Bioactive Profile of Various Salvia officinalis L. Preparations
Abstract
1. Introduction
2. Methodology of Review
3. Production of Sage Extracts
3.1. Hydrodistillation
3.2. Soxhlet Extraction
3.3. Infusion
3.4. Solid–Liquid Extraction
3.4.1. Ultrasound-Assisted Extraction (UAE)
3.4.2. Microwave-Assisted Extraction (MAE)
3.5. Supercritical CO2 Extraction (SC-CO2)
4. Analysis of Different Extraction Methods
4.1. Hydrodistillation/Steamdistillation
4.2. Soxhlet Extraction
4.3. Infusions
4.4. Solid–Liquid Extraction
4.5. Supercritical CO2 Extraction
5. Chemical Composition of Sage Products
6. Sage and Health Benefits
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dinç, M.; Pinar, N.M.; Dogu, S.; Yildirimli, S. Micromorphological studies of Lallemantia l. (Lamiaceae) species growing in Turkey. Acta Biol. Crac. Ser. Bot. 2009, 51, 45–54. [Google Scholar]
- Walker, J.B.; Sytsma, K.J. Staminal Evolution in the Genus Salvia (Lamiaceae): Molecular Phylogenetic Evidence for Multiple Origins of the Staminal Lever. Ann. Bot. 2007, 100, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Ulubelen, A. Chemical constituents: Terpenoids in the genus Salvia. In Medicinal and Aromatic Plants-Industrial Profiles; Kintzios, S.E., Ed.; Harwood Academic: Reading, UK, 2000; Volume 14, pp. 55–68. [Google Scholar]
- Hedge, I.C.; Salvia, L. Flora Europaea; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1972; Volume 3, p. 188. [Google Scholar]
- Šilić, Č. Atlas Drveća i Grmlja; Korene, Z., Mitić, V., Eds.; Zavod za Izdavanje Udžbenika: Sarajevo, Bosnia and Herzegovina, 1973; Volume 4, p. 174. [Google Scholar]
- Herbalpedia. Available online: http://www.herbworld.com/learningherbs/sage.pdf (accessed on 15 August 2018).
- Fu, Z.; Wang, H.; Hu, X.; Sun, Z.; Han, C. The Pharmacological Properties of Salvia Essential Oils. J. Appl. Pharm. Sci. 2013, 3, 122–127. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef] [PubMed]
- El-Feky, A.M.; Aboulthana, W.M. Phytochemical and Biochemical Studies of Sage (Salvia officinalis L.). UK J. Pharm. Biosci. 2016, 4, 56–62. [Google Scholar] [CrossRef]
- Grdiša, M.; Jug-Dujaković, M.; Lončarić, M.; Carović-Stanko, K.; Ninčević, T.; Liber, Z.; Radosavljević, I.; Šatović, Z. Dalmatian Sage (Salvia officinalis L.): A Review of Biochemical Contents, Medical Properties and Genetic Diversity. Agric. Conspec. Sci. 2015, 80, 69–78. [Google Scholar]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M. Chemistry, Pharmacology and Medicinal Property of Sage (Salvia) to Prevent and Cure Illnesses such as Obesity, Diabetes, Depression, Dementia, Lupus, Autism, Heart Disease and Cancer. J. Tradit. Complement. Med. 2014, 4, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.T.; Wang, M.; Wei, G.J.; Huang, T.C.; Huang, M.T. Chemistry and antioxidative factors in rosemary and sage. BioFactors 2000, 13, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Foo, L.Y. Polyphenolics of Salvia—A review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Miguel, G.; Cruz, C.; Faleiro, M.L.; Simõesc, M.T.F.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Salvia officinalis L. essential oils: Effect of hydrodistillation time on the chemical composition, antioxidant and antimicrobial activities. Nat. Prod. Res. 2011, 25, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Dapkevicius, A.; Venskutonis, R.; van Beek, A.T.; Linssen, J.P.H. Antioxidant Activity of Extracts Obtained by Different Isolation Procedures from some Aromatic Herbs Grown in Lithuania. J. Sci. Food Agric. 1998, 77, 140–146. [Google Scholar] [CrossRef]
- Ollanketo, M.; Peltoketo, A.; Hiltunen, K.H.R.; Riekkola, M-L. Extraction of sage (Salvia officinalis L.) by pressurized hot water and conventional methods: Antioxidant activity of the extracts. Eur. Food Res. Technol. 2002, 215, 158–163. [Google Scholar] [CrossRef]
- Raal, A.; Orav, A.; Arak, E. Composition of the essential oil of Salvia officinalis L. from various European countries. Nat. Prod. Res. 2007, 21, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Lakušić, B.S.; Ristić, M.S.; Slavkovska, V.N.; Stojanović, D.LJ.; Lakušić, D.V. Variations in essential oil yields and compositions of Salvia officinalis (Lamiaceae) at different developmental stages. Bot. Serb. 2013, 37, 127–139. [Google Scholar]
- Ahl, H.S.A.; Hussein, M.S.; Gendy, A.S.H.; Tkachenko, K.G. Quality of Sage (Salvia officinalis L.) Essential Oil Grown in Egypt. Int. J. Plant Sci. Ecol. 2015, 1, 119–123. [Google Scholar]
- Kuštrak, D.; Kuftinec, J.; Blažević, N. Yields and Composition of Sage Oils from Different Regions of the Yugoslavian Adriatic Coast. J. Nat. Prod. 1984, 47, 520–524. [Google Scholar] [CrossRef]
- Menaker, A.; Kravets, M.; Koel, M.; Orav, A. Identification and characterization of supercritical fluid extracts from herbs. C. R. Chim. 2004, 7, 629–633. [Google Scholar] [CrossRef]
- Politeo, O.; Jukić, M.; Miloš, M. Chemical Composition and Antioxidant Activity of Essential Oils of Twelve Spice Plants. Croat. Chem. Acta 2006, 79, 545–552. [Google Scholar]
- Farhat, M.B.; Jordán, M.J.; Chaouech-Hamada, R.; Landoulsi, A.; Sotomayor, J.A. Variations in Essential Oil, Phenolic Compounds, and Antioxidant Activity of Tunisian Cultivated Salvia officinalis. J. Agric. Food Chem. 2009, 57, 10349–10356. [Google Scholar] [CrossRef] [PubMed]
- Al-Tawaha, A.; Al-Karaki, G.; Massadeh, A. Antioxidant activity, total phenols and variation of chemical composition from essential oil in sage (Salvia officinalis L.) grown under protected soilless condition and open field conditions. J. Environ. Biol. 2013, 7, 894–901. [Google Scholar]
- Tosun, A.; Khan, S.; Kim, Y.S.; Calín-Sánchez, A.; Hysenaj, X.; Carbonell-Barrachina, A.A. Essential Oil Composition and Anti-Inflammatory Activity of Salvia officinalis L. (Lamiaceae) in Murin Macrophages. Trop. J. Pharm. Res. 2014, 13, 937–942. [Google Scholar] [CrossRef]
- Zawislak, G. Yield and chemical composition of essential oil from Salvia officinalis L. in third year of cultivation. Herba Pol. 2014, 60, 13–22. [Google Scholar] [CrossRef]
- Chalchat, J.C.; Michet, A.; Pasquier, B. Study of Clones of Salvia officinalis L. Yields and Chemical Composition of Essential Oil. Flavour Fragr. J. 1998, 13, 68–70. [Google Scholar] [CrossRef]
- Langa, E.; Della Porta, G.; Palavra, A.M.F.; Urieta, J.S.; Mainara, A.M. Supercritical fluid extraction of Spanish sage essential oil: Optimization of the process parameters and modelling. J. Supercrit. Fluids 2009, 49, 174–181. [Google Scholar] [CrossRef]
- Glisic, S.; Ivanovic, J.; Ristic, M.; Skala, D. Extraction of sage (Salvia officinalis L.) by supercritical CO2: Kinetic data, chemical composition and selectivity of diterpenes. J. Supercrit. Fluids 2010, 52, 62–70. [Google Scholar] [CrossRef]
- Maksimovic, S.; Kesic, Ž.; Lukic, I.; Milovanovic, S.; Ristic, M.; Skala, D. Supercritical fluid extraction of curry flowers, sage leaves, and their mixture. J. Supercrit. Fluids 2013, 84, 1–13. [Google Scholar] [CrossRef]
- Abdelkader, M.; Ahcen, B.; Rachid, D.; Hakim, H. Phytochemical Study and Biological Activity of Sage (Salvia officinalis L.). Int. J. Biol. Biomol. Agricult. Food Biotechnol. Eng. 2014, 8, 1253–1257. [Google Scholar]
- Putievsky, E.; Ravid, U.; Dudai, N. The influence of Season and Harvest Frequency on Essential Oil and Herbal Yields from a Pure Clone of Sage (Salvia officinalis) Grown Under Cultivated Conditions. J. Nat. Prod. 1986, 49, 326–329. [Google Scholar] [CrossRef]
- Occhipinti, A.; Capuzzo, A.; Arceusz, A.; Maffei, M.E. Comparative analysis of α- and β-thujone in the essential oil and supercritical CO2 extract of sage (Salvia officinalis L.). J. Essent. Oil Res. 2014, 26, 85–90. [Google Scholar] [CrossRef]
- Aleksovski, S.A.; Sovová, H. Supercritical CO2 extraction of Salvia officinalis L. J. Supercrit. Fluids 2007, 40, 239–245. [Google Scholar] [CrossRef]
- Koubaa, F.G.; Abdennabi, R.; Soussi Ben Salah, A.; El Feki, A. Microwave extraction of Salvia officinalis essential oil and assessment of its GC-MS identification and protective effects versus vanadium-induced nephrotoxicity in Wistar rats models. Arch. Physiol. Biochem. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Farhat, M.B.; Landoulsi, A.; Chaouch-Hamada, R.; Sotomayor, J.A.; Jordán, M.J. Characterization and quantification of phenolic compounds and antioxidant properties of Salvia species growing in different habitats. Ind. Crops Prod. 2013, 49, 904–914. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, V.; Chiliment, S.; Oprea, E. Capillary gas chromatography–mass spectrometry of volatile and semi-volatile compounds of Salvia officinalis. J. Chromatogr. A 2004, 1027, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2015, 170, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, B.F.; Walch, S.G.; Tinzoh, L.N.; Stühlinger, W.; Lachenmeier, D.W. Rapid UHPLC determination of polyphenols in aqueous infusions of Salvia officinalis L. (sage tea). J. Chromatogr. B 2011, 879, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Dent, M.; Dragović-Uzelac, V.; Penić, M.; Brnčić, M.; Bosiljkov, T.; Levaj, B. The Effect of Extraction Solvents, Temperature and Time on the Composition and Mass Fraction of Polyphenols in Dalmatian Wild Sage (Salvia officinalis L.) Extracts. Food Technol. Biotechnol. 2013, 51, 84–91. [Google Scholar] [CrossRef]
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol-water mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- Duletić-Laušević, S.; Alimpić, A.; Pavlović, D.; Marin, P.D.; Lakušić, D. Salvia officinalis of different origins Antioxidant activity, phenolic and flavonoid content of extracts. Agro Food Ind. Hi Tech 2016, 27, 52–55. [Google Scholar]
- Roby, M.H.; Sarhan, M.A.; Selim, K.A.H.; Khael, K.I. Evaluation of antioxidant activity, total polyphenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.) and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Sališová, M.; Toma, S.; Mason, T.J. Comparison of conventional and ultrasonically assisted extractions of pharmaceutically active compounds from Salvia officinalis. Ultrason. Sonochem. 1997, 4, 131–134. [Google Scholar] [CrossRef]
- Veličković, D.T.; Randjelović, N.V.; Ristić, M.S.; Veličković, A.S.; Šmelcerović, A.A. Chemical constituents and antimicrobial activity of the ethanol extracts obtained from the flower, leaf and stem of Salvia officinalis L. J. Serb. Chem. Soc. 2003, 68, 17–24. [Google Scholar] [CrossRef]
- Dragović-Uzelac, V.; Garofuli, I.E.; Jukić, M.; Penić, M.; Dent, M. The Influence of Microwave-Assisted Extraction on the Isolation of Sage (Salvia officinalis L.) Polyphenols. Food Technol. Biotechnol. 2012, 50, 377–383. [Google Scholar]
- Putnik, P.; Kovačević, D.B.; Penić, M.; Fegeš, M.; Dragović-Uzelac, V. Microwave-Assisted Extraction (MAE) of Dalmatian Sage Leaves for the Optimal Yield of Polyphenols: HPLC-DAD Identification and Quantification. Food Anal. Methods 2016, 9, 2385–2394. [Google Scholar] [CrossRef]
- Brunner, G. Supercritical fluids: Technology and application to food processing. J. Food Eng. 2005, 67, 21–33. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Damjanovic, B.; Lepojevic, Z.; Zivkovic, V.; Tolic, A. Extraction of fennel (Foeniculum vulgare Mill.) seeds with supercritical CO2: Comparison with hydrodistillation. Food Chem. 2005, 92, 143–149. [Google Scholar] [CrossRef]
- Zinnai, A.; Sanmartin, C.; Taglieri, I.; Andrich, G.; Venturi, F. Supercriticalfluid extraction from microalgae with highcontent of LC-PUFAs. A case of study: Sc-CO2 oil extraction from Schizochytrium sp. J. Supercrit. Fluids 2016, 116, 126–131. [Google Scholar] [CrossRef]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Andrich, G.; Zinnai, A. A Simplified Method to Estimate Sc-CO2 Extraction of Bioactive Compounds from Different Matrices: Chili Pepper vs. Tomato By-Products. Appl. Sci. 2017, 7, 361. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibánez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Taddeo, R. Extraction of Sage Oil by Supercritical CO2: Influence of Some Process Parameters. J. Supercrit. Fluids 1995, 8, 302–309. [Google Scholar] [CrossRef]
- Daukšas, E.; Venskutonis, P.R.; Povilaityte, V.; Sivik, B. Rapid screening of antioxidant activity of sage (Salvia officinalis L.) extracts obtained by supercritical carbon dioxide at different extraction conditions. Nahrung/Food 2001, 45, 338–341. [Google Scholar] [CrossRef]
- Fornari, T.; Ruiz-Rodriguez, A.; Vicente, G.; Vázquez, E.; García-Risco, M.R.; Reglero, G. Kinetic study of the supercritical CO2 extraction of different plants from Lamiaceae family. J. Supercrit. Fluids 2012, 64, 1–8. [Google Scholar] [CrossRef]
- Fellah, S.; Diouf, P.N.; Petrissans, M.; Barth, D.; Romdahne, M.; Perrin, D.; Abderrabba, M. Supercritical CO2, Hydrodistillation extractions of Salvia Officinalis L. Influence of Extraction Process on Antioxidant Properties. In Proceedings of the 10th European Meeting on Supercritical Fluids, Reactions, Materials and Natural Products, Strasbourg/Colmar, France, 12–14 December 2005. [Google Scholar]
- Mičić, V.; Lepojević, Ž.; Jotanović, M.; Tadić, G.; Pejović, B. Supercritical Extraction of Salvia officinalis L. J. Appl. Sci. 2011, 11, 3630–3634. [Google Scholar]
- Jokić, S.; Molnar, M.; Jakovljević, M.; Aladić, K.; Jerković, I. Optimization of supercritical CO2 extraction of Salvia officinalis L. leaves targeted on Oxygenated monoterpenes, α-humulene, viridiflorol and manool. J. Supercrit. Fluids 2018, 133, 253–262. [Google Scholar] [CrossRef]
- Glisic, S.B.; Ristic, M.; Skala, D.U. The combined extraction of sage (Salvia officinalis L.): Ultrasound followed by supercritical CO2 extraction. Ultrason. Sonochem. 2011, 18, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 196. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 17, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Singh, J. Maceration, Percolation and Infusion Techniques for the Extraction of Medicinal and Aromatic Plants. In Extraction Technologies for Medicinal and Aromatic Plants; Handa, S.S., Khanuja, S.P.S., Longo, G., Rakesh, D.D., Eds.; ICS-UNIDO: Trieste, Italy, 2008; pp. 67–82. [Google Scholar]
- Danlami, J.M.; Arsad, A.; Zaini, M.A.A.; Sulaiman, H. A comparative study of various oil extraction techniques from plants. Rev. Chem. Eng. 2014, 30, 605–626. [Google Scholar] [CrossRef]
- Bubalo Cvjetko, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Pereira, C.G.; Meireles, M.A.A. Economic analysis ofrosemary, fennel and anise essential oils obtained by supercritical fluid extraction. Flavour Fragr. J. 2007, 22, 407–413. [Google Scholar] [CrossRef]
- Shariaty-Niassar, M.; Aminzadeh, B.; Azadi, P.; Soltanali, S. Economic evaluation of herb extraction using supercritical fluid. Chem. Ind. Chem. Eng. Q. 2009, 15, 143–148. [Google Scholar] [CrossRef]
- Rój, E.; Gagoś, M.; Dobrzyńska-Inger, A. Cost optimization of extract production in supercritical extraction process with the use of CO2-a novel approach. Procedia Eng. 2012, 42, 356–362. [Google Scholar] [CrossRef]
- Rhyu, H.Y. Gas chromatographic characterization of sages of various geographic origin. J. Food Sci. 1979, 44, 758–762. [Google Scholar] [CrossRef]
- Pitarevic, I.; Kuftinec, J.; Blažević, N.; Kuštrak, D. Seasonal variation of essential oil yield and composition of Dalmatian sage, Salvia officinalis. J. Nat. Prod. 1984, 47, 409–412. [Google Scholar] [CrossRef]
- Svoboda, K.P. A Study of the Variability of Rosemary and Sage and their Volatile Oils on the British Market: Their Antioxidative Properties. Flavour Fragr. J. 1992, 7, 81–87. [Google Scholar] [CrossRef]
- Piccaglia, R.; Marotti, M.; Dellacecca, V. Effect of Planting Density and Harvest Date on Yield and Chemical Composition of Sage Oil. J. Essent. Oil Res. 1997, 9, 187–191. [Google Scholar] [CrossRef]
- Perry, N.B.; Anderson, R.E.; Brennan, N.J.; Douglas, M.H.; Heaney, A.J.; McGimpsey, J.A.; Smallfield, B.M. Essential Oils from Dalmatian Sage (Salvia officinalis L.): Variations among Individuals, Plant Parts, Seasons, and Sites. J. Agric. Food Chem. 1999, 27, 2048–2054. [Google Scholar] [CrossRef]
- Santos-Gomes, P.C.; Fernandes-Ferreira, M. Organ- and Season-Dependent Variation in the Essential Oil Composition of Salvia officinalis L. Cultivated at Two Different Sites. J. Agric. Food Chem. 2001, 49, 2908–2916. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, I.; Zakhama, N.; Aidi Wannes, W.; Kchouk, M.E.; Marzouk, B. Water deficit effects on Salvia officinalis fatty acids and essential oils composition. Sci. Hortic. 2009, 120, 271–275. [Google Scholar] [CrossRef]
- Sellami, I.H.; Rebey, I.B.; Sriti, J.; Rahali, F.Z.; Limam, F.; Marzouk, B. Drying Sage (Salvia officinalis L.) Plants and Its Effects on Content, Chemical Composition, and Radical Scavenging Activity of the Essential Oil. Food Bioprocess Technol. 2012, 5, 2978–2989. [Google Scholar] [CrossRef]
- Arraiza, M.P.; Arrabal, C.; López, J.V. Seasonal Variation of Essential Oil Yield and Composition of Sage (Salvia officinalis L.) Grown in Castilla-La Mancha (Central Spain). Not. Bot. Horti Agrobot. 2012, 40, 106–108. [Google Scholar] [CrossRef]
- Cvetkovikj, I.; Stefkov, G.; Karapandzova, M.; Kulevanova, S.; Satovic, Z. Essential Oils and Chemical Diversity of Southeast European Populations of Salvia officinalis L. Chem. Biodivers. 2015, 12, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.O.; Maciarello, M.J. Essential oils of cultivars of dalmatian sage. J. Essent. Oil Res. 1990, 2, 139–144. [Google Scholar] [CrossRef]
- Jug-Dujaković, M.; Ristić, M.; Pljevljakušić, D.; Dajić-Stevanović, Z.; Liber, Z.; Hančević, K.; Radić, T.; Šatović, Z. High diversity of indigenous populations of Dalmatian sage (Salvia officinalis L.) in essential-oil composition. Chem. Biodivers. 2012, 9, 2309–2323. [Google Scholar] [CrossRef] [PubMed]
- Craft, J.; Satyal, P.; Setzer, W.N. The Chemotaxonomy of Common Sage (Salvia officinalis) Based on the Volatile Constituents. Medicines 2017, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Ivanović, J.; Đilas, S.; Jadranin, M.; Vajs, V.; Babović, N.; Petrović, S.; Žižović, I. Supercritical carbon dioxide extraction of antioxidants from rosemary (Rosmarinus officinalis L.) and sage (Salvia officinalis L.). J. Serb. Chem. Soc. 2009, 74, 717–732. [Google Scholar] [CrossRef]
- Andrade, P.B.; Seabra, R.M.; Valentão, P.; Areias, F. Simultaneous determination of flavonoids, phenolic acids and coumarins in seven medicinal species by HPLC/Diode array detector. J. Liq. Chromatogr. Relat. Technol. 1998, 21, 2813–2820. [Google Scholar] [CrossRef]
- Veličković, D.T.; Milenović, D.M.; Ristić, M.S.; Veljković, V.B. Kinetics of ultrasonic extraction of extractive substances from garden (Salvia officinalis L.) and glutinous (Salvia glutinosa L.) sage. Ultrason. Sonochem. 2006, 13, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L. Salvia (Sage): A Review of its Potential Cognitive-Enhancing and Protective Effects. Drugs R. D. 2017, 17, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Dal Prá, V.; Bisol, L.B.; Detoni, S.; Denti, M.; Grando, J.; Pollo, C.; Pasquali, T.R.; Hofmann Júnio, A.E.; Mazzuti, M.A.; Macedo, S.M. Antiinflammatory activity of fracionated extracts of Salvia officinalis L. J. Appl. Pharm. Sci. 2011, 7, 67–71. [Google Scholar]
- Baricevic, D.; Sosa, S.; Della Loggia, R.; Tubaro, A.; Simonovska, B.; Krasna, A.; Zupancic, A. Topical antiinflammatory activity of Salvia officinalis L. leaves: The relevance of ursolic acid. J. Ethnopharmacol. 2001, 75, 125–132. [Google Scholar] [CrossRef]
- Juhás, S.; Cikos, S.; Czikková, S.; Veselá, J.; Il’ková, G.; Hájek, T.; Domaracká, K.; Domaracký, M.; Bujnáková, D.; Rehák, P.; et al. Effects of Borneol and Thymoquinone on TNBS-Induced Colitis in Mice. Folia Biol. 2008, 54, 1–7. [Google Scholar]
- Ninomiya, K.; Matsuda, H.; Shimoda, H.; Nishida, N.; Kasajima, N.; Yoshino, T.; Morikawa, T.; Yoshikawa, M. Carnosic acid, a new class of lipid absorption inhibitor from sage. Bioorg. Med. Chem. Lett. 2004, 14, 1943–1946. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.; Rouse, M.; Moss, L. Aromas of Salvia Species Enhance Everyday Prospective Memory Performance in Healthy Young Adults. Adv. Chem. Engineer. Sci. 2014, 4, 339–346. [Google Scholar] [CrossRef]
- Hernandez-Saavedra, D.; Perez-Ramirez, I.F.; Ramos-Gomez, M.; Mendoza-Diaz, S.; Loarca-Pina, G.; Reynoso-Camacho, R. Phytochemical characterization and effect of Calendula officinalis, Hypericum perforatum, and Salvia officinalis infusions on obesity associated cardiovascular risk. Med. Chem. Res. 2016, 25, 163–172. [Google Scholar] [CrossRef]
- Eidi, A.; Eidi, M. Antidiabetic effects of sage (Salvia officinalis L.) leaves in normal and streptozotocininduced diabetic rats. Diabetes Metab. Syndr. Clin. Res. Rev. 2009, 3, 40–44. [Google Scholar] [CrossRef]
- Shahrzad, K.; Mahya, N.; Fatemeh, T.B.; Maryam, K.; Mohammadreza, F.B.; Jahromy, M.H. Hepatoprotective and Antioxidant Effects of Salvia officinalis L. Hydroalcoholic Extract in Male Rats. Chin. Med. J. 2014, 5, 130–136. [Google Scholar] [CrossRef]
- Sá, C.M.; Ramos, A.A.; Azevedo, M.F.; Lima, C.F.; Fernandes Ferreira, M.; Pereira-Wilson, C. Sage tea drinking improves lipid profile and antioxidant defences in humans. Int. J. Mol. Sci. 2009, 10, 3937–3950. [Google Scholar] [CrossRef] [PubMed]
- Pedro, D.; Ramos, A.; Lima, C.; Baltazar, F.; Pereira-Wilson, C. Modulation of DNA damage prevention and signaling pathways in diet induced colon cancer prevention. BMC Proc. 2010, 4, 53. [Google Scholar] [CrossRef]
- Bauer, J.; Kuehnl, J.; Rollinger, J.M.; Scherer, O.; Northoff, H.; Stuppner, H.; Werz, O.; Koeberle, A. Carnosol and carnosic acids from Salvia officinalis inhibit microsomal prostaglandin E2 synthase-1. J. Pharmacol. Exp. Ther. 2012, 342, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Osman, N.N.; Abd El–Azime, A. Salvia officinalis L. (sage) Ameliorates Radiation-Induced Oxidative Brain Damage in Rats. Arab J. Nucl. Sci. Appl. 2013, 46, 297–304. [Google Scholar]
- Badiee, P.; Nasirzadeh, A.R.; Motaffaf, M. Comparison of Salvia officinalis L. essential oil and antifungal agents against candida species. J. Pharm. Technol. Drug Res. 2012, 1–7. [Google Scholar] [CrossRef]
- Hayouni, E.A.; Chraief, I.; Abedrabba, M.; Bouix, M.; Leveau, J.-Y.; Mohammed, H.; Hamdi, M. Tunisian Salvia officinalis L. and Schinus molle L. essential oils: Their chemical compositions and their preservative effects against Salmonella inoculated in minced beef meat. Int. J. Food Microbiol. 2008, 125, 242–251. [Google Scholar] [CrossRef] [PubMed]
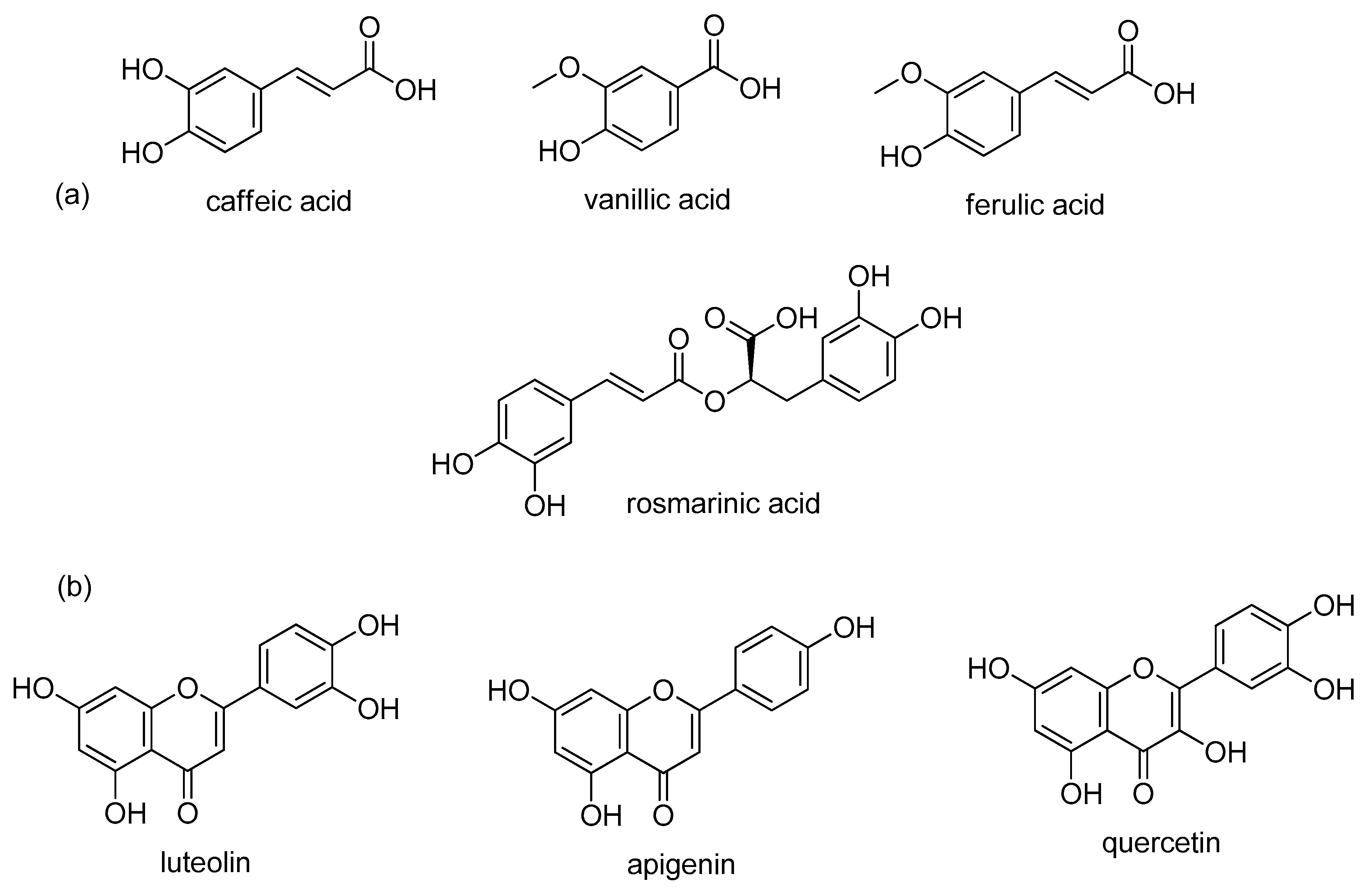
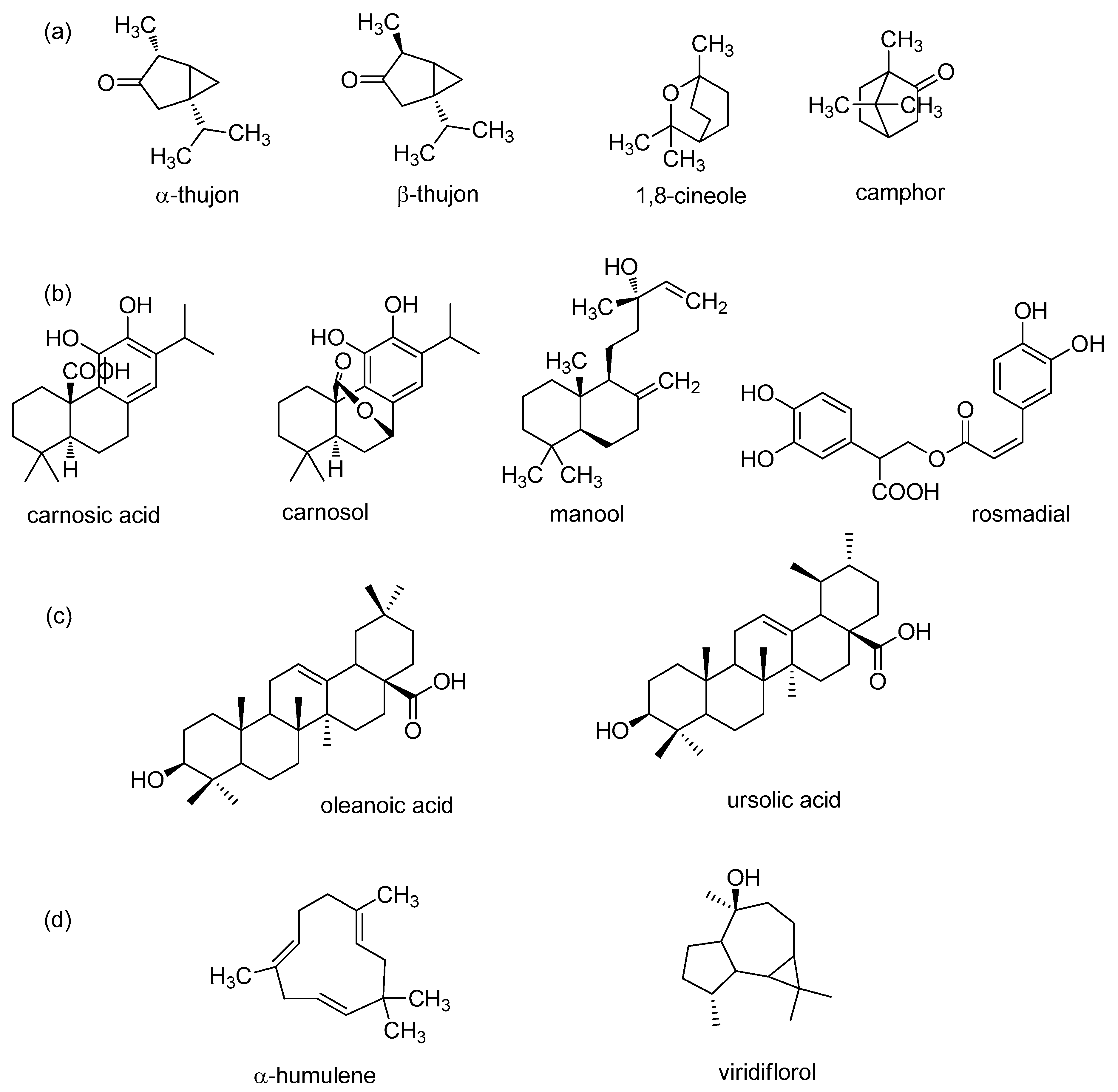
| Plant Part | Yield | Bioactive Compounds | Country | Reference |
|---|---|---|---|---|
| Herba | 24.8 mL/kg | α-Pinene (3.5%), Camphene (5.3%), 1,8-Cineole (11.9%), α-Thujone (21.0%), β-Thujone (10.1%), Camphor (23.9%), Borneol (2.6%), Bornyl acetate (2.6%), (E)-b-Caryophyllene (3.4%), α-Humulene (3.3%), Viridiflorol (5.6%) | France | [17] |
| 10.0 mL/kg | α-Pinene (5.8%), Camphene (5.1%), 1,8-Cineole (14.6%), α-Thujone (18.6%), β-Thujone (6.6%), Camphor (13.7%), Borneol (5.0%), Bornyl acetate (1.2%), (E)-b-Caryophyllene (2.9%), α-Humulene (2.6%), Viridiflorol (8.2%) | Hungary | [17] | |
| 15.0 mL/kg | α-Pinene (5.1%), Camphene (6.8%), 1,8-Cineole (12.6%), α-Thujone (19.6%), β-Thujone (5.4%), Camphor (19.2%), Borneol (2.0%), Bornyl acetate (1.7%), (E)-b-Caryophyllene (1.1%), α-Humulene (1.4%), Viridiflorol (10.4%) | Belgium | [17] | |
| 12.8 mL/kg | α-Pinene (4.4%), Camphene (7.1%), 1,8-Cineole (17.0%), α-Thujone (16.2%), β-Thujone (7.1%), Camphor (28.5%), Borneol (2.8%), Bornyl acetate (1.9%), (E)-b-Caryophyllene (0.9%), α-Humulene (2.0%), Viridiflorol (4.5%) | Russia | [17] | |
| 21.8 mL/kg | α-Pinene (5.1%), Camphene (5.9%), 1,8-Cineole (45.3%), α-Thujone (3.0%), β-Thujone (1.5%), Camphor (11.3%), Borneol (1.6%), Bornyl acetate (0.1%), (E)-b-Caryophyllene (4.9%), α-Humulene (0.4%), Viridiflorol (1.1%) | Greece | [17] | |
| 21.1 mL/kg | α-Pinene (3.7%), Camphene (3.6%), 1,8-Cineole (1.6%), α-Thujone (13.7%), β-Thujone (11.6%), Camphor (12.9%), Borneol (3.0%), Bornyl acetate (1.9%), (E)-b-Caryophyllene (2.7%), α-Humulene (2.1%), Viridiflorol (7.9%) | Ukraine | [17] | |
| 4.2 mL/kg | α-Pinene (0.2%), Camphene (0.2%), 1,8-Cineole (9.1%), α-Thujone (6.8%), β-Thujone (1.6%), Camphor (29.8%), Borneol (11.8%), Bornyl acetate (7.8%), (E)-b-Caryophyllene (1.6%), α-Humulene (1.8%), Viridiflorol (4.5%) | Scotland | [17] | |
| 2.2 mL/kg | α-Pinene (0.1%), Camphene (0.1%), 1,8-Cineole (2.7%), α-Thujone (18.7%), β-Thujone (11.7%), Camphor (12.7%), Borneol (2.4%), Bornyl acetate (1.9%), (E)-b-Caryophyllene (7.5%), α-Humulene (7.5%), Viridiflorol (15.7%) | Moldavia | [17] | |
| 5.1–15.2 mL/kg | α-Pinene (0.6–6.4%), Camphene (0.6–5.5%), 1,8-Cineole (5.3–14.6%), α-Thujone (15.2–26.6%), β-Thujone (5.2–12.9%), Camphor (16.4–20.0%), Borneol (1.8–4.9%), Bornyl acetate (2.1–2.2%), (E)-b-Caryophyllene (2.4–4.5%), α-Humulene (5.3–8.5%), Viridiflorol (4.0–8.5%) | Estonia | [17] | |
| Leaves, dried | 1.4%–3.5% | α-Pinene (5.73–6.64%), Camphene (6.16–8.13%), β-Pinene (1.42–2.68%), Myrcene (1.01–1.27%), Limonene (1.82–2.63%) 1,8-Cineole (8.95–10.43%), α-Thujone (23.61–26.17%), β-Thujone (3.91–4.38%), Camphor (20.50–23.14%), Linalool (0.37–2.40%), Bornyl acetate (2.12–2.3.52%), Isoborneol (0.04–2.80%), Borneol (9.99–11.03%) | Croatia (mainland) | [20] |
| 1.4%–3.5% | α-Thujene (0.18–1.38%), α-Pinene (2.65–4.90%), Camphene (2.40–8.48%), β-Pinene (1.07–3.38%), Myrcene (0.46–1.57%), Limonene (1.03–3.64%), 1,8-Cineole (7.84–22.46%), α-Thujone (7.17–36.33%), β-Thujone (3.94–31.89%), Camphor (6.99–19.61%), Linalool (0.32–4.66%), Bornyl acetate (0.59–5.32%), Isoborneol (0.14–2.12%), Borneol (6.45–15.54%) | Croatia (island) | [20] | |
| 2.4%–3.2% | α-Pinene (5.4–6.6%), Camphene (4.2–5.3%), β-Pinene (2.6–3.3%), Limonene (1.3–1.7%), 1,8-Cineole (13.4–16.8%), α-Thujone (1.1–1.5%), β-Thujone (15.0–17.7%), Camphor (27.0–32.2%), Bornyl acetate (0.9–1.8%), β-Caryophyllene (3.5–4.3%), Terpinen-4-ol (0.1–1.1%), α-Humulene (0.9–1.8%), Borneol (1.7–3.7%), iso-Borneol (0.3–2.0%), Ledol (1.7–2.8%) | Portugal | [27] | |
| 2.2%–3.1% | α-Pinene (1.9–2.7%), Camphene (3.9–5.9%), β-Pinene (1.1–1.6%), Limonene (0.6–1.5%), 1,8-Cineole (7.1–9.7%), α-Thujone (19.6–24.3%), β-Thujone (1.7–2.9%), Camphor (23.8–27.9%), Bornyl acetate (2.8–3.9%), β-Caryophyllene (1.7–2.4%), α-Humulene (7.6–12.4%), Borneol (2.9–4.3%), iso-Borneol (0.3–2.0%), Ledol (3.7–7.5%), Caryophyllen-8-ol (1.1–2.6%) | Hungary | [27] | |
| 2.0%–2.5% | α-Pinene (5.7–9.0%), Camphene (2.2–2.9%), β-Pinene (2.3–4.4%), Limonene (0.8–1.4%), 1,8-Cineole (3.6–6.0%), (E)-β-Ocimene (0.1–1.3%), α-Thujone (18.9–26.6%), β-Thujone (5.0–8.3%), Camphor (18.2–27.3%), Linalol (0.3–1.4%), Bornyl acetate (0.6–1.4%), β-Caryophyllene (1.0–1.6%), Terpinen-4-ol (0.4–1.2%), α-Humulene (7.3–10.5%), Borneol (1.8–3.3%), Ledol (1.8–3.6%),Caryophyllen-8-ol (1.3–4.3%) | Romania | [27] | |
| 1.8%–2.7% | α-Pinene (1.0–1.3%), Camphene (2.1–3.8%), β-Pinene(1.1–1.6%), Limonene (1.0–1.4%), 1,8-Cineole (9.5–13.3%), β-Thujone (24.4–25.9%), Camphor (20.8–27.1%), β-Caryophyllene (4.8–8.0%), α-Humulene (3.5–5.2%), Thujyl alcohol (1.1–1.3%), Borneol (2.8–3.7%), Ledol (4.9–6.8%), Caryophyllen-8-ol (0.9–1.6%) | Czech Republic | [27] | |
| 1.3%–2.5% | α-Pinene (0.8–2.9%), Camphene (2.0–3.2%), β-Pinene (1.0–2.8%), Limonene (0.5–1.5%), 1,8-Cineole (8.6–11.6%), α-Thujone (22.2–31.9%), β-Thujone (2.7–8.9%), Camphor (15.8–24.0%), Bornyl acetate (0.4–2.8%), β-Caryophyllene (1.0–4.4%), α-Humulene (5.5–7.6%), Borneol (1.9–4.5%), Ledol (3.0–4.1%), Caryophyllen-8-ol (1.9–4.1%) | France | [27] | |
| / | α-Pinene (4.9%), Camphene (5.0%), β-Pinene (3.4%), 1,8-Cineole (12.1%), α-Thujone+linalool (21.2%), β-Thujone (4.4%), Camphor (23.6%), Borneol (5.6%), E-caryophyllene (2.7%), α-Humulene (5.2%), Viridiflorol (3.0%) | Estonia | [21] | |
| / | α-trans-Ocimene (1.69%), Camphene (1.66%), 1-Octen-3-ol (8.50%), 1,8-Cineole (6.72%), α-Thujone (21.85%), β-Thujone (5.51%), Camphor (11.25%), 1-Borneol (2.58%), Bornyl acetate (3.22%), β-Caryophyllene (3.54%), α-Humulene (4.51%), α-Farnesene (1.15%), Viridiflorol (11.71%), Citronellyl propionate (1.22%), Manool (9.15%) | Romania | [38] | |
| 1.02% | α-Thujone (19.02%), Viridiflorol (18.96%), 1,8-Cineole (8.58%), Limonene (6.56%), trans-Carryophyllene (5.20%), β-Thujone (4.09%), α-Thujene (3.42%), β-Pinene (2.19%), Camphor (2.10%), Linalool (2.02%) | Tunisia | [58] | |
| / | α-Pinene (4.5%), Camphene (2.8%), β-Pinene (1.5%), 1,8-Cineole (14.1%), Thujone (56.5%), Camphor (5.7%), α-Humulene (6.9%) | Croatia | [22] | |
| 27 mL/kg | α-Pinene (4.35%), Camphene (7.61), p-Cymene (2.77%), 1,8-Cineole (7.96%), α-Thujone (24.29%), β-Thujone (4.03%), Camphor (23.72%), Borneol (2.21%), Bornyl acetate (2.73%), β-Caryophyllene (2.25%), α-Humulene (2.83%), Viridiflorol (6.41%), Manool (4.07%) | Albania | [34] | |
| 1.08%–1.37% | α-Pinene (8.26%), Camphene (7.27%), 1,8-Cineole (20.13%), cis-Thujone (26.85%), Camphor (16.66%), Borneol (2.76%), Bornyl acetate (1.90%), E-Caryophyllene (1.31%), α-Humulene (1.83%), Viridiflorol (1.08%) | Croatia | [30] | |
| 97 (±3.7) mg/kg leaf dry weight | α-Pinene (1.1 ± 0.09%), Camphene (2.3 ± 0.18%), β-Pinene (1.6 ± 0.32%), Myrcene (1.4 ± 0.11%), Limonene (1.3 ± 0.03%), 1,8-cineole (10.4 ± 1.79%), α-Thujone (17.3 ± 2.94%), Camphor (29.2 ± 2.84%), β-Thujone (4.9 ± 0.64%), β-Caryophyllene (6.4 ± 1.21%), α-Humulene (3.7 ± 1.94%), Caryophyllene oxide (1.9 ± 0.78%), Viridiflorol (11.6 ± 2.23%) | Poland | [33] | |
| Leaves, fresh, completely and incompletely developed | 0.3%–2.9% | Camphor (7.0–32.7%), cis-Thujone (6.7–20.0%), α-Humulene (3.4-18.9%), Viridiflorol (5.7–12.4%), Manool (1.4–14.5%), Camphene (3.6–8.6%), 1,8-Cineole (3.0–6.9%), Limonene (2.2–9.1%), β-Pinene (2.7–13.5%), trans-Thujone (0.7–2.4%), α-Pinene (3.4–5.2%), Myrcene (0.6–1.2%), cis-β-Ocimene (0.0–3.2%), Borneol (1.3–3.0%), Bornyl acetate (0.1–1.7%), β-Caryophyllene (1.0–4.7%) | Serbia | [18] |
| 0.2%–2.1% | Camphor (1.9–30.4%), cis-Thujone (10.6–28.5%), α-Humulene (4.5–33.3%), Viridiflorol (2.9–10.7%), Manool (1.7–9.2%), Camphene (0.2–3.6%), 1,8-Cineole (1.2–19.4%), Limonene (0.5–3.6%), β-Pinene (0.4–6.5%), trans-Thujone (1.4–14.5%), α-Pinene (0.4–1.9%), Myrcene (0.6–1.3%), cis-β-Ocimene (0.0–4.8%), trans- β-Ocimene (0.0–1.7%), Borneol (0.3–1.9%), β-Caryophyllene (0.4–2.5%), α-Terpinene (0.1–1.5%), p-Cymene (0.3–3.6%), γ-Terpinene (0.4–2.9%), cis-Sabinene hydrate (0.4–1.5%), cis-Pinocamphone (0.0–1.9%), Terpinen-4-ol (0.1–2.2%) | Croatia | [18] | |
| Leaves and flowers | 1.59%–1.87% | α-Pinene (3.54%), Camphene (5.63%), Myrcene (5.47%), 1,8-Cineole (19.6%), Camphor (46.1%), Borneol (4.54%), Viridiflorol (0.26%) | Spain | [28] |
| Dried aerial parts | 2.0%–2.1% | α-Pinene (6.5–8.2%), Camphene (2.4–2.9%), β-Pinene (2.8–3.4%), Myrcene (2.0–2.1%), p-Cymene (1.5–1.7%), 1,8-Cineole (64.3–67.1%), α-Thujone (1.2–1.4%), β-Thujone (2.3–2.8%), Camphor (5.3–6.1%), α-Terpineol (1.0–1.2%), β-Caryophyllene (1.4–1.6%) | Portugal | [14] |
| 4.0% | Cineole (13.69%), Borneol (13.77%), α-Thujone (12.46%), Ledene (11.05%), β-Pinene (7.00%), α-Humulene (6.92%), Trans-caryophyllene (5.28%), β-Thujone (4.56%), Camphor (3.58%), Naphthalene (3.27%), Camphene (2.86%), Bicyclo (1.75%) | Iran | [100] | |
| 2.13%–3.3% | α-Thujone (0.300–0.378 µgg−1), Camphor (5.88–16.3 µgg−1), β-Thujone (0.300–0.378 µgg−1), Carvacrol (2.28–51.1 µgg−1), 1,8-Cineole (20.1–37.9 µgg−1) | Jordan | [24] | |
| 0.58% | 1,8-Cineole (33.27%), β-Thujone (18.40%), α-Thujone (13.45%), Borneol (7.39%), β-Elemene (4.82%), Camphor (3.31%), α-Pinene (2.74%), Fenchyl acetate (1.6%), α-Muurolol (1.41%), Camphene (1.03%), | Tunis | [101] | |
| 1.1%–1.2% | α-Pinene (1.05–1.63%), β-Pinene (1.81–3.80%), Myrcene (1.00–1.07%), 1,8-Cineole (8.85–15.6%), α-Thujone (11.55–19.23%), β-Thujone (5.45–6.17%), Camphor (5.08–15.06%), Borneol (1.35–2.87%), β-Caryophyllene (2.63–9.24%), α-Humulene (1.93–8.94%), Viridiflorol (9.94–19.46%), Manool (5.52–13.06%) | Tunisia | [36] | |
| 0.5% | α-Pinene (4.60%), Camphene (3.61%), β-Pinene (1.18%), Limonene (1.52%), 1.8-Cineole (10.03%), β-Thujone (33.15%), α-Thujone (8.73%), Camphor (13.16%), Borneol (2.98%), Bornyl acetate (1.02%), Viridiflorol (3.13%), Humuleneepoxide II (1.21%), Manool (1.48%) | Croatia | [29] | |
| Fresh aerial part | 0.29%–0.39% | α-Pinene (0.27–3.86%), Camphene (1.14–5.65%), β-Pinene (0.23–2.02%), Limonene (tr.-1.42%), Eucalyptol (4.98–13.4%), α-Thujone (35.9–45.8%), β-Thujone (4.35–9.60%), Camphor (15.5–21.1%), Borneol (0.74–3.20%), β-Caryophyllene (tr.-3.78%), α-Humulene (tr.-3.85%) | Italy | [78] |
| Fresh plant | / | Cis-Salvene (1.46%), α–Pinene (4.12%), Camphene (2.87%), β-Pinene (6.06%), Eucalyptol (11.17%), Thujone (35.86%), Camphor (8.13%), Terpinen-4-ol (1.53%), α-Terpineol (1.09%), Linalylacetate (1.87%), α-Humulene (3.25%), Cadinene (1.04%) | Greece | [35] |
| Plant Part | Extraction Parameters | Yield | Bioactive Compounds | Country | Reference |
|---|---|---|---|---|---|
| Leaves, dried | pressure 15 MPa, temperature 40 °C, CO2 flow rate 0.48-0.53 kg h−1, time 1.70–1.82 h | 4.453% | α-Pinene (1.80%), Camphene (1.54%), 1,8-Cineole (6.57%), cis-Thujone (10.03%), Camphor (10.76%), α-Humulene (3.90%), Viridiflorol (7.70%), Manool (17.70%), Labda-7,14-diene-13-ol (2.97%), Abietol (2.36%), Heneicosane (1.02%), Octacosane (2.77%), Triacontane (4.43%) | Croatia | [30] |
| pressure 25 MPa, temperature 60 °C, CO2 flow rate 6 kg h−1, time 90 min, co-solvent (95% ethanol - 2% w/w) | 90 (±7.5) mg kg−1 leaf dry weight | α-Pinene (1.0 ± 0.10%), Camphene (1.4 ± 0.58%), 1,8-Cineole (4.6 ± 0.95%), α-Thujone (7.5 ± 1.07%), β-Thujone (4.9 ± 0.88%), Borneol (8.4 ± 1.53%), Menthol (1.3 ± 0.37%), Camphor (16.4 ± 1.11%), Bornyl acetate (2.2 ± 0.58%), α-Humulene (6.4 ± 1.36%), Viridiflorol (22.51 ± 1.99%), Humuleneepoxide II (2.4 ± 0.49%), β-Caryophyllene (6.4 ± 1.53%), Caryophylleneoxide (1.5 ± 0.80%) | Poland | [33] | |
| pressure 30 MPa, temperature 40 °C CO2 flow rate 2.4 kg h−1, time 1.5–4.5 h, fractionation in 2 separators (s1, s2) | 1.39% 3.23% | s1 = 1,8Cineole (13.97–14.32%), Cis sabinene hydrate (-,1.17%), Linalool (-,1.79%), Cissabinol (-,2.24%), α-Terpineol (-,1.39%), Geraniol (-,1.48%), Camphor (43.46–59.03%), Borneol (6.91–14.08%), Linalyl acetate (-,6.48%), Endobornyl acetate (-,4.70%) Sabinyl acetate (5.15–12.92%), α-Terpinenyl (-,3.36%), E-Caryophyllene (-,2.31%), Humulene (-,1.56%), Geranylpropionate (-,1.91%), Spathulenol (-,1.63%), Viridiflorol (2.29%); s2 = 1,8-Cineole (4.27–17.12%), Trans Sabinenehydrate (0.50–10.92%), Linalool (-,1.34%), Cissabinol (2.37–3.16%), Camphor (30.79–43.07%), Borneol (7.29–12.50%), α-Terpineol (1-40-3.10%), Geraniol (1.16,3.0%), Linalyl acetate (2.65–4.78%), Endobornyl acetate (1.65–3.21%), Sabinyl acetate (4.84–23.90%), α-Terpinenyl (-,3.46%), E-Caryophyllene (1.98–2.56%), Humulene (-,1.42%), Geranylpropionate (-,1.30%), Spathulenol (-,2.45%), Viridiflorol (1.98–5.42%) | Spain | [57] | |
| pressure (80, 100, 150, 200, 300 MPa), temperature 40 °C, CO2 flow rate 3.23 × 10−3 kg min−1, time 4 h | 0.76%–4.65% | α-Thujone (0.66–5.15%), Camphor (1.43–15.24%), Isoborneole (6.80–11.29%), Terpineol-L-4 (0.25–2.08%), Bornyl–acetate (2.01–5.90%), Sabinyl-acetate (0.41–1.05%), Isocaryophyllene (0.84–2.74%), α-Gurjunene (0.44–1.45%), γ-Elemene (7.02–24.98%), Selina-3,7(11) diene (11.25–13.83%), 1,11-Epoxyhumulene (1.98–3.67%), Caryophylleneoxide (0.87–1.73%), Phyllocladene (4.19–23.37%) | Bosnia and Herzegovina | [59] | |
| pressure 80–100 MPa, temperature 45–60 °C, CO2 flow rate 0.95 kg h−1, 2 separators | 1.35% | α-Pinene (2.37%), Camphene (1.02%), β-Pinene (2.44%), β-Myrcene (2.29%), Cymene-orrho (1.82%), 1,8-Cineole (54.36%), α-Thujone (1.38%), β-Thujone (1.42%), Camphor (5.74%), α-Terpineol (1.61%), Caryophyllene (7.06%), α-Humulene (1.27%), β-Bisabolene (1.04%), y-Cadinene (1.46%), Manool (1.79%), 1,8-Cineole (54.36%), Camphor (5.74%), Caryophyllene (7.06%), α-Pinene (2.37%) β-Pinene (2.44%), β-Myrcene (2.29%) | Italy | [55] | |
| pressure 17.2 MPa, temperature 45 °C, CO2 flow rate 1 mL min−1, time 60 min | 13.2%, 7.6% | α-Pinene (5.3%), Camphene (6.1%), β-Pinene (9.5%), 1,8-Cineole (9.7%), α-Thujone+linalool (27.1%), β-Thujone (4.4%), Camphor (15.6%), Borneol (2.1%), E-caryophyllene (2.2%), α-Humulene (4.9%), Viridiflorol (1.6%) | Estonia | [21] | |
| pressure 9 MPa, temperature 25 and 50 °C, CO2 flow rate 0.35 g min−1, time 3 h | 2.7%–4.8% | α-Pinene (1.45, 4.28%), Camphene (2.54, 7.16%), β-Myrcene (1.06, 1.11%), p-Cymene (1.22, 3.14%), 1,8-Cineole (3.45, 9.54%), α-Thujone (17.49, 26.52%), β-Thujone (2.54, 4.23%), Camphor (19.08, 27.26%), Borneol (2.34%, tr.), Bornyl acetate (2.00, 2.25%), β-Caryophyllene (4.06, 3.83%), β-Gurjunene (1.00%, tr.), Aromadendrene (1.34, tr.), α-Humulene (4.73, 3.82%), Viridiflorol (6.64%, -), Manool (15.28, 0.65%), Sclareol (0.71, 1.52%), Heneicosane (3.92, 2.04%), Hentriacontane (4.66%, tr.) | Albania | [34] | |
| pressure 65–160 MPa, temperature 50 °C, flow rate 3.5–4 g min−1, time 5 h | / | Manool (32.39–56.49%), Ledene (4.43–7.63%), Viridiflorol (4.50–24.69%), 5,8-Dimethoxy-2-methyl-4H-Naphtho[2,3-b]pyran-4,6,9-trione (0.03–7.18%) Camphor (1.00–4.45%), Estra-1,3,5(10),9(11)-teraen-17-one (0.02–2.84%), β-Caryophyllene (1.13–2.33%), 1,8-Cineole (0.80–1.79%), α-Thujone (1.00–1.57%), Aromadendrene (0.65–1.01%) | Tunisia | [58] | |
| pressure 10–30 MPa, temperature 40–60 °C, CO2 flow rate 1–3 kg h−1, time 90 min | 0.242%–7.361% | 1,8-Cineole (6.56–25.52 mg CE g−1), α-/β-Thujone (11.56–34.68 mg CE g−1), Camphor (38.23–102.97 mg g−1), α-Humulene (39.90–90.73 mg CE g−1), Viridiflorol (48.07–97.01 mg CE g−1), Manool (113.90–335.36 mg CE g−1), α-Pinene (0.47–9.09 mg CE g−1), Camphene (0.35–7.87 mg CE g−1), β-Pinene (0.24–1.15 mg CE g−1), β-Myrcene (0.24–1.15 mg CE g−1), p-Cymene (0.24–1.15 mg CE g−1), Limonene (0.24–1.68 mg CE g−1), Linalool (0.24–2.30 mg CE g−1), Borneol (7.32–31.01 mg CE g−1), Terpinen-4-ol (0.24–2.30 mg CE g−1), p-Cymen-8-ol (0.24–1.35 mg CE g−1), α-Terpineol (0.48–1.35 mg CE g−1), Myrtenol (0.00–1.15 mg CE g−1), Bornyl acetate (3.54–14.93 mg CE g−1), trans-β-caryophyllene (3.54–16.08 mg CE g−1), 6-Oxobornyl acetate (3.07–14.93 mg CE g−1), Alloaromadendrene (0.24–2.30 mg CE g−1), Ledene (0.47–2.30 mg CE g−1) | Croatia | [60] | |
| Leaves and flowers | pressure 90 and 100 MPa, temperature 40 and 50 °C, particle diameter (0.3, 0.5, 0.8 mm), CO2 flow rate (0.72, 1.02, 1.32 kg h−1 | 1.27%–1.88% | α-Pinene (1.33,1.54%), Camphene (1.73,2.42%), Myrcene (2.65,3.89%), 1,8-Cineole (16.1,14.2%), Camphor (40.9,48.0%), Borneol (4.62,4.17%), α-Terpineol (1.45, 0.95%), β-Caryophyllene (1.47,1.53%), Methyldodecanoate (1.77,1.85%), Viridiflorol (1.41,0.24%) | Spain | [28] |
| Dried aerial parts | pressure 7,10,15,20,30 MPa, temperature 50 °C, CO2 flow rate 0.4 kg h−1 | 4.82% | α-Pinene (0.77–2.07%), Camphene (0.50–1.54%), 1.8-Cineole (1.88–4.75%), β-Thujone (7.95–16.56%), α-Thujone (3.26–8.12%), Camphor (7.95–10.64%), Neo-3-thujanol (2.16–2.51%), Myrtenol (0.66–1.06%), α-Campholenicacid (1.43–2.52%), Acetophloroglucine (0.72–2.54%), Trans-caryophyllene (1.39–2.16%), α-Humulene (1.90–3.08%), Caryophylleneoxide (0.60–2.62%), Viridiflorol (4.14–9.58%), Humuleneepoxide II (1.16–4.78%), Muurola-4.10(14)-dien-1-β-ol (0.50–1.04%), Manool (13.15–21.75%), Carnosol derivative (6.25–13.09%), Trans-ferruginol (0.08–1.71%), Methylhexadecanoate (0.74–1.98%), Methyloleate (0.07–1.19%), Methyloctadecanoate (0.07–2.46%), Octacosane (0.35–2.17%), Untriacontane (0.00–1.33%), Olean-18-ene (0.52–4.24%), Lupeol (0.70–3.04%) | Croatia | [29] |
| Type of Extraction | Plant Part | Extraction Parameters | Yield | Bioactive Compounds | Country | Reference |
|---|---|---|---|---|---|---|
| INFUSION | Flowering aerial parts | / | Luteolin diglucuronide (11.89 ± 0.15 mg g−1), 6-Hydroxyluteolin 7-O-glucuronide (2.53 ± 0.08 mg g−1), Sagecoumarin (1.11 ± 0.05 mg g−1), Luteolin 7-O-rutinoside (9.35 ± 0.20 mg g−1), Luteolin 7-O-glucuronide (88.12 ± 0.36 mg g−1), Luteolin 7-O-glucoside (37.41 ± 0.65 mg g−1), Sagerinic acid (2.92 ± 0.08 mg g−1), cis-Rosmarinic acid (0.97 ± 0.07 mg g−1), trans-Rosmarinic acid (73.97 ± 0.15 mg g-1), Apigenin 7-O-glucoside (5.40 ± 0.01 mg g−1), Luteolin acetylglucoside (15.56 ± 0.33 mg g−1), Hispidulin glucuronide (10.53 ± 0.25 mg g−1), Hispidulin (1.01 ± 0.03 mg g−1) | Spain | [39] | |
| Teas (commercial brands) or sage leaves | / | Saponin (3.8 ± 0.77-12.9 ± 0.25 mg L−1), Luteolin-diglucuronide (5.1 ± 1.20-44.0 ± 1.99 mg L−1), Hydroxyluteolin-glucuronide (6.7 ± 1.57 mg L−1), Apigenin-diglucuronide (1.1 ± 0.22-9.1 ± 0.05 mg L−1), Luteolin-7-O-glucoside (3.5 ± 0.71-8.4 ± 0.22 mg L−1), Luteolin-rutinoside (4.9 ± 0.31-10.7 ± 0.60 mg L−1), Luteolin-7-O-glucuronide (37.9 ± 2.17-166.3 ± 1.65 mg L−1), Rosmarinic acid (30.5 ± 1.00-295.7 ± 9.71 mg L−1), Apigenin-glucuronide (8.6 ± 0.39-41.1 ± 1.15 mg L−1), Salvianolic acid K (6.8 ± 0.18-56.4 ± 2.95 mg L−1), Carnosic acid (9.1 ± 1.20-32.9 ± 3.71 mg L−1) | Germany | [40] | ||
| MACERATION | Leaves, dried | methanol, 72 h, room temperature | 23.41% ± 2.65% | Chlorogenic acid (1.22%), Caffeic acid (1.98%), Quinic acid (1.19%), p-Coumaric acid (1.2%), Caffeoyl quinic acid derivative (1.07%), Quercetin-7-O-glucoside (2.52%), Ferulic acid (18.79%), Carnosic acid (3.77%), Cinnamic acid (2.57%), Rosmarinic acid (17.85%), Apigenin (14.32%), Luteolin-7-O-rutinose (8.61%) | Egypt | [44] |
| SOXHLET | Leaves, dried (commercial samples) | hexane and ethyl acetate, 6 h | / | Rosmarinic acid (10.0 ± 0.92 mg g−1), Apigenin (2.5 ± 0.38 mg g−1), Hispidulin (6.3 ± 0.58 mg g−1), Carnosol (31.1 ± 1.00 mg g−1), Rosmadial (6.8 ± 0.42 mg g−1), Carnosic acid (42.9 ± 3.05 mg g−1), Methyl carnosate (8.6 ± 0.22 mg g−11), Oleanolic acid (171.9 ± 10.6 mg g−1), Ursolic acid (358.8 ± 14.2 mg g−1) | [37] | |
| Dried aerial parts | ethanol and water (70:30 v/v), 4 h | 26.5% | α-Campholenic acid (1.21%), Cis-α-Bergamotene (1.61%), Viridiflorol (4.25%), Humuleneepoxide II (1.31%), Manool (13.15%), Carnosol derivative (15.21%), Trans-ferruginol (1.49%), Methylhexadecanoate (1.08%), Heptacosane (1.10%), Nonacosane (6.21%), Untriacontane (7.05%), t-Sitosterol (1.25%), Olean-18-ene (24.76%), Lupeol (8.01%) | Croatia | [29] | |
| methanol, 2 h under nitrogen atmosphere | / | Caffeic acid (222.24 ± 11.23-695.04 ± 18.21 µg g−1 DM), Ferulic acid (312.43 ± 2.53-703.29 ± 17.74 µg g−1 DM), Rosmaric acid (13,680.22 ± 101.77-18,378.00 ± 393.26 µg g−1 DM), Gallic acid (14.49 ± 2.41-29.74 ± 1.05 µg g−1 DM), p-hydroxy benzoic acid (121.15 ± 2.16-122.31 ± 2.65 µg g−1 DM), Carnosic acid (3278.30 ± 227.59-6001.75 ± 390.12 µg g−1 DM), Carnosol (5045.42 ± 318.10-5947.03 ± 173.45 µg g−1 DM), Methyl carnosate (4816.59 ± 199.40-7174.00 ± 73.27 µg g−1 DM), Luteolin-7-O-glucoside (386.63 ± 0.39-661.04 ± 65.60 µg g−1 DM), Apigenin-7-glucoside (210.01 ± 0.70-913.90 ± 166.89 µg g−1 DM), Luteolin (21.41 ± 0.54-66.44 ± 1.90 µg g−1 DM), Apigenin (55.77 ± 6.65-77.51 ± 4.22 µg g−1 DM), Genkwanin (21.57 ± 0.80-25.60 ± 4.58 µg g−1 DM), Naringin (485.77 ± 32.41-857.92 ± 8.41 µg g−1 DM) | Tunisia | [36] | ||
| EXTRACTION | Herba, dried | ethanol, 1 h–7 days, Temperature 20, 30, 50 °C | / | Cineole (6.8–43.3 mg kg−1), Thujone (48.2–269.2 mg kg−1), Borneol (2.5–7.6 mg kg−1) | Slovakia | [45] |
| Herba, dried | ethanol, stirring with and without ultrasound, temperature 20 °C | / | Cineole (14.4–33.4 mg kg−1), Thujone (95.0–232.9 mg kg−1), Borneol (5.3–8.8 mg kg−1) | Slovakia | [45] | |
| Herba, dried | ethanol ultrasound, 1 h–7 days, temperature 20, 30, 50 °C | / | Cineole (9.8–40.3 mg kg−1), Thujone (63.9–258.2 mg kg−1), Borneol (2.9–7.2 mg kg−1) | Slovakia | [45] | |
| Commercially available plant samples | methanol, acidification and elution through column, | / | Syringic acid, p-Coumaric acid, Ferulic acid, Sinapic acid, Luteolin, Apigenin | [85] | ||
| Flowering aerial parts | methanol:water (80:20, v/v), 1 h, temperature 25 °C | / | Caffeic acid (2.00 ± 0.01 mg g−1), Luteolin diglucuronide (4.94 ± 0.01 mg g−1), 6-Hydroxyluteolin 7-O-glucuronide (1.72 ± 0.09 mg g−1), Sagecoumarin (0.76 ± 0.09 mg g−1), Luteolin 7-O-rutinoside (12.57 ± 0.03 mg g−1), Luteolin 7-O-glucuronide (94.73 ± 2.55 mg g−1), Luteolin 7-O-glucoside (56.09 ± 3.45 mg g−1), Sagerinic acid (3.35 ± 0.31 mg g−1), cis-Rosmarinic acid (1.20 ± 0.01 mg g−1), trans-Rosmarinic acid (93.22 ± 0.12 mg g−1), Apigenin-7-O-glucoside (7.47 ± 0.06 mg g−1), Luteolin acetylglucoside (21.73 ± 0.78 mg g−1), Hispidulin glucuronide (15.08 ± 0.14 mg g−1), Apigenin acetylglucoside (7.47 ± 0.06 mg g−1), Hispidulin (2.24 ± 0.13 mg g−1) | Spain | [39] | |
| Leaves, dried | 30, 50 or 70% aqueous ethanol, acetone and water, 30, 60, 90 min, Temperature 60, 90 °C | / | Vanillic (3.04 ± 0.11-14.01 ± 0.80 mg 100 g−1 DM), Caffeic (8.05 ± 0.22-125.31 ± 8.32 mg 100 g−1 DM), Syringic (41.20 ± 0.89-70.32 ± 1.78 mg 100 g−1 DM), Rosmarinic (1759.10 ± 12.02-3634.12 ± 33.30 mg 100 g−1 DM), Salvianolic K (18.10 ± 1.00-50.14 ± 0.97 mg 100 g−1 DM), Salvianolic I acids (12.45 ± 0,82-26.12 ± 0.97 mg 100 g−1 DM), Methyl rosmarinate (9.06 ± 1.60-100.01 ± 5.47 mg 100 g−1 DM), 6-Hydroxyluteolin-7-glucoside (38.10 ± 2.00- 202.13 ± 0.89 mg 100 g−1 DM), Luteolin-7-glucuronide (109.63 ± 10.99- 356.20 ± 25.60 mg 100 g−1 DM), Luteolin-7-glucoside (23.65 ± 1.45-233.23 ± 10.73 mg 100 g−1 DM), Luteolin-3-glucuronide (470.31 ± 5.43-998.12 ± 20.01 mg 100 g−1 DM), Apigenin-7-glucuronide (59.55 ± 5.22-291.10 ± 0.65 mg 100 g−1 DM), Apigenin-7-glucoside (32.12 ± 1.65-147.26 ± 1.30 mg/100 g−1 DM) | Croatia | [41] | |
| Leaves, infusion | Infusion and elution through column, dichloromethane | / | 1,8-Cineole (16.16%), α-Thujone (25.78%), β-Thujone (7.07%), Camphor (24.94%), 1-Borneol (5.38%), exo-2-Hydroxycineole acetate (1.73%), 6-Oxobornyl acetate (7.99%) | Romania | [38] | |
| Leaves | Liquid-liquid extraction of infusion, dichloromethane; hexane | / | 1,8-Cineole (10.66; 11.37%), α-Thujone (22.61;23.95%), β-Thujone (4.58; 5.13%), Camphor (25.88; 24.93%), 1-Borneol (5.66; 5.62%), Dihydrocamphene carbinol (1.74; 0.81%), Bornyl acetate (1.02; 0.59%), 6-Oxobornyl acetate (9.18; 10.59%), Shyobunol (3.29; 0.73%) | Romania | [38] | |
| Leaves | Microwave power (500, 600, 700 W), 30, 50 or 70% aqueous ethanol, acetone (v/v) and water, 3, 5, 7, 9 11 min temperature 80 °C | / | Rosmarinic acid (7.1 ± 0.2-32.3 ± 0.6 mg g−1), sum of vanillic, caffeic, syringic, sagerinic, salvianolic acid K and salvianolic acid I (1.8 ± 0.1-2.6 ± 0.1 mg g−1), 6-hidroxyluteolin-7-glucoside (1.8 ± 0.1-2.5 ± 0.1 mg g−1), luteolin-3′-glucuronide (3.8 ± 0.1-7.5 ± 0.2 mg g−1), sum of luteolin-7-glucuronide, luteolin-7-glucoside, apigenin-7-glucuronide and apigenin-7-glucoside (1.6 ± 0.1-6.1 ± 0.1 mg g−1) | Croatia | [47] | |
| Leaves | Microwave power (100–500 W), 30, 50 or 70% aqueous ethanol, acetone (v/v) and water, 3, 5, 7, 9, 10 min, temperature 30, 50, 60, 80 °C, addition of 10% HCl | / | Rosmarinic acid (384.35 ± 30.78-1521.54 ± 38.44 mg 100 g−1), Methyl rosmarinate (0.72 ± 3.04-90.44 ± 3.04 mg 100 g−1), Syringic acid (5.93 ± 1.06-22.35 ± 1.06 mg 100 g−1), Salvianolic acid (1.32 ± 2.62-15.86 ± 2.62 mg 100 g−1), Caffeic acid (5.19 ± 2.51-21.23 ± 2.51 mg/100 g−1), Vanillic acid (0.80 ± 0.35-4.57 ± 0.50 mg/100 g−1) | Croatia | [48] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakovljević, M.; Jokić, S.; Molnar, M.; Jašić, M.; Babić, J.; Jukić, H.; Banjari, I. Bioactive Profile of Various Salvia officinalis L. Preparations. Plants 2019, 8, 55. https://doi.org/10.3390/plants8030055
Jakovljević M, Jokić S, Molnar M, Jašić M, Babić J, Jukić H, Banjari I. Bioactive Profile of Various Salvia officinalis L. Preparations. Plants. 2019; 8(3):55. https://doi.org/10.3390/plants8030055
Chicago/Turabian StyleJakovljević, Martina, Stela Jokić, Maja Molnar, Midhat Jašić, Jurislav Babić, Huska Jukić, and Ines Banjari. 2019. "Bioactive Profile of Various Salvia officinalis L. Preparations" Plants 8, no. 3: 55. https://doi.org/10.3390/plants8030055
APA StyleJakovljević, M., Jokić, S., Molnar, M., Jašić, M., Babić, J., Jukić, H., & Banjari, I. (2019). Bioactive Profile of Various Salvia officinalis L. Preparations. Plants, 8(3), 55. https://doi.org/10.3390/plants8030055








