Seed Pretreatment and Foliar Application of Proline Regulate Morphological, Physio-Biochemical Processes and Activity of Antioxidant Enzymes in Plants of Two Cultivars of Quinoa (Chenopodium quinoa Willd.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatment
2.2. Plant Growth
2.3. Chlorophyll Contents
2.4. Hydrogen Peroxide (H2O2) Contents
2.5. Malondialdehyde (MDA) Contents
2.6. Free Proline Contents
2.7. Ascorbic Acid
2.8. Antioxidant Enzymes
2.9. Catalase (CAT) and Peroxidase (POD)
2.10. Superoxide Dismutase (SOD)
2.11. Total Soluble Sugars
2.12. Total Phenolics
2.13. Free Amino Acids
2.14. Statistical Analysis
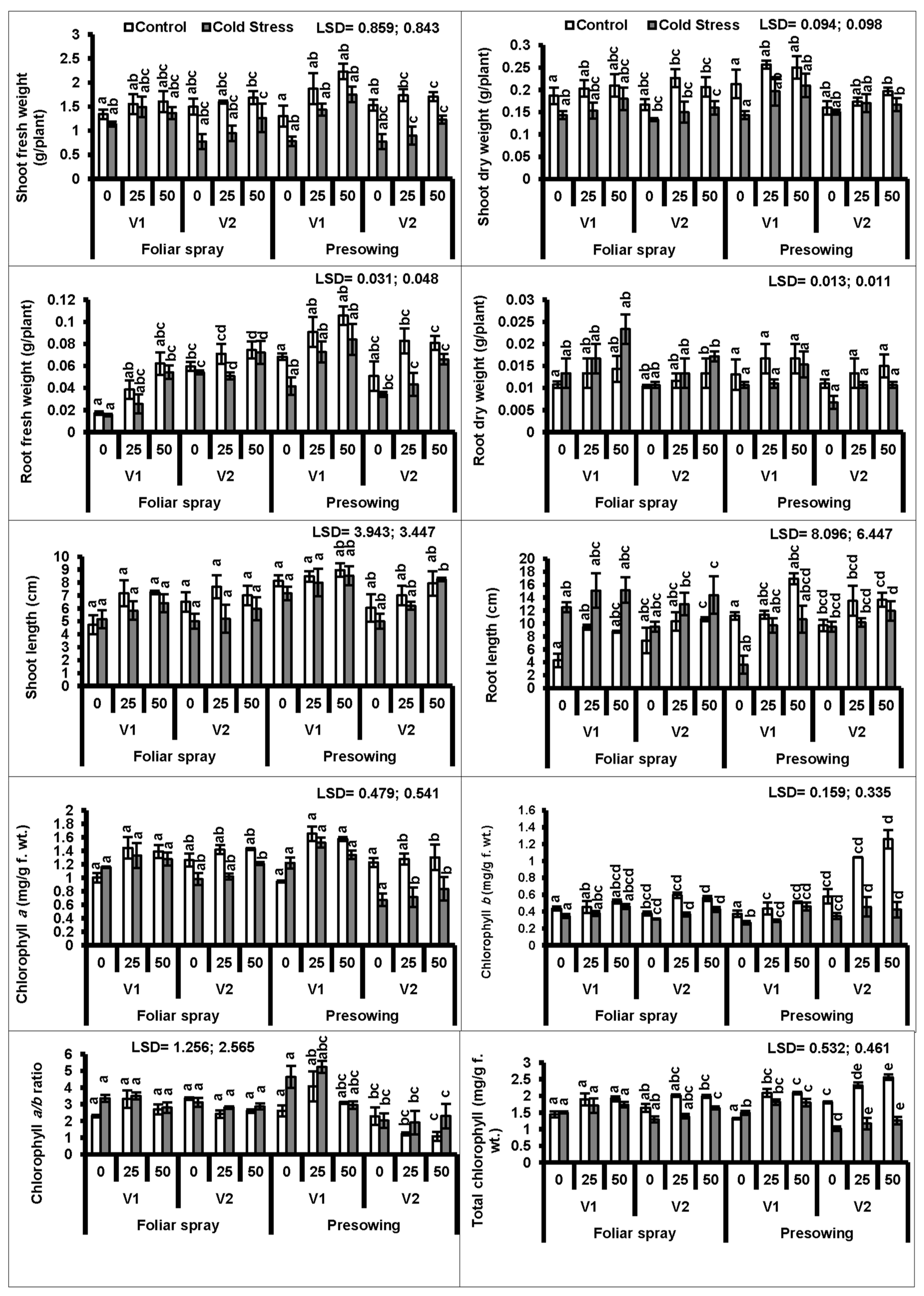
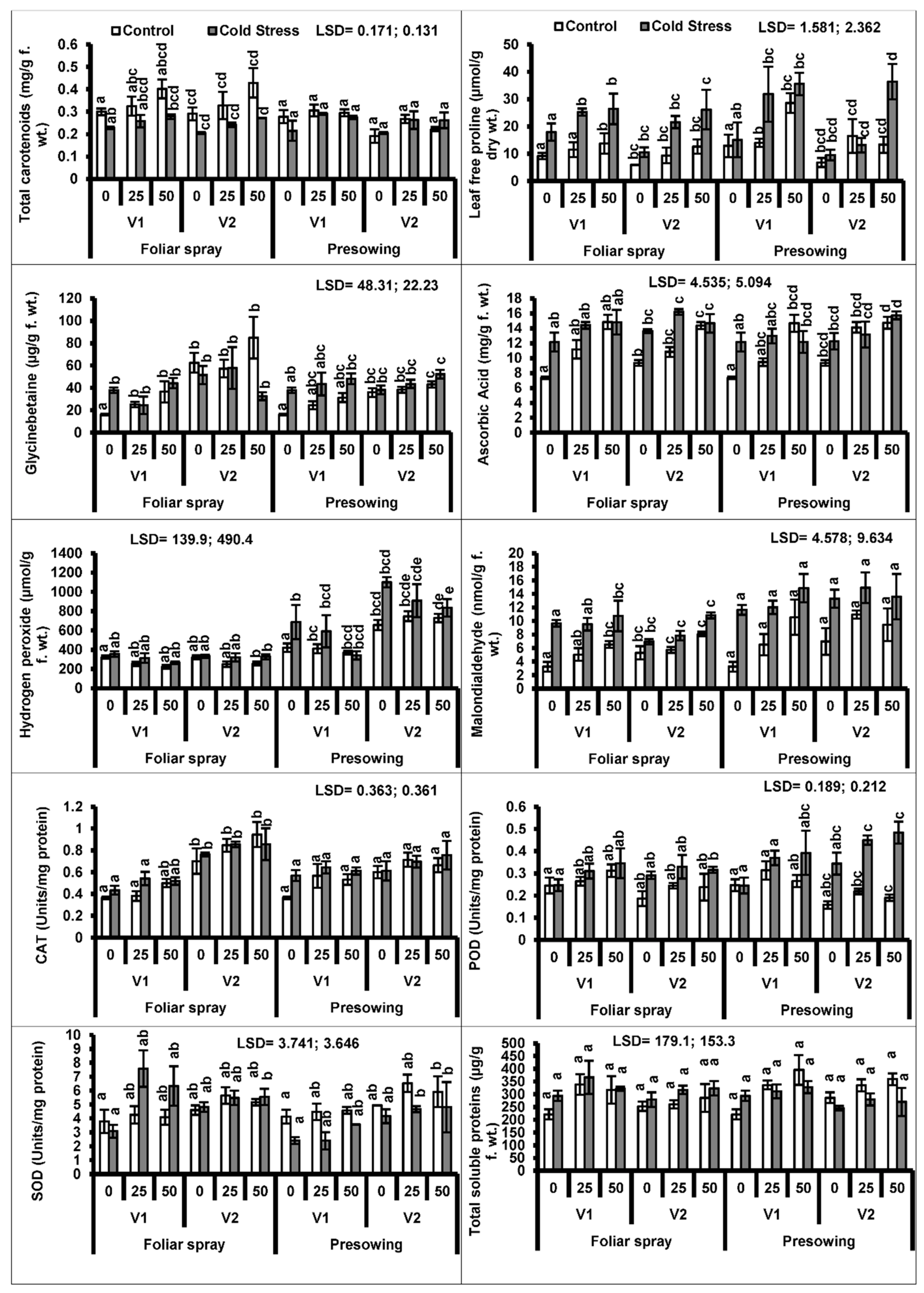
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shahbaz, M.; Ashraf, M.; Al-Qurainy, F.; Harris, P.J.C. Salt tolerance in selected vegetable crops. Crit. Rev. Plant Sci. 2012, 31, 303–320. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Basu, S.; Sengupta, D.N. Antioxidants and stress-related metabolites in the seedlings of two indica rice varieties exposed to cadmium chloride toxicity. Acta Physiol. Plant. 2011, 34, 835–847. [Google Scholar] [CrossRef]
- Shahbaz, M.; Ashraf, M. Improving salinity tolerance in cereals. Crit. Rev. Plant Sci. 2013, 32, 237–249. [Google Scholar] [CrossRef]
- Blokhina, O. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Schwartz, M.D.; Ahas, R.; Aasa, A. Onset of spring starting earlier across the Northern Hemisphere. Global Change Biol. 2006, 12, 343–351. [Google Scholar] [CrossRef]
- Ahmad, T.; Shahzad, J.e.S. Low temperature stress effect on wheat cultivars germination. Afr. J. Microbiol. Res. 2012, 6, 1265–1269. [Google Scholar] [CrossRef][Green Version]
- Ghadirnezhad, R.; Fallah, A. Temperature effect on yield and yield components of different rice cultivars in flowering stage. Int. J. Agron. 2014, 2014, 1–4. [Google Scholar] [CrossRef]
- Clayton, S.; Neves, P.C. Country snapshot: Brazil. Rice Today 2011, 10, 16–17. [Google Scholar]
- De Lima, J.C.; Loss-Morais, G.; Margis, R. MicroRNAs play critical roles during plant development and in response to abiotic stresses. Genet. Mol. Biol. 2012, 35, 1069–1077. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K.; Seki, M. Regulatory network of gene expression in the drought and cold stress responses. Curr. Opin. Plant Biol. 2003, 6, 410–417. [Google Scholar] [CrossRef]
- Sanghera, G.; Wani, S.; Hussain, W.; Singh, N. Engineering cold stress tolerance in crop plants. Curr. Genom. 2011, 12, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Sun, L.; Liu, P.; Liu, G.; Tian, J.; Liao, H. Malate synthesis and secretion mediated by a manganese-enhanced malate dehydrogenase confers superior manganese tolerance in Stylosanthes guianensis. Plant Physiol. 2015, 167, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Foolad, M.R. Crop breeding for salt tolerance in the era of molecular markers and marker-assisted selection. Plant Breed. 2013, 132, 10–20. [Google Scholar] [CrossRef]
- Wu, D.; Cai, S.; Chen, M.; Ye, L.; Chen, Z.; Zhang, H.; Dai, F.; Wu, F.; Zhang, G. Tissue metabolic responses to salt stress in wild and cultivated barley. PLoS ONE 2013, 8, e55431. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Soshinkova, T.N.; Radyukina, N.L.; Korolkova, D.V.; Nosov, A.V. Proline and functioning of the antioxidant system in Thellungiella salsuginea plants and cultured cells subjected to oxidative stress. Russ. J. Plant Physiol. 2013, 60, 41–54. [Google Scholar] [CrossRef]
- Molla, M.R.; Ali, M.R.; Hasanuzzaman, M.; Al-Mamun, M.H.; Ahmed, A.; Nazim-Ud-Dowla, M.A.N.; Rohman, M.M. Exogenous proline and betaine-induced upregulation of glutathione transferase and glyoxalase I in lentil (Lens culinaris) under drought stress. Not. Bot. Hort. Agrobot. Cluj-Napoca 2014, 42. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Giri, J. Glycinebetaine and abiotic stress tolerance in plants. Plant Signal. Behav. 2011, 6, 1746–1751. [Google Scholar] [CrossRef]
- Kumar, S.; Nayyar, H.; Bhanwara, R.; Upadhyaya, H. Chilling stress effects on reproductive biology of chickpea. J. SAT Agric. Res. 2010, 8, 1–14. [Google Scholar]
- Mattioli, R.; Marchese, D.; D’Angeli, S.; Altamura, M.M.; Costantino, P.; Trovato, M. Modulation of intracellular proline levels affects flowering time and inflorescence architecture in Arabidopsis. Plant Mol. Biol. 2008, 66, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Funck, D.; Winter, G.; Baumgarten, L.; Forlani, G. Requirement of proline synthesis during Arabidopsis reproductive development. BMC Plant Biol. 2012, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.; Funck, D.; Szabados, L.; Rentsch, D. Proline metabolism and transport in plant development. Amino Acids 2010, 39, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ashraf, M.; Athar, H.U.R. Exogenously applied proline at different growth stages enhances growth of two maize cultivars grown under water deficit conditions. Pak. J. Bot. 2007, 39, 1133–1144. [Google Scholar]
- Okuma, E.; Murakami, Y.; Shimoishi, Y.; Tada, M.; Murata, Y. Effects of exogenous application of proline and betaine on the growth of tobacco cultured cells under saline conditions. Soil Sci. Plant Nutr. 2004, 50, 1301–1305. [Google Scholar] [CrossRef]
- Talat, A.; Nawaz, K.; Hussian, K.; Bhatti, K.H.; Siddiqi, E.H.; Khalid, A.; Anwer, S.; Sharif, M.U. Foliar application of proline for salt tolerance of two wheat (Triticum aestivum L.) cultivars. World Appl. Sci. J. 2013, 22, 547–554. [Google Scholar]
- Ben Ahmed, C.; Magdich, S.; Ben Rouina, B.; Sensoy, S.; Boukhris, M.; Ben Abdullah, F. Exogenous proline effects on water relations and ions contents in leaves and roots of young olive. Amino Acids 2010, 40, 565–573. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martínez, E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: a review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef]
- Stikic, R.; Glamoclija, D.; Demin, M.; Vucelic-Radovic, B.; Jovanovic, Z.; Milojkovic-Opsenica, D.; Jacobsen, S.-E.; Milovanovic, M. Agronomical and nutritional evaluation of quinoa seeds (Chenopodium quinoa Willd.) as an ingredient in bread formulations. J. Cereal Sci. 2012, 55, 132–138. [Google Scholar] [CrossRef]
- Jacobsen, S.E. The worldwide potential for quinoa (Chenopodium quinoa Willd.). Food Rev. Int. 2003, 19, 167–177. [Google Scholar] [CrossRef]
- Jacobsen, S.E. The situation for quinoa and its production in Southern Bolivia: From economic success to environmental disaster. J. Agron. Crop. Sci. 2011, 197, 390–399. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Biondi, S.; Martínez, E.A.; Orsini, F.; Antognoni, F.; Jacobsen, S.E. Quinoa—A model crop for understanding salt-tolerance mechanisms in halophytes. Plant Biosys. 2015, 150, 357–371. [Google Scholar] [CrossRef]
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.E. Nutritional value and use of the Andean crops quinoa (Chenopodium quinoa) and Kañiwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Llorente, J. Quinoa. Available online: http://dsalud.com/index.php.
- Kamran, M.; Shahbaz, M.; Ashraf, M.; Akram, N.A. Alleviation of drought-induced adverse effects in spring wheat (Triticum aestivum L.) using proline as a pre-sowing seed treatment. Pak. J. Bot. 2009, 41, 621–632. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts, polyphenoxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Meth. Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Julkunen-Tiitto, R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Van Slyke, D.D.; Lemish, S. The gasometric determination of free amino acids in blood filtrates by the ninhydrin-carbon dioxide method. J. Biol. Chem. 1943, 150, 231–250. [Google Scholar]
- Kahlaoui, B.; Hachicha, M.; Misle, E.; Fidalgo, F.; Teixeira, J. Physiological and biochemical responses to the exogenous application of proline of tomato plants irrigated with saline water. J. Saudi Soc. Agric. Sci. 2018, 17, 17–23. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Cerdán, M.; Sánchez-Sánchez, A.; Jordá, J.D.; Juárez, M.; Sánchez-Andreu, J. Effect of commercial amino acids on iron nutrition of tomato plants grown under lime-induced iron deficiency. J. Plant Nutr. Soil Sci. 2013, 176, 859–866. [Google Scholar] [CrossRef]
- Elewa, T.A.; Sadak, M.S.; Saad, A.M. Proline treatment improves physiological responses in quinoa plants under drought stress. Biosci. Res. 2017, 14, 21–33. [Google Scholar]
- Ali, H.; Siddiqui, M.; Al-Whaibi, M.; Basalah, M.; Sakran, A.; El-Zaidy, M. Effect of proline and abscisic acid on the growth and physiological performance of faba bean under water stress. Pak. J. Bot. 2013, 45, 933–940. [Google Scholar]
- Deivanai, S.; Xavier, R.; Vinod, V.; Timalata, K.; Lim, O. Role of exogenous proline in ameliorating salt stress at early stage in two rice cultivars. J. Stress Physiol. Biochem. 2011, 7. [Google Scholar]
- Abd Elhamid, E.M.; Sadak, M.S.; Tawfik, M. Physiological response of Fenugreek plant to the application of proline under different water regimes. Res. J. Pharmaceut. Biol. Chem. Sci. 2016, 7, 580–594. [Google Scholar]
- Yan, Z.; Guo, S.; Shu, S.; Sun, J.; Tezuka, T. Effects of proline on photosynthesis, root reactive oxygen species (ROS) metabolism in two melon cultivars (Cucumis melo L.) under NaCl stress. Afr. J. Biotechnol. 2011, 10, 18381–18390. [Google Scholar] [CrossRef]
- Çelik, Ö.; Ayan, A.; Atak, Ç. Enzymatic and non-enzymatic comparison of two different industrial tomato (Solanum lycopersicum) varieties against drought stress. Bot. Stud. 2017, 58. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Massacci, A.; Nabiev, S.M.; Pietrosanti, L.; Nematov, S.K.; Chernikova, T.N.; Thor, K.; Leipner, J. Response of the photosynthetic apparatus of cotton (Gossypium hirsutum) to the onset of drought stress under field conditions studied by gas-exchange analysis and chlorophyll fluorescence imaging. Plant Physiol. Biochem. 2008, 46, 189–195. [Google Scholar] [CrossRef]
- Smolikova, G.N.; Laman, N.A.; Boriskevich, O.V. Role of chlorophylls and carotenoids in seed tolerance to abiotic stressors. Russ. J. Plant Physiol. 2011, 58, 965–973. [Google Scholar] [CrossRef]
- Pandey, H.; Baig, M.; Bhatt, R. Effect of moisture stress on chlorophyll accumulation and nitrate reductase activity at vegetative and flowering stage in Avena species. Agric. Sci. Res. J. 2012, 2, 111–118. [Google Scholar]
- Sadak, M.S.; Mostafa, H.A. Physiological role of pre-sowing seed with proline on some growth, biochemical aspects, yield quantity and quality of two sunflower cultivars grown under seawater salinity stress. Sci. Agric. 2015, 9, 60–69. [Google Scholar]
- Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. BioMed Res. Int. 2014, 2014, 1–17. [Google Scholar] [CrossRef]
- Nawaz, K.; Ashraf, M. Exogenous application of glycinebetaine modulates activities of antioxidants in maize plants subjected to salt stress. J. Agron. Crop. Sci. 2010, 196, 28–37. [Google Scholar] [CrossRef]
- Agami, R.A. Applications of ascorbic acid or proline increase resistance to salt stress in barley seedlings. Biol. Plant. 2014, 58, 341–347. [Google Scholar] [CrossRef]
- Mazid, M.; Khan, T.A.; Khan, Z.H.; Quddusi, S.; Mohammad, F. Occurrence, biosynthesis and potentialities of ascorbic acid in plants. Int. J. Plant Anim. Environ. Sci. 2011, 1, 167–184. [Google Scholar]
- Sakr, M.T.; El-Sarkassy, N.M.; Fuller, M.P. Osmoregulators proline and glycine betaine counteract salinity stress in canola. Agron. Sustain. Dev. 2012, 32, 747–754. [Google Scholar] [CrossRef]
- Abdelhamid, M.T.; Rady, M.M.; Osman, A.S.; Abdalla, M.A. Exogenous application of proline alleviates salt-induced oxidative stress in Phaseolus vulgaris L. plants. J. Hort. Sci. Biotechnol. 2013, 88, 439–446. [Google Scholar] [CrossRef]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef]
- Garg, N.; Manchanda, G. ROS generation in plants: Boon or bane? Plant Biosyst. 2009, 143, 81–96. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Sakran, A.M.; Ali, H.M.; Basalah, M.O.; Faisal, M.; Alatar, A.; Al-Amri, A.A. Calcium-induced amelioration of boron toxicity in radish. J. Plant Growth Regul. 2012, 32, 61–71. [Google Scholar] [CrossRef]
- Osman, H.S. Enhancing antioxidant–yield relationship of pea plant under drought at different growth stages by exogenously applied glycine betaine and proline. Ann. Agric. Sci. 2015, 60, 389–402. [Google Scholar] [CrossRef]
- Azevedo, R.A.; Gratão, P.L.; Monteiro, C.C.; Carvalho, R.F. What is new in the research on cadmium-induced stress in plants? Food Energy Secur. 2012, 1, 133–140. [Google Scholar] [CrossRef]
- Sgherri, C.; Cosi, E.; Navari-Izzo, F. Phenols and antioxidative status of Raphanus sativus grown in copper excess. Physiol. Plant. 2003, 118, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ben Ahmed, C.; Ben Rouina, B.; Sensoy, S.; Boukhriss, M.; Ben Abdullah, F. Exogenous proline effects on photosynthetic performance and antioxidant defense system of young olive tree. J. Agric. Food Chem. 2010, 58, 4216–4222. [Google Scholar] [CrossRef] [PubMed]
- Zali, G.A.; Ehsanzadeh, P. Exogenous proline improves osmoregulation, physiological functions, essential oil, and seed yield of fennel. Ind. Crops Prod. 2018, 111, 133–140. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Hasanloo, T.; Mohammadi, S. Physiological characteristics, antioxidant enzyme activities, and gene expression in 2 spring canola (Brassica napus L.) cultivars under drought stress conditions. Turk. J. Agric. Forest. 2014, 39, 413–420. [Google Scholar] [CrossRef]

| Source of Variation | df | Shoot Fresh Weight | Shoot Dry Weight | Root Fresh Weight | Root Dry Weight | Shoot Length | Root Length |
|---|---|---|---|---|---|---|---|
| Cultivars (Cv) | 1 | 0.132 ns | 0.0002 ns | 0.0005.8 ns | 0.00005 ns | 0.16 ns | 0.0011 ns |
| Chilling Stress (CS) | 1 | 1.311 *** | 0.0196 *** | 0.0072 *** | 0.0001.1 * | 11.33 * | 207.36 *** |
| Foliar Spray (FS) | 2 | 0.273 ns | 0.0034 ns | 0.0015 *** | 0.0043 * | 5.94 ns | 54.5 ** |
| Cv x CS | 1 | 0.409 * | 0.00024 ns | 0.00054 ns | 0.00002 ns | 2.66 ns | 34.41 * |
| Cv x FS | 2 | 0.044 ns | 0.0005.5 ns | 0.000009 ns | 0.00003 ns | 1.05 ns | 1.02 ns |
| CS x FS | 2 | 0.014 ns | 0.0006 ns | 0.00002 ns | 0.00002 ns | 1.48 ns | 0.955 ns |
| Cv x CS x FS | 2 | 0.036 ns | 0.0002.6 ns | 0.0002 ns | 0.000002 ns | 0.564 ns | 2.62 ns |
| Error | 24 | 0.0001 | 0.00002 | 1.82 | 7.683 | ||
| Chl. a | Chl. b | Chl. a/b | Total Chl. | Carotenoids | Proline | ||
| Cultivars (Cv) | 1 | 0.019 ns | 0.0003 ns | 0.174 ns | 0.014 ns | 0.0001 ns | 79.59 ns |
| Chilling Stress (CS) | 1 | 0.232 ** | 0.104 *** | 0.837 * | 0.648 *** | 0.0876 *** | 1070.26 *** |
| Foliar Spray (FS) | 2 | 0.184 ** | 0.045 *** | 0.308 ns | 0.407 *** | 0.0245 ** | 244.5 ** |
| Cv x CS | 1 | 0.169 * | 0.0097 ns | 0.193 ns | 0.26 ** | 0.001 ns | 5.93 ns |
| Cv x FS | 2 | 0.0352 ns | 0.0093 ns | 1.107 ** | 0.0088 ns | 0.00005 ns | 15.75 ns |
| CS x FS | 2 | 0.0269 ns | 0.0045 ns | 0.0308 ns | 0.052 ns | 0.0039 ns | 40.36 ns |
| Cv x CS x FS | 2 | 0.0214 ns | 0.00563 ns | 0.52 ns | 0.0165 ns | 0.000065 ns | 4.64 ns |
| Error | 24 | 0.0269 | 0.0029 | 0.185 | 0.9332 | 0.0033 | 35.04 |
| Glycine Betaine | Ascorbic Acid | Hydrogen Peroxide | MDA | Catalase | Peroxidase | ||
| Cultivars (Cv) | 1 | 6553.30 *** | 4.54 ns | 1463.05 ns | 2.9221e-4 ns | 1.24 *** | 0.0035 ns |
| Chilling Stress (CS) | 1 | 288.79 ns | 80.14 *** | 20928.4 ** | 116.7 *** | 0.013 ns | 0.030 * |
| Foliar Spray (FS) | 2 | 259.63 ns | 50.41 *** | 13452.4 ** | 24.30 *** | 0.059 * | 0.0117 ns |
| Cv x CS | 1 | 2106.43 * | 0.978 ns | 28.44 ns | 18.48 * | 0.182 ns | 0.0087 ns |
| Cv x FS | 2 | 175.61 ns | 3.16 ns | 2696.2 ns | 1.54 ns | 0.0033 ns | 0.0023 ns |
| CS x FS | 2 | 648.77 ns | 18.22 ns | 1711.5 ns | 0.449 ns | 0.0124 ns | 0.0001 ns |
| Cv x CS x FS | 2 | 710.5 ns | 1.37 ns | 464.9 ns | 2.12 ns | 0.0041 ns | 0.0009 ns |
| Error | 24 | 274.01 | 2.414 | 2299.2 | 2.45 | 0.0155 | 0.0042 |
| SOD | Total Soluble Sugars | Total Phenolics | Total Free Amino Acids | ||||
| Cultivars (Cv) | 1 | 1.104 ns | 62.93 * | 39.27 ns | 0.0262 ** | ||
| Chilling Stress (CS) | 1 | 7.02 * | 228.01 *** | 686.44 *** | 0.0162 * | ||
| Foliar Spray (FS) | 2 | 8.909 * | 114.3 *** | 122.06 ** | 0.0078 * | ||
| Cv x CS | 1 | 4.867 ns | 0.694 ns | 78.91 ns | 0.0008 ns | ||
| Cv x FS | 2 | 2.111 ns | 17.3 ns | 15.57 ns | 0.0001 ns | ||
| CS x FS | 2 | 2.91 ns | 18.73 ns | 34.22 ns | 0.0006 ns | ||
| Cv x CS x FS | 2 | 3.76 ns | 54.72 ns | 42.4 ns | 0.0012 ns | ||
| Error | 24 | 1.642 | 11.51 | 18.57 | 0.002 |
| Source of Variation | df | Shoot Fresh Weight Shoot Fresh Weight | Shoot Dry Weight | Root Fresh Weight | Root Dry Weight | Shoot Length | Root Length |
|---|---|---|---|---|---|---|---|
| Cultivars (Cv) | 1 | 0.558 * | 0.016 *** | 0.0027 ** | 0.000006 ns | 19.65 *** | 6.33 ns |
| Chilling Stress (CS) | 1 | 3.091 *** | 0.0113 ** | 0.0047 *** | 0.00001 * | 2.89 ns | 107.8 *** |
| Presowing (Pre) | 2 | 1.216 *** | 0.0052 * | 0.0038 *** | 0.00005 ns | 10.11 ns | 69.21 *** |
| Cv x CS | 1 | 0.098 ns | 0.004 ns | 0.00005 ns | 0.0000009 ns | 0.027 ns | 26.18 * |
| Cv x Pre | 2 | 0.32 * | 0.0008 ns | 0.00006 ns | 0.000000015 ns | 1.66 ns | 8.07 ns |
| CS x Pre | 2 | 0.0261 ns | 0.00005 ns | 0.000008 ns | 0.000001 ns | 0.663 ns | 2.09 ns |
| Cv x CS x Pre | 2 | 0.0305 ns | 0.0005 ns | 0.00002 ns | 0.0000007 ns | 0.231 ns | 15.93 ns |
| Error | 24 | 0.0834 | 0.00113 | 0.000002 | 0.00001 | 1.394 | 4.87 |
| Chl. a | Chl. b | Chl. a/b | Total Chl. | Carotenoids | Proline | ||
| Cultivars (Cv) | 1 | 1.23 *** | 0.777 *** | 34.58 *** | 0.0532 ns | 0.015 * | 445.49 * |
| Chilling Stress (CS) | 1 | 0.703 *** | 0.951 *** | 5.49 * | 3.29 *** | 0.0006 ns | 611.02 ** |
| Presowing (Pre) | 2 | 0.273 ** | 0.223 *** | 1.80 ns | 0.933 *** | 0.011 ** | 909.4 *** |
| Cv x CS | 1 | 0.559 *** | 0.462 *** | 0.497 ns | 2.04 *** | 0.0054 ns | 5.33 ns |
| Cv x Pre | 2 | 0.159 * | 0.052 * | 2.83 * | 0.039 ns | 0.00001 ns | 3.88 ns |
| CS x Pre | 2 | 0.044 ns | 0.060 * | 0.136 ns | 0.205 ** | 0.000008 ns | 120.9 ns |
| Cv x CS x Pre | 2 | 0.069 ns | 0.0809 ** | 2.46 ns | 0.0035 ns | 0.0009 ns | 262.5 * |
| Error | 24 | 0.0342 | 0.0131 | 0.772 | 0.024 | 0.002 | 72.99 |
| Glycine Betaine | Ascorbic Acid | Hydrogen Peroxide | MDA | Catalase | Peroxidase | ||
| Cultivars (Cv) | 1 | 633.52 ** | 28.13 ** | 1158762.5 *** | 26.82 ns | 0.142 ** | 0.00002 ns |
| Chilling Stress (CS) | 1 | 1386.6 *** | 19.09 * | 317579.2 ** | 266.2 *** | 0.049 ns | 0.199 *** |
| Presowing (Pre) | 2 | 414.3 ** | 49.14 *** | 67016.8 ns | 34.64 ns | 0.050 ns | 0.0302 ** |
| Cv x CS | 1 | 424.9 * | 1.98 ns | 21085.4 ns | 3.5 ns | 0.019 ns | 0.07 ** |
| Cv x Pre | 2 | 6.90 ns | 1.392 ns | 10304.3 ns | 19.68 ns | 0.0015 ns | 0.00001 ns |
| CS x Pre | 2 | 0.648 ns | 15.92 * | 75875.6 ns | 8.54 ns | 0.0047 ns | 0.0104 ns |
| Cv x CS x Pre | 2 | 27.11 ns | 12.34 * | 7958.5 ns | 0.75 ns | 0.0079 ns | 0.00008 ns |
| Error | 24 | 58.01 | 3.04 | 28230.2 | 10.89 | 0.0152 | 0.00008 |
| SOD | Total Soluble Sugars | Total Phenolics | Total Free Amino Acids | ||||
| Cultivars (Cv) | 1 | 22.35 *** | 244.92 * | 570.6 *** | 0.003 ns | ||
| Chilling Stress (CS) | 1 | 18.34 ** | 121.4 ns | 1348.4 *** | 0.022 ** | ||
| Presowing (Pre) | 2 | 2.14 ns | 72.27 ns | 41.28 ns | 0.005 ns | ||
| Cv x CS | 1 | 0.292 ns | 6.33 ns | 0.307 ns | 0.0037 ns | ||
| Cv x Pre | 2 | 0.782 ns | 29.98 ns | 1.195 ns | 0.00001 ns | ||
| CS x Pre | 2 | 0.682 ns | 93.94 ns | 12.003 ns | 0.0002 ns | ||
| Cv x CS x Pre | 2 | 0.206 ns | 22.75 ns | 0.284 ns | 0.00008 ns | ||
| Error | 24 | 1.56 | 36.22 | 18.87 | 0.0019 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaqoob, H.; Akram, N.A.; Iftikhar, S.; Ashraf, M.; Khalid, N.; Sadiq, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Seed Pretreatment and Foliar Application of Proline Regulate Morphological, Physio-Biochemical Processes and Activity of Antioxidant Enzymes in Plants of Two Cultivars of Quinoa (Chenopodium quinoa Willd.). Plants 2019, 8, 588. https://doi.org/10.3390/plants8120588
Yaqoob H, Akram NA, Iftikhar S, Ashraf M, Khalid N, Sadiq M, Alyemeni MN, Wijaya L, Ahmad P. Seed Pretreatment and Foliar Application of Proline Regulate Morphological, Physio-Biochemical Processes and Activity of Antioxidant Enzymes in Plants of Two Cultivars of Quinoa (Chenopodium quinoa Willd.). Plants. 2019; 8(12):588. https://doi.org/10.3390/plants8120588
Chicago/Turabian StyleYaqoob, Hira, Nudrat A. Akram, Samrah Iftikhar, Muhammad Ashraf, Noman Khalid, Muhammad Sadiq, Mohammed Nasser Alyemeni, Leonard Wijaya, and Parvaiz Ahmad. 2019. "Seed Pretreatment and Foliar Application of Proline Regulate Morphological, Physio-Biochemical Processes and Activity of Antioxidant Enzymes in Plants of Two Cultivars of Quinoa (Chenopodium quinoa Willd.)" Plants 8, no. 12: 588. https://doi.org/10.3390/plants8120588
APA StyleYaqoob, H., Akram, N. A., Iftikhar, S., Ashraf, M., Khalid, N., Sadiq, M., Alyemeni, M. N., Wijaya, L., & Ahmad, P. (2019). Seed Pretreatment and Foliar Application of Proline Regulate Morphological, Physio-Biochemical Processes and Activity of Antioxidant Enzymes in Plants of Two Cultivars of Quinoa (Chenopodium quinoa Willd.). Plants, 8(12), 588. https://doi.org/10.3390/plants8120588







