The Induction and Roles Played by Phi Thickenings in Orchid Roots
Abstract
1. Introduction
2. Results
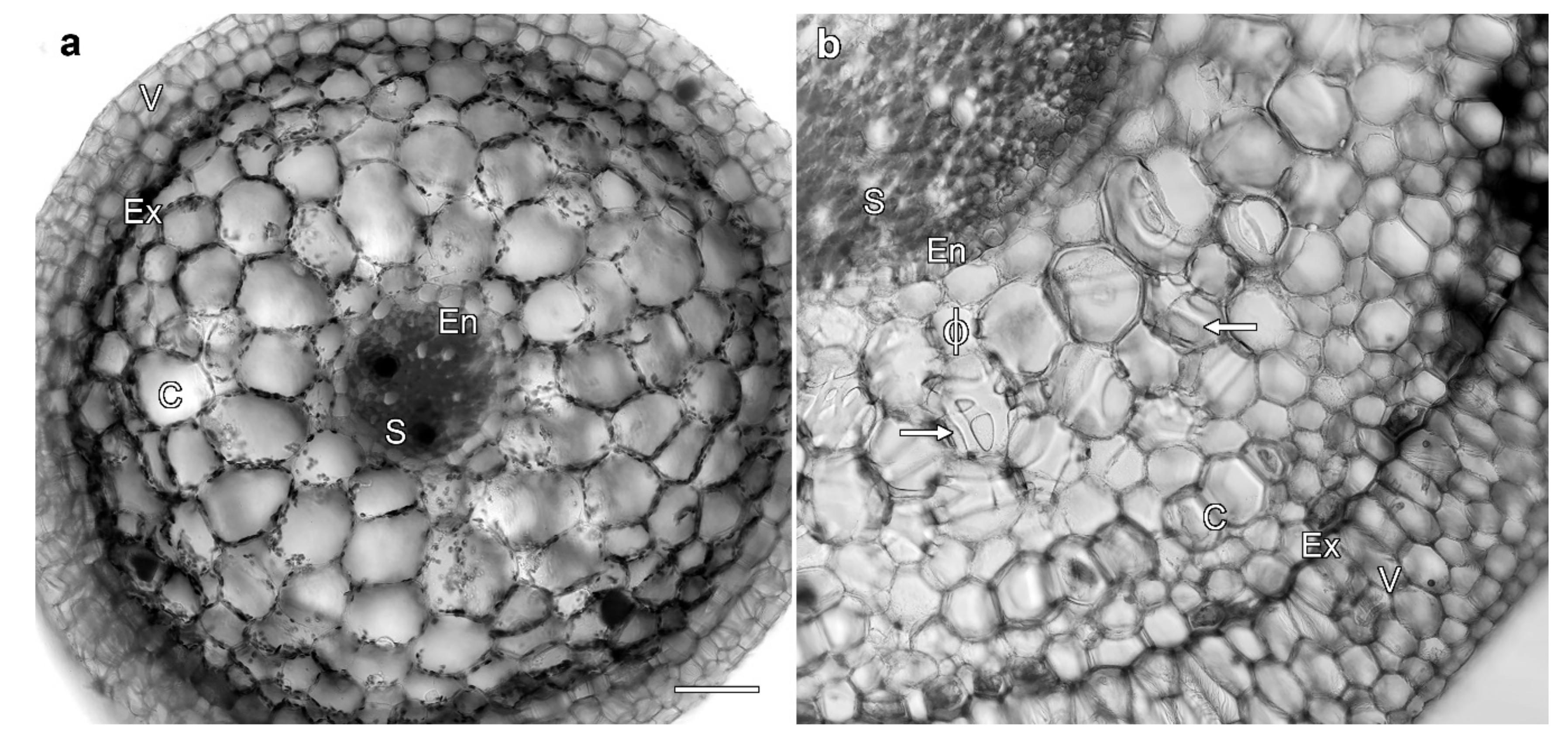
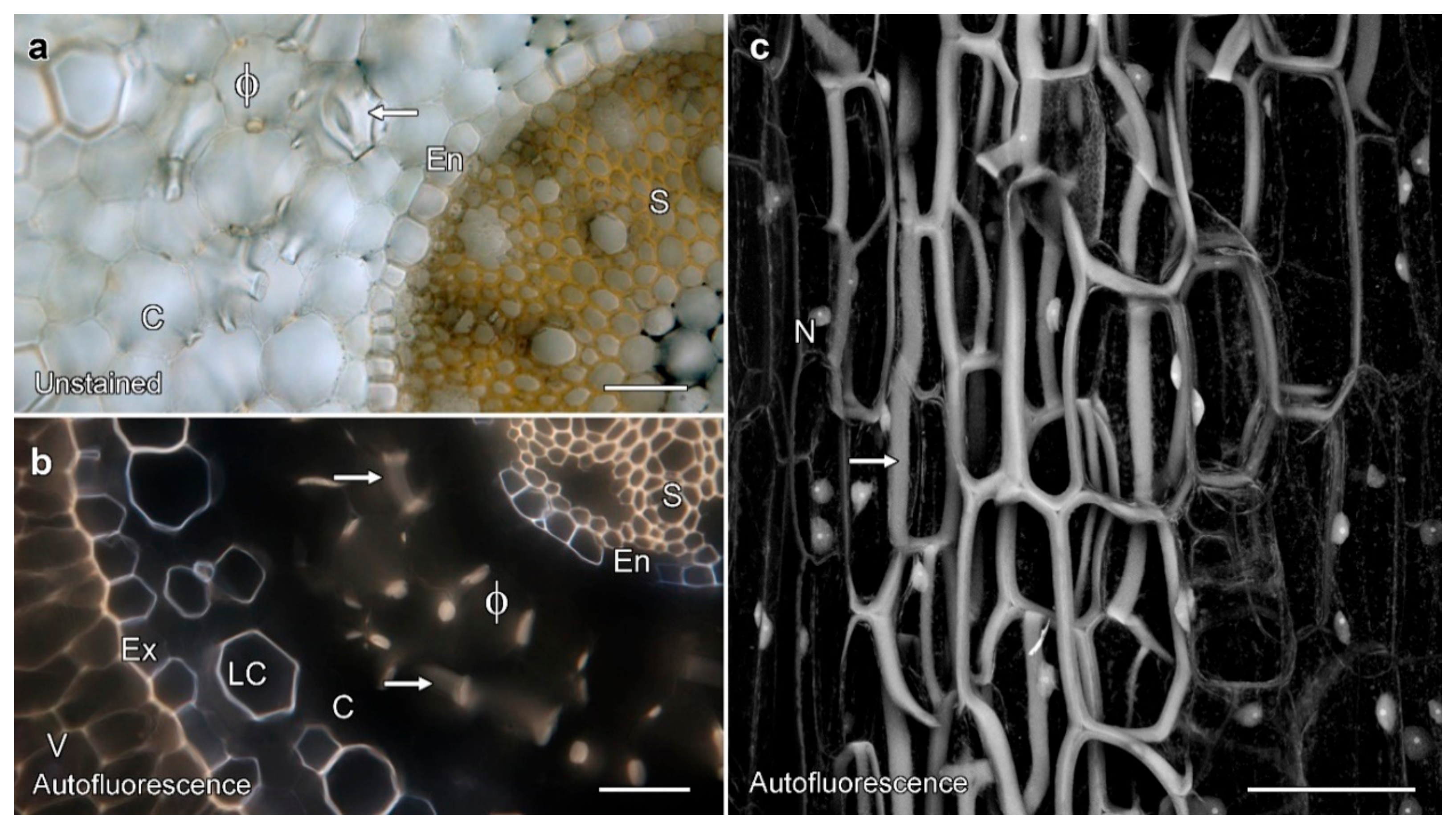
2.1. Miltoniopsis Orchid Roots Can Contain Phi Thickenings
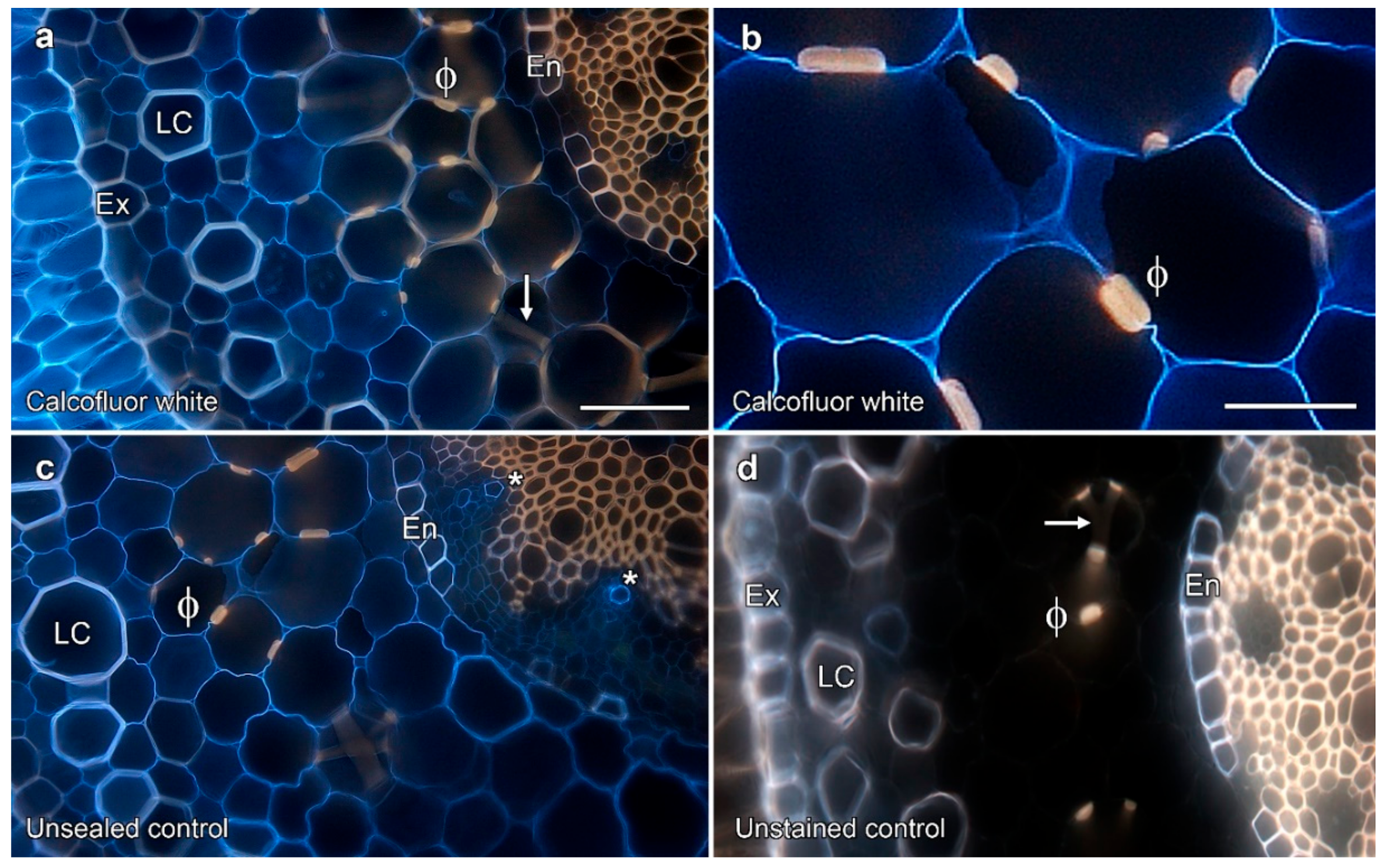
2.2. Permeability Tests on Phi Thickenings
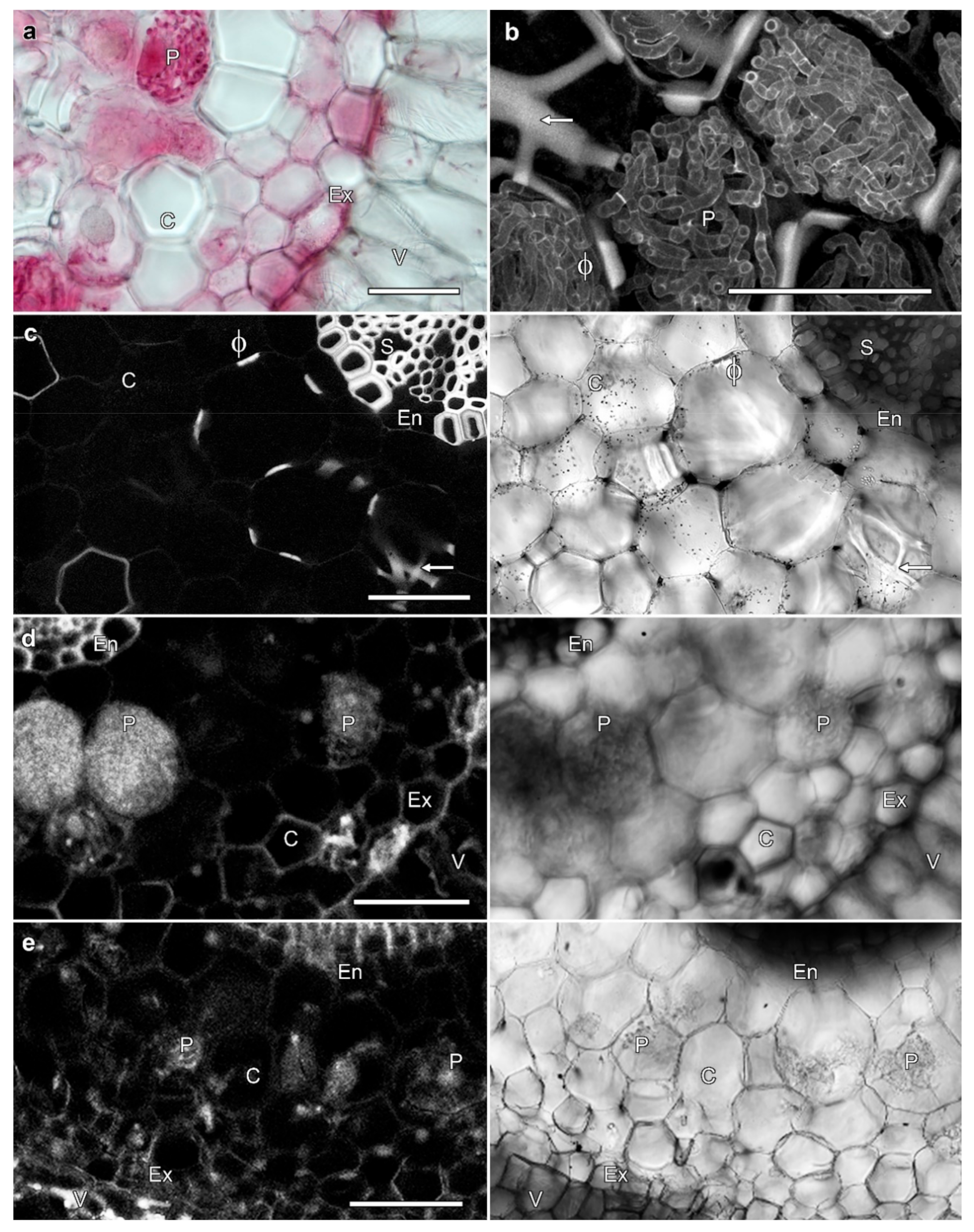
2.3. Fungal Infections Are Not Required to Induce Phi Thickenings
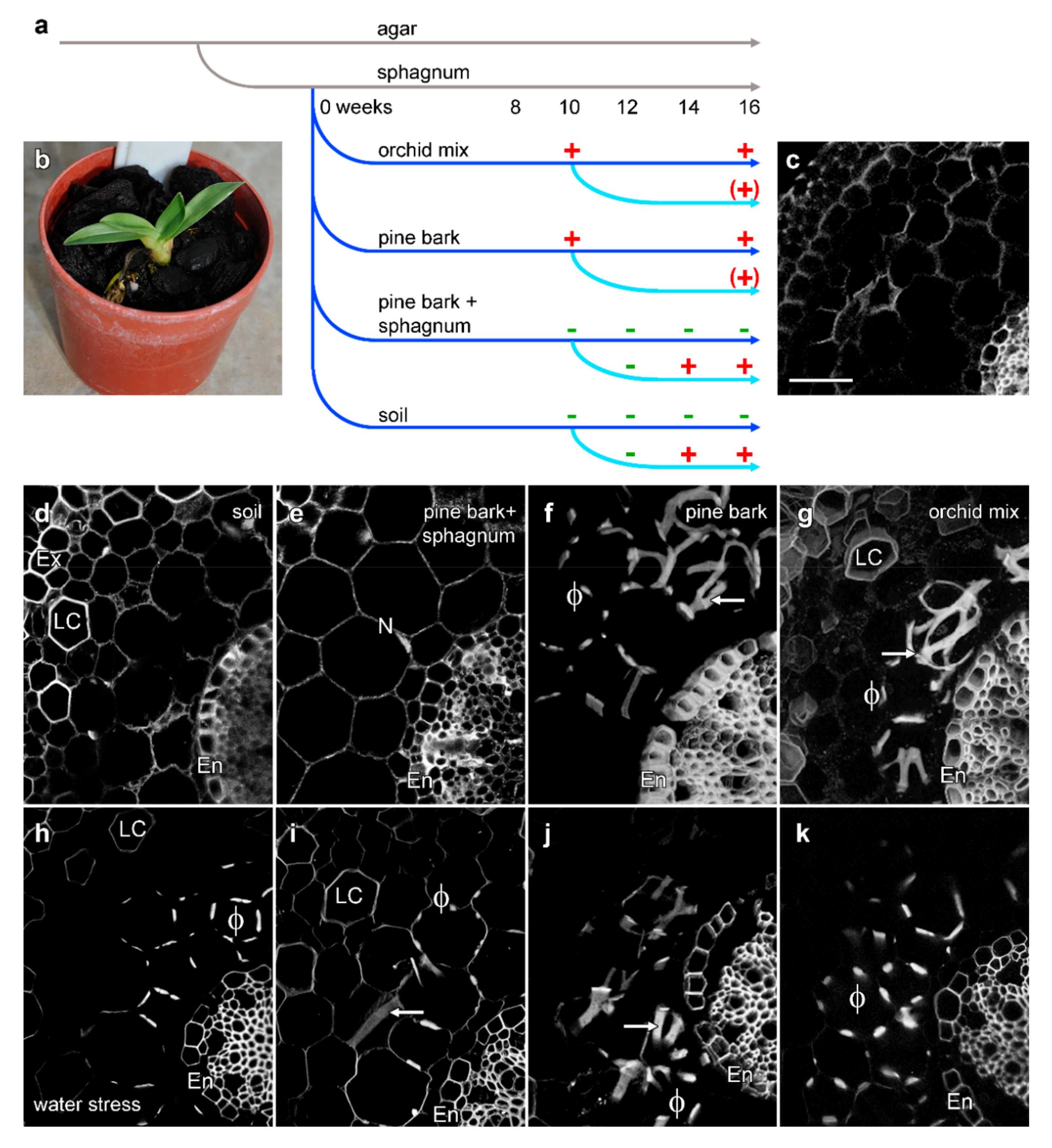
2.4. Induction of Phi Thickenings
- Commercial orchid mix, made of big chunks of fir bark with finer pieces. This provided the least compaction and lowest water retention.
- Medium-sized pieces of fir bark.
- Medium-sized fir bark with pieces of sphagnum moss.
- Soil that consisted of fine fir bark that passed through a 6 mm diameter soil sieve. This mix provided the most compaction and best water retention.
3. Discussion
3.1. Orchid Phi Thickenings Do Not Regulate Solute Uptake
3.2. Orchid Phi Thickenings Do Not Regulate Fungal Penetration
3.3. Are Phi Thickenings a Response to Environmental Stress?
4. Materials and Methods
4.1. Plant Material
4.2. Fungal Reinfection Experiments
4.3. Microscopy
4.4. Permeability of Phi Thickenings
4.5. Phi Thickening Induction Experiments
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aleamotu’a, M.; McCurdy, D.W.; Collings, D.A. Phi thickenings in roots—Novel secondary wall structures responsive to biotic and abiotic stresses. J. Exp. Bot. 2019, 70, 4631–4641. [Google Scholar] [CrossRef]
- Van Tieghem, P.E.L. Mémoire sur la racine. Ann. Sci. Nat. Bot. Ser. 5 1871, 13, 187–195. [Google Scholar]
- De Melo, H.C. Espessamento em fi de parede celular. Hoehnea 2011, 3, 1–7. [Google Scholar] [CrossRef]
- Fernandez-Garcia, N.; Lopez-Berenguer, C.; Olmos, E. Role of phi cells under abiotic stress in plants. In Root Engineering: Basic and Applied Concepts; Morte, A., Varma, A., Eds.; Springer: Berlin, Germany, 2014; pp. 23–38. [Google Scholar]
- Wilcox, H.E.; Wang, C.J.K. Mycorrhizal and pathological associations of dematiaceous fungi in roots of 7-month-old tree seedlings. Can. J. For. Res. 1987, 17, 884–899. [Google Scholar] [CrossRef]
- Melville, L.H.; Massicotte, H.B.; Peterson, R.L. Ontogeny of early stages of ectomycorrhizae synthesized between Dryas integrifolia and Hebeloma cylindrosporum. Bot. Gaz. 1987, 148, 332–341. [Google Scholar] [CrossRef]
- Van Fleet, D.S. A comparison of histochemical and anatomical characteristics of the hypodermis with the endodermis in vascular plants. Am. J. Bot. 1950, 37, 721–725. [Google Scholar] [CrossRef]
- Mackenzie, K.A.D. The development of the endodermis and phi layer of apple roots. Protoplasma 1979, 100, 21–32. [Google Scholar] [CrossRef]
- Haas, D.L.; Carothers, Z.B.; Robbins, R.R. Observations on the phi-thickenings and Casparian strips in Pelargonium roots. Am. J. Bot. 1974, 63, 863–867. [Google Scholar] [CrossRef]
- Peterson, C.A.; Emanuel, M.E.; Weerdenburg, C.A. The permeability of phi thickenings in apple (Pyrus malus) and geranium (Pelargonium hortorum) roots to an apoplastic fluorescent dye tracer. Can. J. Bot. 1981, 59, 1107–1110. [Google Scholar] [CrossRef]
- Degenhardt, B.; Gimmler, H. Cell wall adaptations to multiple environmental stresses in maize roots. J. Exp. Bot. 2000, 51, 595–603. [Google Scholar] [CrossRef]
- López-Pérez, L.; Fernandez-Garcia, N.; Olmos, E.; Carvajal, M. The phi thickening in roots of broccoli plants: An acclimation mechanism to salinity? Int. J. Plant Sci. 2007, 168, 1141–1149. [Google Scholar] [CrossRef]
- Van Tieghem, P.E.L. Sur le réseau de soutien de l’ecorce de la racine. Ann. Sci. Nat. Bot. Ser. 7 1888, 8, 375–378. [Google Scholar]
- Soukup, A.; Malá, J.; Hrubcová, M.; Kálal, J.; Votrubová, O.; Cviková, M. Differences in anatomical structure and lignin content of roots of pedunculate oak and wild cherry-tree plantlets during acclimation. Biol. Plant 2004, 48, 481–489. [Google Scholar] [CrossRef]
- Marin, J.A.; Arbeloa, A.; Castillo, M.; Andreu, P. Root acclimatization of the micropropagated fruit tree rootstock ‘Adafuel’ (Prunus Dulcis (Mill.) D.A. Webb x P. persica (L.) Batsch). In International Symposium on Acclimatization and Establishment of Micropropagated Plants; Romano, A., Ed.; International Society for Horticultural Science: Leuven, The Netherlands, 2008; pp. 403–408. [Google Scholar]
- Fernandez-Garcia, N.; Lopez-Perez, L.; Hernandez, M.; Olmos, E. Role of Phi cells and the endodermis under salt stress in Brassica oleracea. New Phytol. 2009, 181, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Aleamotu’a, M.; Tai, Y.-T.; McCurdy, D.W.; Collings, D.A. Developmental biology and induction of phi thickenings by abiotic stress in roots of the Brassicaceae. Plants 2018, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Scialabba, A.; Melati, M.R. The effect of NaCl on growth and xylem differentiation of radish seedlings. Bot. Gaz. 1990, 151, 516–521. [Google Scholar] [CrossRef]
- Pan, C.Z.; Nakao, Y.; Nii, N. Anatomical development of phi thickening and the Casparian strip in loquat roots. J. Jpn. Soc. Hortic. Sci. 2006, 75, 445–449. [Google Scholar] [CrossRef]
- Gerrath, J.M.; Matthes, U.; Purich, M.; Larson, D.W. Root environmental effects on phi thickening production and root morphology in three gymnosperms. Can. J. Bot. 2005, 83, 379–385. [Google Scholar] [CrossRef]
- Idris, N.A.; Collings, D.A. The life of phi: The development of phi thickenings in roots of the orchids of the genus Miltoniopsis. Planta 2015, 241, 489–506. [Google Scholar] [CrossRef]
- Moreira, A.S.; Filho, J.P.; dos Santos Isaias, R.M. Structural adaptations of two sympatric epiphytic orchids (Orchidaceae) to a cloudy forest environment in rocky outcrops of Southeast Brazil. Rev. Biol. Trop. 2013, 61, 1053–1065. [Google Scholar]
- Burr, B.; Barthlott, W. On a velamen-like tissue in the root cortex of orchids. Flora 1991, 185, 313–323. [Google Scholar] [CrossRef]
- Olatunji, O.A.; Nengim, R.O. Occurrence and distribution of tracheoidal elements in the Orchidaceae. Bot. J. Linn. Soc. 1980, 80, 357–370. [Google Scholar] [CrossRef]
- Idris, N.A.; Collings, D.A. Cell wall development in the velamen layer of the orchid Miltoniopsis investigated by confocal microscopy. Malays. J. Micro 2014, 10, 20–26. [Google Scholar]
- Weerdenburg, C.A.; Peterson, C.A. Structural changes in phi thickenings during primary and secondary growth in roots. 1. Apple (Pyrus malus) Rosaceae. Can. J. Bot. 1983, 61, 2570–2576. [Google Scholar] [CrossRef]
- Geldner, N. The endodermis. Ann. Rev. Plant Biol. 2013, 64, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Doblas, V.G.; Geldner, N.; Barberon, M. The endodermis, a tightly controlled barrier for nutrients. Curr. Opin. Plant Biol. 2017, 39, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Naseer, S.; Lee, Y.; Lapierre, C.; Franke, R.; Nawrath, C.; Geldner, N. Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proc. Natl. Acad. Sci. USA 2012, 109, 10101–10106. [Google Scholar] [CrossRef]
- Barberon, M.; Vermeer, J.E.M.; de Bellis, D.; Wang, P.; Naseer, S.; Andersen, T.G.; Humbel, B.M.; Nawrath, C.; Takano, J.; Salt, D.E.; et al. Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 2012, 164, 447–459. [Google Scholar] [CrossRef]
- van der Mortel, J.E.; Villaneuva, L.A.; Schat, H.; Kwekkeboom, J.; Coughlan, S.; Moerland, P.D.; van Themaat, E.V.L.; Koorneef, M.; Aarts, M.G.M. Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiol. 2006, 142, 1127–1147. [Google Scholar] [CrossRef]
- Zelko, I.; Lux, A.; Czibula, K. Difference in the root structure of hyperaccumulator Thlaspi caerulescens and non-hyperaccumulator Thlaspi arvense. Int. J. Environ. Pollut. 2008, 33, 123–132. [Google Scholar] [CrossRef]
- Massicotte, H.B.; Melville, L.H.; Peterson, R.L.; Unestam, T. Comparative studies of ectomycorrhiza formation in Alnus glutinosa and Pinus resinosa with Paxillus involutus. Mycorrhiza 1999, 8, 229–240. [Google Scholar] [CrossRef]
- Pratikakis, E.; Rhizopoulou, S.; Psaras, G.K. A phi layer in roots of Ceratonia siliqua L. Bot. Acta 1998, 111, 93–98. [Google Scholar] [CrossRef]
- Miyazaki, J.; Tan, B.H.; Errington, S.G. Eradication of endophytic bacteria via treatment for axillary buds of Petunia hybrida using Plant Preservative Mixture (PPMTM). Plant Cell Tissue Organ Cult. 2010, 102, 365–372. [Google Scholar] [CrossRef]
- Currah, R.; Zelmer, C.; Hambleton, S.; Richardson, K. Fungi from orchid mycorrhiza. In Orchid Biology; Arditti, J., Pridgeon, A., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 117–170. [Google Scholar]
- Beardmore, J.; Pegg, G. A technique for the establishment of mycorrhizal infection in orchid tissue grown in aseptic culture. New Phytol. 1981, 87, 527–535. [Google Scholar] [CrossRef]
- Kaminskyj, S.G.W. Effective and flexible methods for visualizing and quantifying endorhizal fungi. In Mycorrhizae: Sustainable Agriculture and Forestry; Siddiqui, Z.A., Akhtar, M.S., Futai, K., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 337–349. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idris, N.A.; Collings, D.A. The Induction and Roles Played by Phi Thickenings in Orchid Roots. Plants 2019, 8, 574. https://doi.org/10.3390/plants8120574
Idris NA, Collings DA. The Induction and Roles Played by Phi Thickenings in Orchid Roots. Plants. 2019; 8(12):574. https://doi.org/10.3390/plants8120574
Chicago/Turabian StyleIdris, Nurul A., and David A. Collings. 2019. "The Induction and Roles Played by Phi Thickenings in Orchid Roots" Plants 8, no. 12: 574. https://doi.org/10.3390/plants8120574
APA StyleIdris, N. A., & Collings, D. A. (2019). The Induction and Roles Played by Phi Thickenings in Orchid Roots. Plants, 8(12), 574. https://doi.org/10.3390/plants8120574





