environmentally induced variation in foliar dark respiration among eighteen southeastern deciduous tree species. Tree Physiol. 1999, 19, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Pooter, H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Friend, D.J.C.; Lydon, J. Effects of daylength on flowering, growth, and CAM of pineapple (Ananas comosus L. Merril). Bot. Gaz. 1979, 140, 280–283. [Google Scholar] [CrossRef]
- Salomon, M.; Christie, J.M.; Knieb, E.; Lempert, U.; Briggs, W.R. Photochemical and mutational analysis of the FMN-binding domains of the plant blue light receptor, phototropin. Biochemistry 2000, 39, 9401–9410. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.M.; Swartz, T.E.; Bogomolni, R.A.; Briggs, W.R. Phototropin LOV domains exhibit distinct roles in regulating photoreceptor function. Plant J. 2002, 32, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, M.; Kagawa, T.; Oikawa, K.; Suetsugu, N.; Miyao, M.; Wada, M. Chloroplast avoidance movement reduces photodamage in plants. Nature 2002, 420, 829–832. [Google Scholar] [CrossRef]
- Chen, M.; Chory, J.; Fankhauser, C. Light signal transduction in higher plants. Annu. Rev. Genet. 2004, 38, 87–117. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.; Bradley, J.M.; Harberd, N.P.; Whitelam, G.C. Photoresponses of light-grown phyA mutants of Arabidopsis: Phytochrome A is required for the perception of day length extensions. Plant Physiol. 1994, 105, 141–149. [Google Scholar] [CrossRef]
- Reed, J.W.; Nagatani, A.; Elich, T.D.; Fagan, M.; Chory, J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994, 104, 1139–1149. [Google Scholar] [CrossRef]
- Neff, M.M.; Chory, J. Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol. 1998, 118, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Takano, M.; Inagaki, N.; Xie, X.; Yuzurihara, N.; Hihara, F.; Ishizuka, T.; Yano, M.; Nishimura, M.; Miyao, A.; Hirochika, H.; et al. Distinct and cooperative function of phytochromes A, B, and C in the control of deetiolation and flowering in rice. Plant Cell 2005, 17, 3311–3325. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T. Plant blue-light receptors. Trends Plant Sci. 2000, 5, 337–342. [Google Scholar] [CrossRef]
- Franklin, K.A.; Quail, P.H. Phytochrome functions in Arabidopsis development. J. Exp. Bot. 2010, 16, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Zaccai, M.; Edri, N. Floral transition in lisianthus (Eustoma grandiflorum). Sci. Hortic. 2002, 95, 333–340. [Google Scholar] [CrossRef]
- Islam, N.; Patil, G.G.; Gislerød, H.R. Effect of photoperiod and light integral on flowering and growth of Eustoma grandiflorum (Raf.) Shinn. Sci. Hortic. 2005, 103, 441–451. [Google Scholar] [CrossRef]

| Photoperiod | Light Quality z (A) | Light Intensity (µmol·m−2·s−1 PPFD) (B) | Fresh Weight (g) | Dry Weight (g) | ||
|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | |||
| LD | 30.1 ± 1.7 | 3.3 ± 0.3 | 3.48 ± 0.2 a y | 0.35 ± 0.1 | ||
| SD | 29.7 ± 1.7 | 2.5 ± 0.1 | 1.85 ± 0.1 cd | 0.26 ± 0.0 | ||
| NI | B | 10 | 27.1 ± 0.5 | 2.4 ± 0.2 | 1.95 ± 0.1 b-d | 0.27 ± 0.0 |
| 20 | 26.9 ± 1.7 | 2.0 ± 0.2 | 2.19 ± 0.1 b | 0.25 ± 0.0 | ||
| R | 10 | 27.9 ± 0.5 | 2.4 ± 0.2 | 2.02 ± 0.1 b-d | 0.24 ± 0.0 | |
| 20 | 25.8 ± 1.8 | 2.1 ± 0.2 | 1.92 ± 0.2 cd | 0.24 ± 0.0 | ||
| W | 10 | 25.7 ± 2.0 | 2.0 ± 0.2 | 1.85 ± 0.2 d | 0.20 ± 0.0 | |
| 20 | 28.2 ± 0.8 | 2.0 ± 0.2 | 2.28 ± 0.1 bc | 0.20 ± 0.0 | ||
| BW | 10 | 27.0 ± 0.7 | 2.2 ± 0.2 | 1.91 ± 0.1 b-d | 0.25 ± 0.0 | |
| 20 | 28.3 ± 2.0 | 2.5 ± 0.2 | 2.48 ± 0.1 b | 0.50 ± 0.2 | ||
| F-test x | Photoperiod | NS | NS | ** | NS | |
| Quality (A) | NS | NS | NS | NS | ||
| Intensity (B) | NS | NS | ** | NS | ||
| A × B | NS | NS | NS | NS | ||
| Photoperiod | Light Quality z (A) | Light Intensity (µmol·m−2·s−1 PPFD) (B) | Fresh Weight (g) | Dry Weight (g) | ||
|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | |||
| LD | 33.2 ± 1.3 b y | 2.9 ± 0.1 b | 3.18 ± 0.1 a | 0.29 ± 0.0 b | ||
| SD | 42.7 ± 0.6 a | 3.3 ± 0.3 b | 2.17 ± 0.0 c-f | 0.26 ± 0.0 bc | ||
| NI | B | 10 | 33.1 ± 1.4 bc | 3.0 ± 0.2 b | 1.98 ± 0.1 c-f | 0.26 ± 0.0 b |
| 20 | 29.9 ± 0.7 cd | 2.4 ± 0.3 b | 2.41 ± 0.1 b | 0.44 ± 0.0 a | ||
| R | 10 | 33.1 ± 1.2 b | 3.9 ± 0.8 b | 2.40 ± 0.2 b-e | 0.30 ± 0.1 c | |
| 20 | 35.3 ± 1.2 b | 4.5 ± 0.2 a | 2.28 ± 0.1 b-d | 0.26 ± 0.0 bc | ||
| W | 10 | 29.6 ± 0.9 d | 2.2 ± 0.4 c | 1.99 ± 0.1 f | 0.21 ± 0.0 d | |
| 20 | 33.0 ± 0.4 bc | 3.0 ± 0.3 b | 1.88 ± 0.0 d-f | 0.22 ± 0.0 bc | ||
| BW | 10 | 31.9 ± 1.0 bc | 2.5 ± 0.1 b | 2.37 ± 0.0 ef | 0.28 ± 0.0 bc | |
| 20 | 37.9 ± 1.1 a | 2.8 ± 0.2 b | 2.45 ± 0.1 bc | 0.27 ± 0.0 bc | ||
| F-test x | Photoperiod | *** | NS | *** | NS | |
| Quality (A) | ** | *** | * | * | ||
| Intensity (B) | ** | NS | * | NS | ||
| A × B | ** | NS | ** | * | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, D.I.; Jeong, H.K.; Park, Y.G.; Jeong, B.R. Flowering and Morphogenesis of Kalanchoe in Response to Quality and Intensity of Night Interruption Light. Plants 2019, 8, 90. https://doi.org/10.3390/plants8040090
Kang DI, Jeong HK, Park YG, Jeong BR. Flowering and Morphogenesis of Kalanchoe in Response to Quality and Intensity of Night Interruption Light. Plants. 2019; 8(4):90. https://doi.org/10.3390/plants8040090
Chicago/Turabian StyleKang, Dong Il, Hai Kyoung Jeong, Yoo Gyeong Park, and Byoung Ryong Jeong. 2019. "Flowering and Morphogenesis of Kalanchoe in Response to Quality and Intensity of Night Interruption Light" Plants 8, no. 4: 90. https://doi.org/10.3390/plants8040090
APA StyleKang, D. I., Jeong, H. K., Park, Y. G., & Jeong, B. R. (2019). Flowering and Morphogenesis of Kalanchoe in Response to Quality and Intensity of Night Interruption Light. Plants, 8(4), 90. https://doi.org/10.3390/plants8040090






