Structural Evidence of Programmed Cell Death Induction by Tungsten in Root Tip Cells of Pisum sativum
Abstract
1. Introduction
2. Results
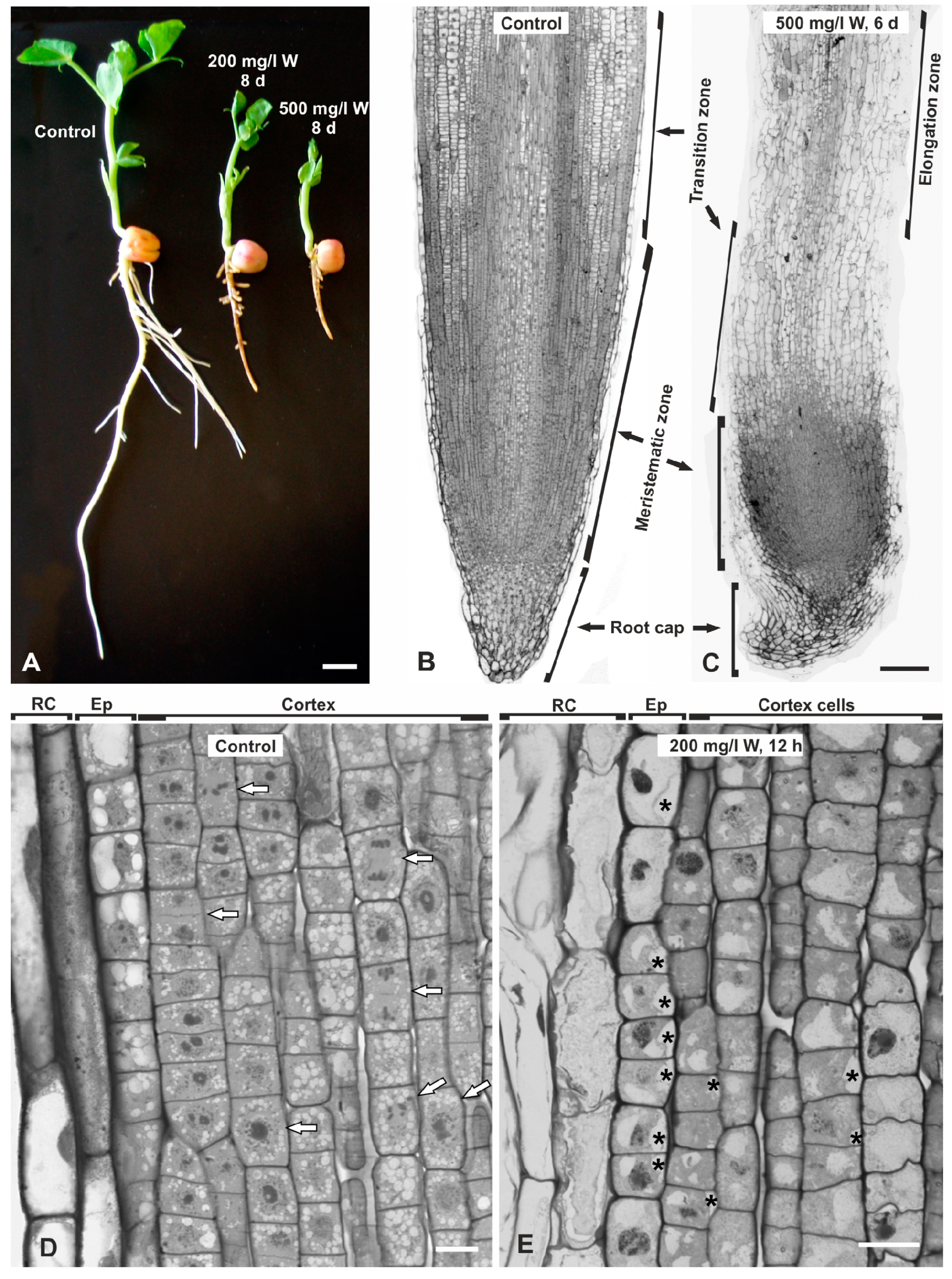
2.1. Macroscopic and Microscopic Effects
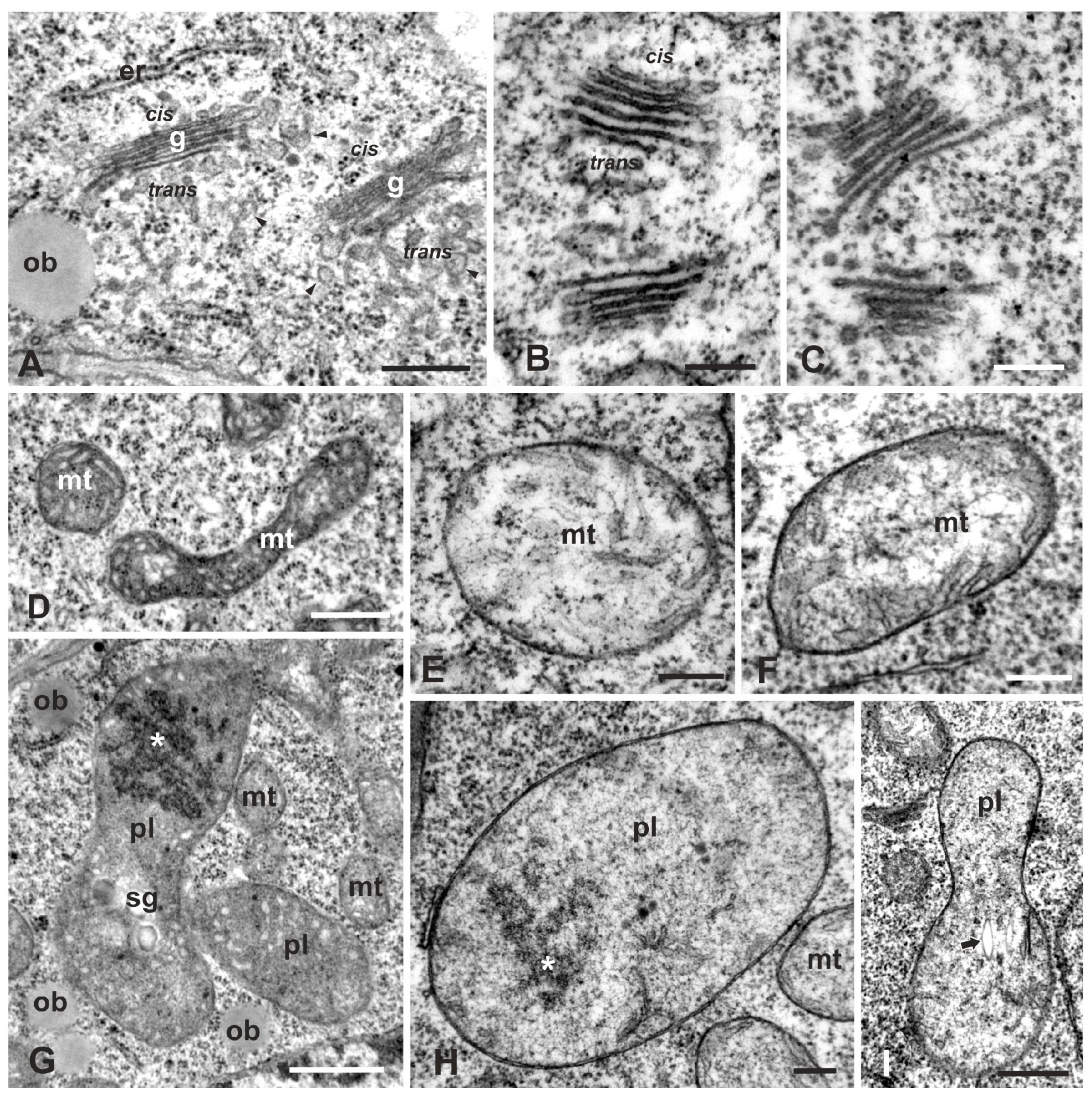
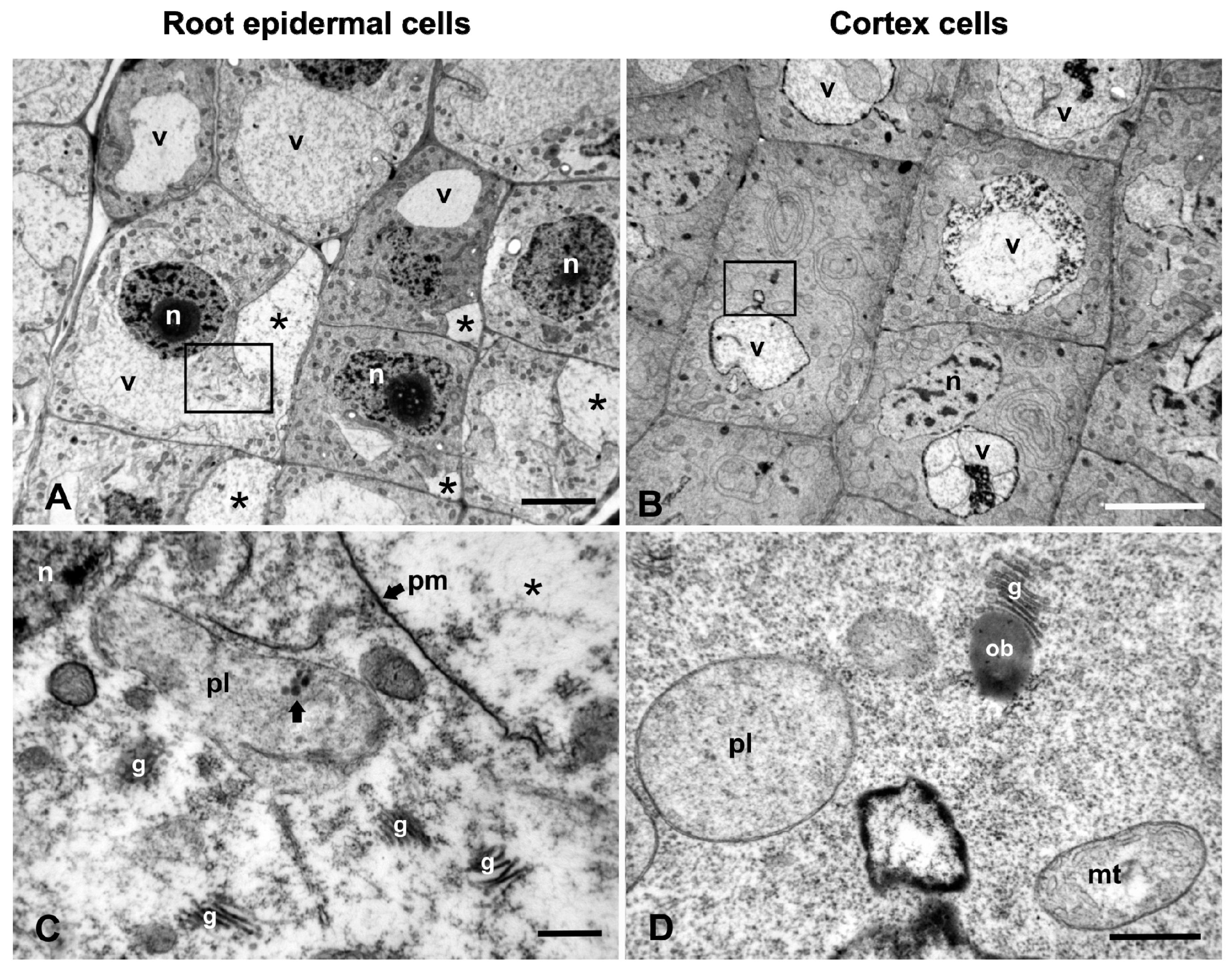
2.2. Ultrastructural Malformations
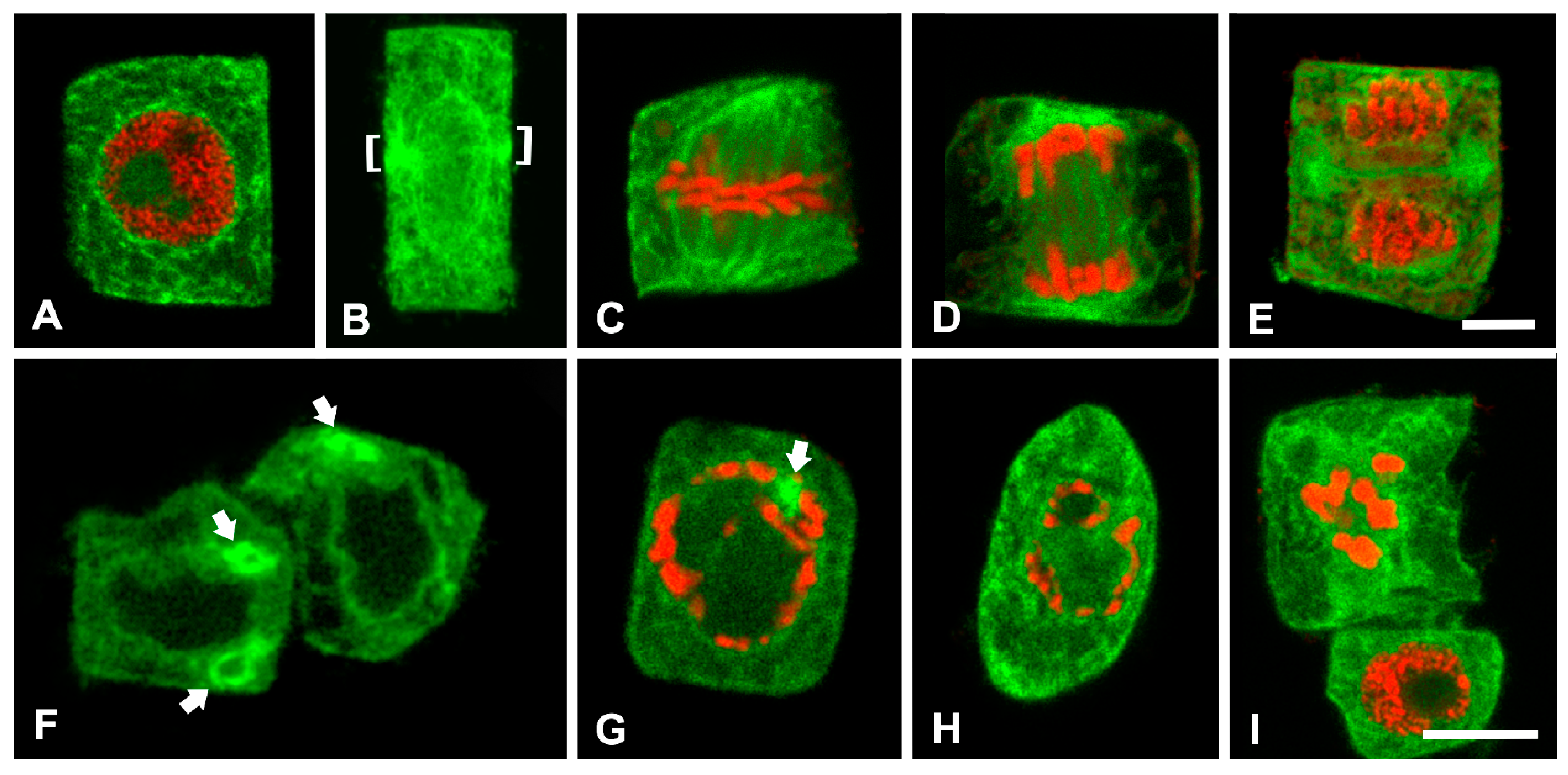
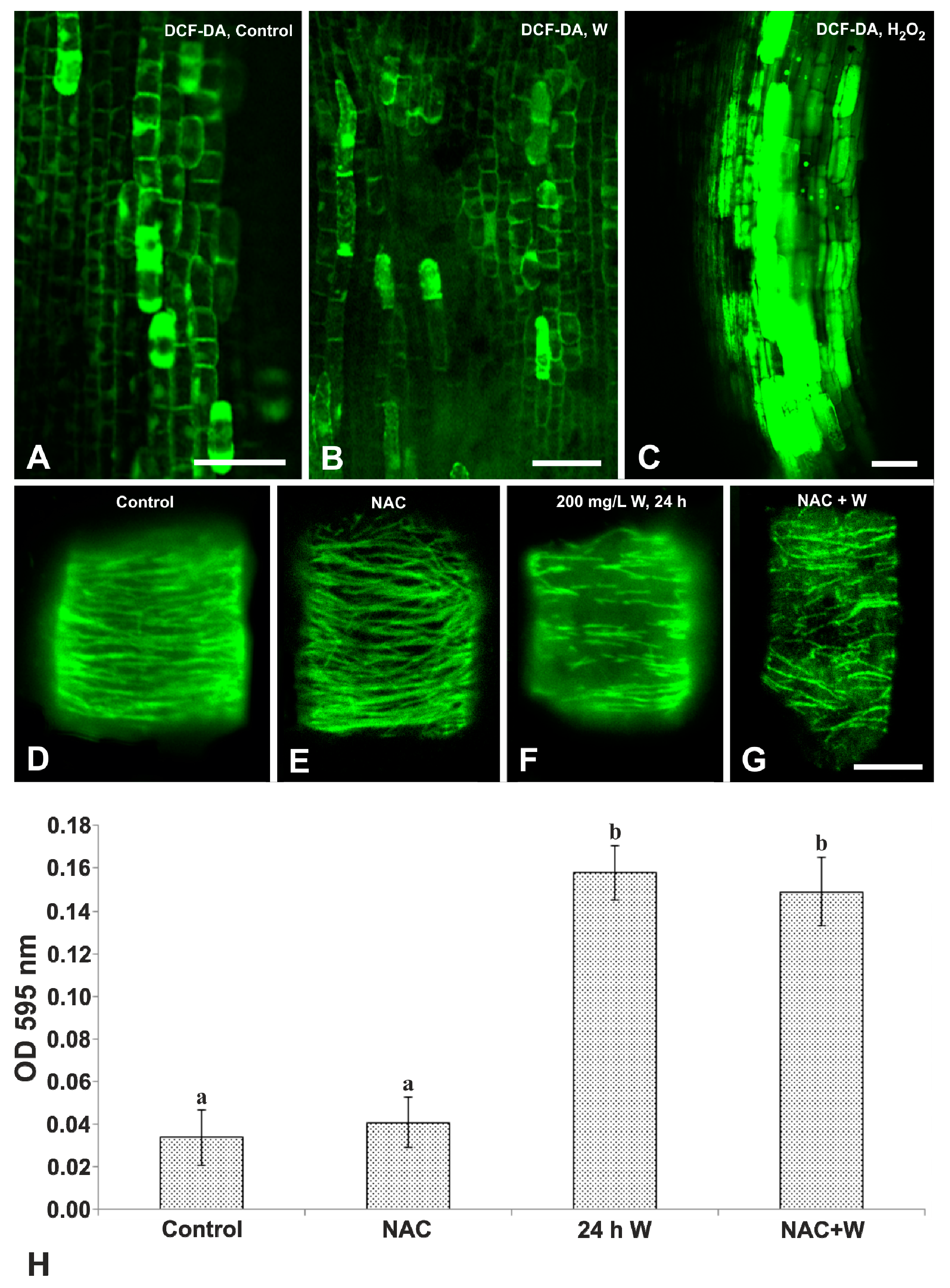
2.3. Effects of W on ER Distribution and ROS Production
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Material Preparation for Light and Electron Microscopy
5.2. ER Immunolocalization
5.3. In Situ Detection of Hydrogen Peroxide with DCF-DA
5.4. Estimation of ROS with NAC and of Dead Cells with Evans Blue
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | actin filament |
| CLSM | confocal laser scanning microscope(y) |
| Cr (VI) | hexavalent chromium |
| DCF-DA | 2,7-dichlorofluorescein diacetate |
| ER | endoplasmic reticulum |
| ER-PPB | preprophase band of ER |
| H2O2 | hydrogen peroxide |
| MT | microtubule |
| MT-PPB | preprophase band of MTs |
| NAC | N-acetylcysteine |
| PCD | programmed cell death |
| ROS | reactive oxygen species |
| TEM | transmission electron microscopy |
| W | tungsten |
References
- Beyersmann, D.; Hartwig, A. Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Arch. Toxicol. 2008, 82, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. (Ed.) Heavy Metals in Soils. In Trace Metals and Metalloids in Soils and Their Bioavailability; Environmental Pollution Book Series; Springer Science+Business Media: Dordrecht, Germany, 2013; Volume 22, pp. 1–564. [Google Scholar] [CrossRef]
- Hossain, M.B.; Ahmed, A.S.S.; Sarker, M.S.I. Human health risks of Hg, As, Mn, and Cr through consumption of fish, Ticto barb (Puntius ticto) from a tropical river, Bangladesh. Environ. Sci. Pollut. Res. 2018, 25, 31727–31736. [Google Scholar] [CrossRef] [PubMed]
- Strigul, N.; Koutsospyros, A.; Arienti, P.; Christodoulatos, C.; Dermatas, D.; Braida, W. Effects of tungsten on environmental systems. Chemosphere 2005, 61, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Koutsospyros, A.; Braida, W.; Christodoulatos, C.; Dermatas, D.; Strigul, N. A review of tungsten: From environmental obscurity to scrutiny. J. Hazard. Mater. 2006, 136, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Li, R.; Cheng, H.; Wang, J.; Shao, X. Tungsten distribution in soil and rice in the vicinity of the world’s largest and longest-operating tungsten mine in China. PLoS ONE 2014, 9, e91981. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S. Dissolved and Suspended Transport of Tungsten, Molybdenum, and Vanadium in Natural Waters. Ph.D. Thesis, Applied Geochemistry, Department of Civil, Environmental and Natural Resources Engineering, Luleå University of Technology, Luleå, Sweden, January 2018; pp. 1–174. [Google Scholar]
- Senesi, N.; Padovaro, G.; Brunetti, G. Scandium, titanium, tungsten and zirconium content in commercial inorganic fertilizers and their contribution to soil. Environ. Technol. Lett. 1998, 9, 1011–1020. [Google Scholar] [CrossRef]
- Clausen, J.L.; Korte, N. Environmental fate of tungsten from military use. Sci. Total Environ. 2009, 407, 2887–2893. [Google Scholar] [CrossRef] [PubMed]
- Karachalios, A.; Wazne, M.; Betancur, J.N.; Christodoulatos, C.; Braida, W.; O’Connor, G. Immobilization of copper, lead, and tungsten in mixed munitions firing range-contaminated soils by various amendments. J. Hazard. Toxic Radioact. Waste 2011, 15, 151–159. [Google Scholar] [CrossRef]
- Sadiq, M.; Mian, A.A.; Althagafi, K.M. Inter-city comparison of metals in scalp hair collected after the Gulf War 1991. J. Environ. Sci. Health Part A 1992, 27, 1415–1431. [Google Scholar] [CrossRef]
- Wilson, B.; Pyatt, F.B. Bio-availability of tungsten in the vicinity of an abandoned mine in the English Lake District and some potential health implications. Sci. Total Environ. 2006, 370, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Steinmaus, C.; Lu, M.; Todd, R.L.; Smith, A.H. Probability estimates for the unique childhood leukemia cluster in Fallon, Nevada, and risks near other U.S. military aviation facilities. Environ. Health Perspect. 2004, 112, 766–771. [Google Scholar] [CrossRef]
- Rubin, C.S.; Holmes, A.K.; Belson, M.G.; Jones, R.L.; Flanders, W.D.; Kieszak, S.M.; Osterloh, J.; Luber, G.E.; Blount, B.C.; Barr, D.B.; et al. Investigating childhood leukemia in Churchill County, Nevada. Environ. Health Perspect. 2007, 115, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, P.R.; Bierman, B.J.; Rhodes, K.; Ridenour, G.; Witten, M.L. Comparison of size and geography of airborne tungsten particles in Fallon, Nevada, and Sweet Home, Oregon, with implications for public health. J. Environ. Public Health 2012. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, C.; Kelly, A.D.R.; Petruccelli, L.A.; Lemaire, M.; Mann, K.K. Exposure to tungsten induces DNA damage and apoptosis in developing B lymphocytes. Leukemia 2011, 25, 1900–1904. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.J.; Johnson, D.R.; Seiter, J.M.; Lindsay, J.H.; Boyd, R.E.; Bednar, A.J.; Allison, P.G. Tungsten toxicity, bioaccumulation, and compartmentalization into organisms representing two trophic levels. Environ. Sci. Technol. 2012, 46, 9646–9652. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.D.; Lemaire, M.; Young, Y.K.; Eustache, J.H.; Guilbert, C.; Molina, M.F.; Mann, K.K. In vivo tungsten exposure alters B-cell development and increases DNA damage in murine bone marrow. Toxicol. Sci. 2013, 131, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, G.; Schulze, J.; Bittner, F.; Eilers, T.; Kuper, J.; Bollmann, G.; Nerlich, A.; Brinkmann, H.; Mendel, R.R. The molybdenum cofactor biosynthetic protein Cnx1 complements molybdate-repairable mutants, transfers molybdenum to the metal binding pterin, and is associated with the cytoskeleton. Plant Cell 2000, 12, 2455–2471. [Google Scholar] [CrossRef] [PubMed]
- Hille, R. Molybdenum and tungsten in biology. Trends Biochem. Sci. 2002, 27, 360–367. [Google Scholar] [CrossRef]
- Adamakis, I.-D.S.; Panteris, E.; Eleftheriou, E.P. Tungsten toxicity in plants. Plants 2012, 1, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Fu, G.; Yang, Y.; Zhu, C.; Tao, L. Tungstate: Is it really a specific nitrate reductase inhibitor in plant nitric oxide research? J. Exp. Bot. 2012, 63, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Adamakis, I.-D.S.; Eleftheriou, E.P.; Rost, T.L. Effects of sodium tungstate on the ultrastructure and growth of pea (Pisum sativum) and cotton (Gossypium hirsutum) seedlings. Environ. Exp. Bot. 2008, 63, 416–425. [Google Scholar] [CrossRef]
- Adamakis, I.-D.S.; Panteris, E.; Eleftheriou, E.P. Tungsten affects the cortical microtubules of Pisum sativum root cells: Experiments on tungsten–molybdenum antagonism. Plant Biol. 2010, 12, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Adamakis, I.-D.S.; Panteris, E.; Eleftheriou, E.P. The cortical microtubules are a universal target of tungsten toxicity among land plant taxa. J. Biol. Res.-Thessaloniki 2010, 13, 59–66. [Google Scholar]
- Adamakis, I.-D.S.; Panteris, E.; Eleftheriou, E.P. The nitrate reductase inhibitor, tungsten, disrupts actin microfilaments in Zea mays L. Protoplasma 2014, 251, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Adamakis, I.-D.S.; Panteris, E.; Eleftheriou, E.P. Tungsten disrupts root growth in Arabidopsis thaliana by PIN targeting. J. Plant Physiol. 2014, 171, 1174–1187. [Google Scholar] [CrossRef] [PubMed]
- Adamakis, I.-D.S.; Panteris, E.; Eleftheriou, E.P. The fatal effect of tungsten on Pisum sativum L. root cells: Indications for endoplasmic reticulum stress-induced programmed cell death. Planta 2011, 234, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Adamakis, I.-D.S. Plant Response Mechanisms against the Toxicity of Tungsten. Ph.D. Thesis, School of Biology, Aristotle University of Thessaloniki, Thessaloniki, Greece, October 2013; pp. 1–284, [In Greek with an English Summary]. Available online: http://ikee.lib.auth.gr/record/133344/files/GRI-2013-11405.pdf (accessed on 7 January 2019).
- Kuriyama, H.; Fukuda, H. Developmental programmed cell death in plants. Curr. Opin. Plant Biol. 2002, 5, 568–573. [Google Scholar] [CrossRef]
- Bagniewska-Zadworna, A.; Arasimowicz-Jelonek, M. The mystery of underground death: Cell death in roots during ontogeny and in response to environmental factors. Plant Biol. (Stuttg) 2016, 18, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, I.B.; Pastor, V.; Mauch-Mani, B. Plant responses to simultaneous biotic and abiotic stress: Molecular mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Tyutereva, E.V.; Voitsekhovskaja, O.V. The role of ion disequilibrium in induction of root cell death and autophagy by environmental stresses. Funct. Plant Biol. 2017. [Google Scholar] [CrossRef]
- Hepler, P.K. Membranes in the mitotic apparatus of barley cells. J. Cell Biol. 1980, 86, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Staehelin, L.A. The plant ER: A dynamic organelle composed of a large number of discrete functional domains. Plant J. 1997, 11, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Adamakis, I.-D.S.; Panteris, E.; Cherianidou, A.; Eleftheriou, E.P. Effects of bisphenol A on the microtubule arrays of Pisum sativum L. root meristematic cells. Mutat. Res.–Genet. Toxic. Environ. Mutat. 2013, 750, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Hatsugai, N.; Kuroyanagi, M.; Nishimura, M.; Hara-Nishimura, I. A cellular suicide strategy of plants: Vacuole-mediated cell death. Apoptosis 2006, 11, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Hara-Nishimura, I.; Hatsugai, N. The role of vacuole in plant cell death. Cell Death Diff. 2011, 18, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Reape, T.J.; McCabe, P.F. Commentary: The cellular condensation of dying plant cells: Programmed retraction or necrotic collapse? Plant Sci. 2013, 207, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, W.G.; Papini, A. Ultrastructure of autophagy in plant cells: A review. Autophagy 2013, 9, 1922–1936. [Google Scholar] [CrossRef] [PubMed]
- Hatsugai, N.; Kuroyanagi, M.; Yamada, K.; Meshi, T.; Tsuda, S.; Kondo, M.; Nishimura, M.; Hara-Nishimura, I. A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science 2004, 305, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriou, E.P.; Adamakis, I.-D.S.; Panteris, E.; Fatsiou, M. Chromium-induced ultrastructural changes and oxidative stress in roots of Arabidopsis thaliana. Intern. J. Mol. Sci. 2015, 16, 15852–15871. [Google Scholar] [CrossRef] [PubMed]
- McCabe, P.F.; Levine, A.; Meijer, P.-J.; Tapon, N.A.; Pennell, R.I. A programmed cell death partway activated in carrot cells cultured at low cell density. Plant J. 1997, 12, 267–280. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant 2008, 133, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.C.; Oelmüller, R. Reactive oxygen species generation and signaling in plants. Plant Sign. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Sangwan, R.S.; Mishra, S.; Jadaun, J.S.; Sabir, F.; Sangwan, N.S. Effect of cadmium stress on inductive enzymatic and nonenzymatic responses of ROS and sugar metabolism in multiple shoot cultures of Ashwagandha (Withania somnifera Dunal). Protoplasma 2014, 251, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- AlQuraidi, A.O.; Mosa, K.A.; Ramamoorthy, K. Phytotoxic and genotoxic effects of copper nanoparticles in coriander (Coriandrum sativum—Apiaceae). Plants 2019, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Keunen, E.; Remans, T.; Bohler, S.; Vangronsveld, J.; Cuypers, A. Metal-induced oxidative stress and plant mitochondria. Ιntern. J. Mol. Sci. 2011, 12, 6894–6918. [Google Scholar] [CrossRef] [PubMed]
- Gunning, B.E.S.; Steer, M.W. Plant Cell Biology, Structure and Function; Jones and Bartlett Publishers: Boston, MA, USA, 1996. [Google Scholar]
- Eleftheriou, E.P.; Adamakis, I.-D.S.; Melissa, P. Effects of hexavalent chromium on microtubule organization, ER distribution and callose deposition in root tip cells of Allium cepa L. Protoplasma 2012, 249, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Cacas, J.L. Devil inside: Does plant programmed cell death involve the endomembrane system? Plant Cell Environ. 2010, 33, 1453–1473. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhang, W.; Li, B.; Wang, Y.; Li, K.; Sodmergen; Han, C.; Zhang, Y.; Li, X. An endoplasmic reticulum response pathway mediates programmed cell death of root tip induced by water stress in Arabidopsis. New Phytol. 2010, 186, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Napier, R.M.; Fowke, L.C.; Hawes, C.; Lewis, M.; Pelham, H.R. Immunological evidence that plants use both HDEL and KDEL for targeting proteins to the endoplasmic reticulum. J. Cell Sci. 1992, 102, 261–271. [Google Scholar] [PubMed]
- Sandalio, L.M.; Rodríguez-Serrano, M.; Romero-Puertas, M.C.; del Río, L.A. Imaging of reactive oxygen species and nitric oxide in vivo in plant tissues. Methods Enzym. 2008, 440, 397–409. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Halliwell, B.; Hoey, B.M.; Butler, J. The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989, 6, 593–597. [Google Scholar] [CrossRef]
- Zafarullah, M.; Li, W.Q.; Sylvester, J.; Ahmad, M. Molecular mechanisms of N-acetylcysteine actions. Cell. Mol. Life Sci. 2003, 60, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.J.; Mock, N.M. An improved method for monitoring cell death in cell suspension and leaf disc assays using Evans blue. Plant Cell Tissue Org. Cult. 1994, 39, 7–12. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamakis, I.-D.S.; Eleftheriou, E.P. Structural Evidence of Programmed Cell Death Induction by Tungsten in Root Tip Cells of Pisum sativum. Plants 2019, 8, 62. https://doi.org/10.3390/plants8030062
Adamakis I-DS, Eleftheriou EP. Structural Evidence of Programmed Cell Death Induction by Tungsten in Root Tip Cells of Pisum sativum. Plants. 2019; 8(3):62. https://doi.org/10.3390/plants8030062
Chicago/Turabian StyleAdamakis, Ioannis-Dimosthenis S., and Eleftherios P. Eleftheriou. 2019. "Structural Evidence of Programmed Cell Death Induction by Tungsten in Root Tip Cells of Pisum sativum" Plants 8, no. 3: 62. https://doi.org/10.3390/plants8030062
APA StyleAdamakis, I.-D. S., & Eleftheriou, E. P. (2019). Structural Evidence of Programmed Cell Death Induction by Tungsten in Root Tip Cells of Pisum sativum. Plants, 8(3), 62. https://doi.org/10.3390/plants8030062




