Optimize, Modulate, and Scale-up Resveratrol and Resveratrol Dimers Bioproduction in Vitis labrusca L. Cell Suspension from Flasks to 20 L Bioreactor
Abstract
1. Introduction
2. Results
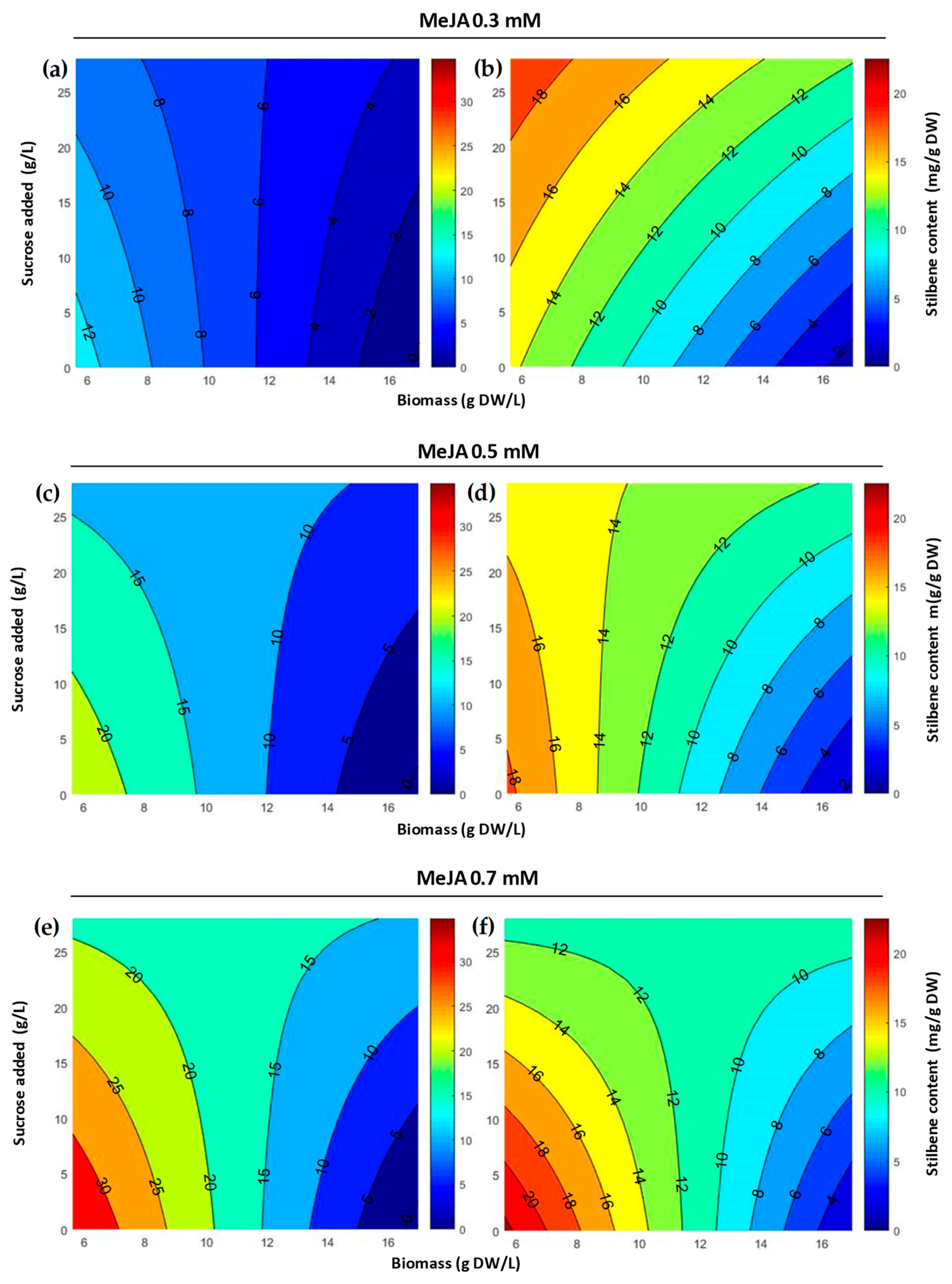
2.1. Optimization of MeJA Elicitation in Flask Experiments
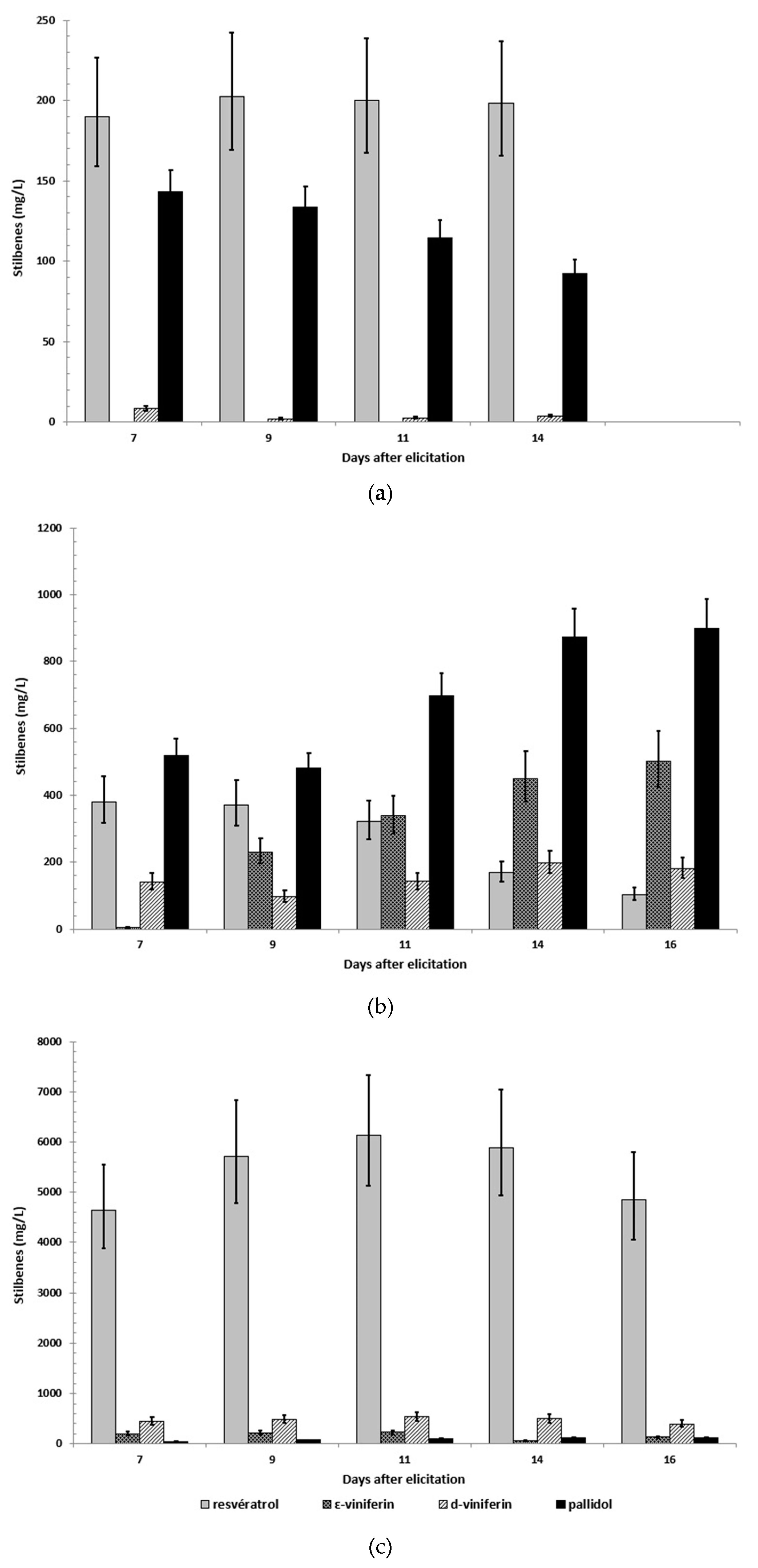
2.2. Production of Stilbenes in 5 L Bioreactor under Optimized MeJA Elicitation
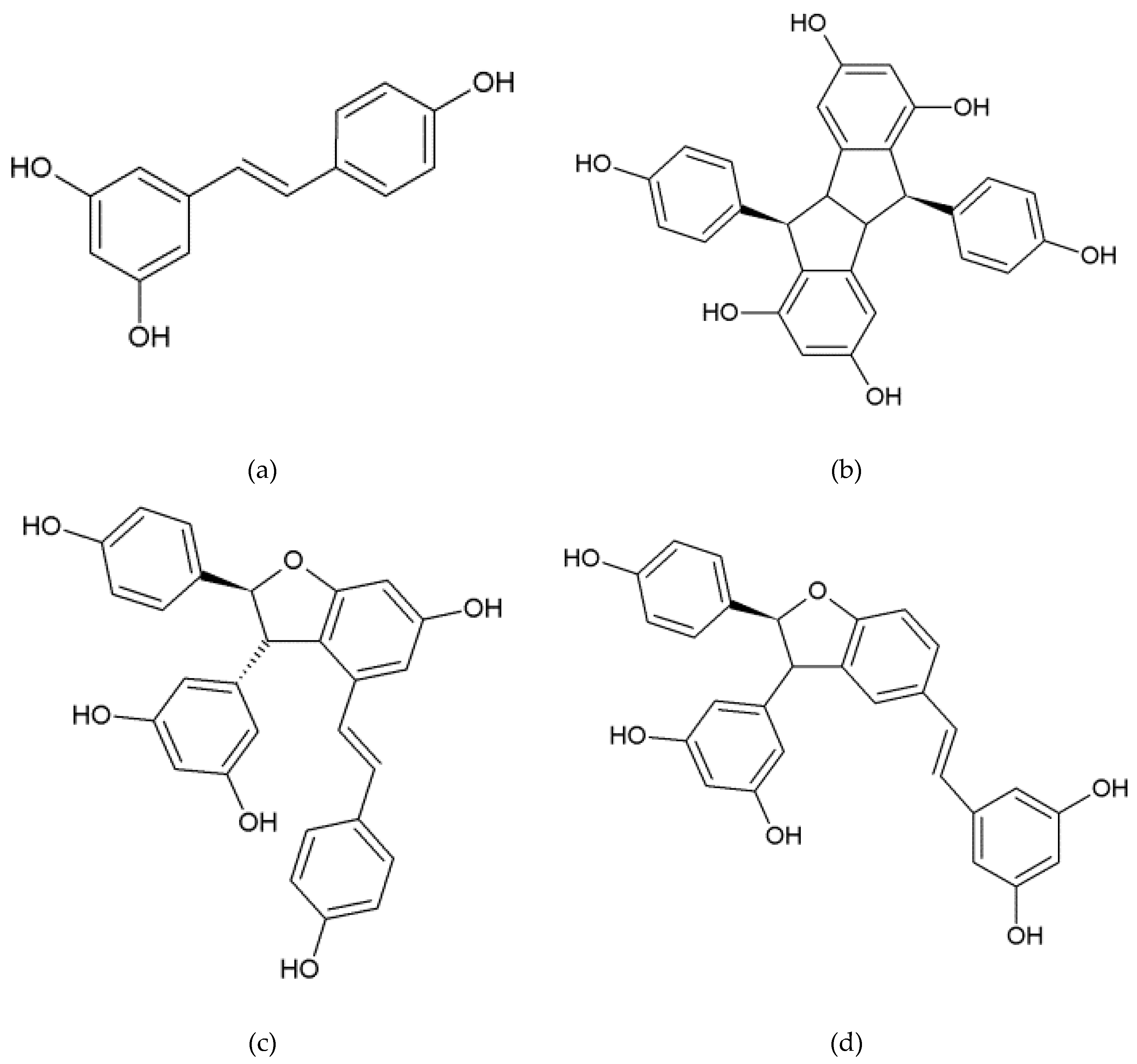
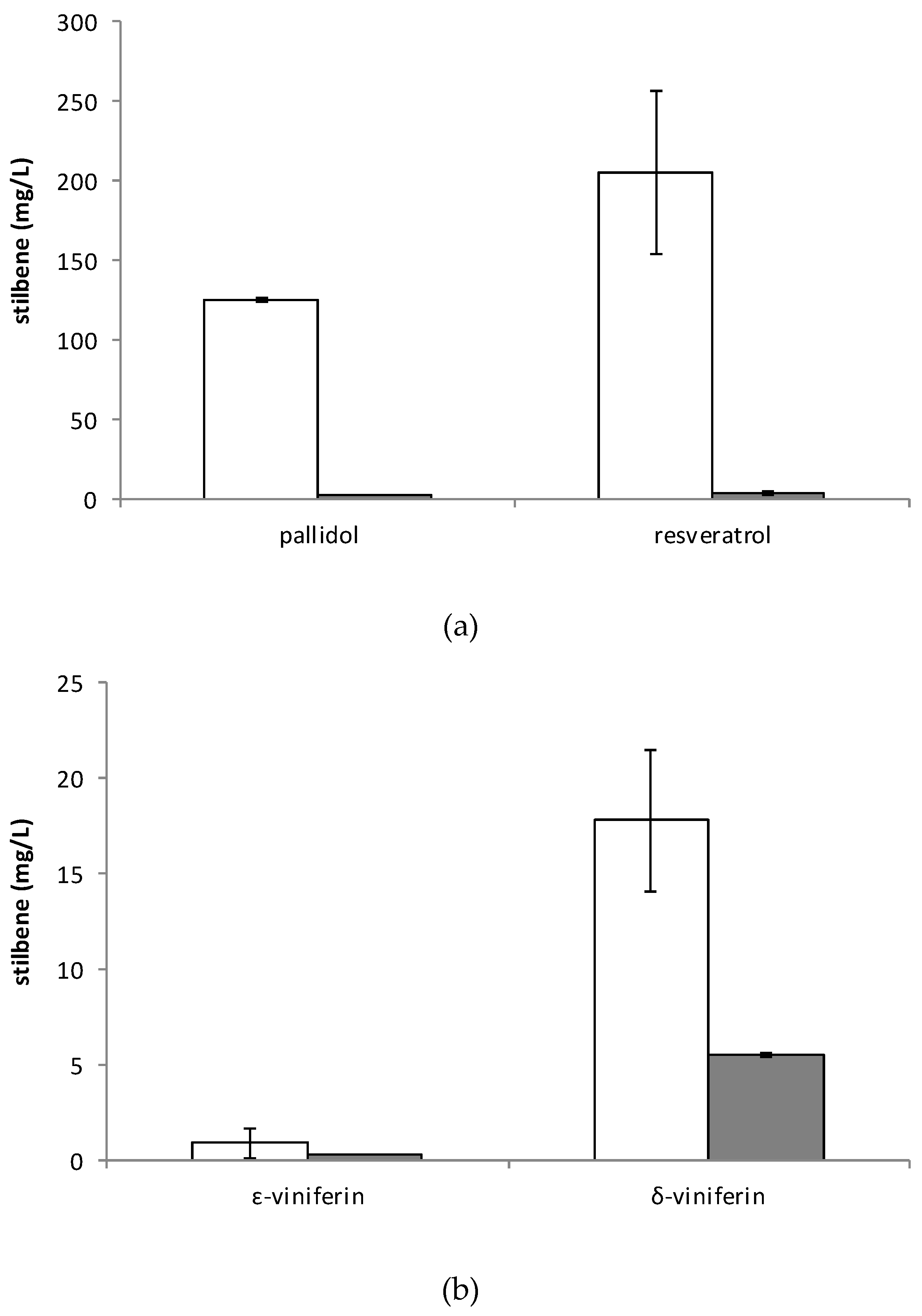
2.3. Stilbene Profile in Cell Suspension in the Presence or Absence of Adsorbent Resin or Methyl Beta-Cyclodextrins and Other Elicitors
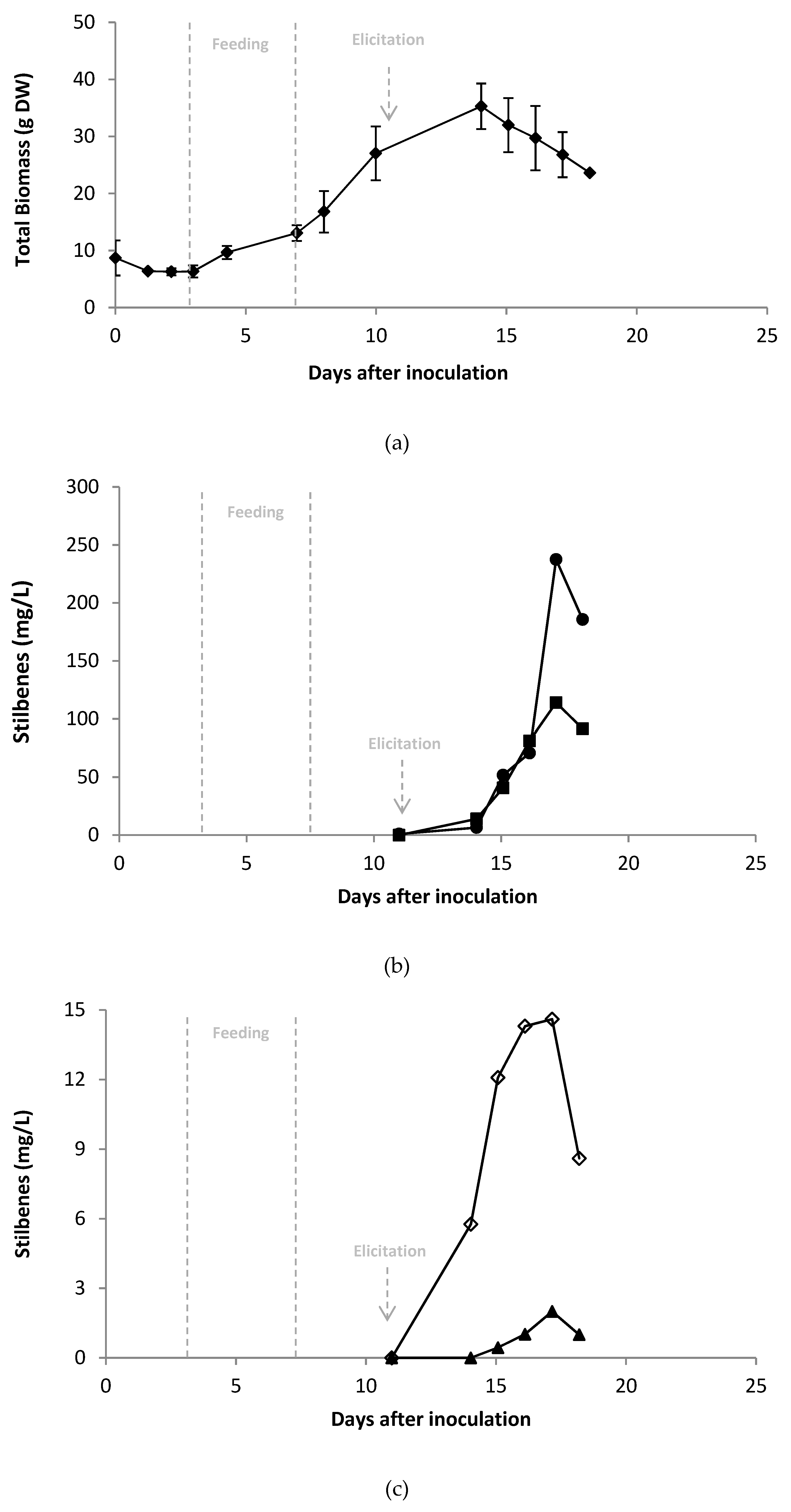
2.4. Scale-up of A Co-Elicitation Methyl-Jasmonate/Methyl-β-Cyclodextrins Culture in 20 L Bioreactor
3. Materials and Methods
3.1. Cell Culture Maintenance
3.2. Statistical Design of Experiment
3.3. Treatment with MeJA Combined with Adsorbent Resin or Methyl-β-Cyclodextrins
3.4. Combined Treatment of Methyl-β-Cyclodextrins and Different Elicitors
3.5. Stirred Bioreactor Cell Culture
3.6. Stilbene Extraction, HPLC Quantification and Identification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pawlus, A.D.; Waffo-Téguo, P.; Shaver, J.; Mérillon, J.-M. Stilbenoid chemistry from wine and the genus Vitis, a review. Oeno One 2012, 46, 57–111. [Google Scholar] [CrossRef]
- Anekonda, T.S. Resveratrol-a boon for treating Alzheimer’s disease? Brain. Res. Rev. 2006, 52, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Nivelle, L.; Hubert, J.; Courot, E.; Jeandet, P.; Aziz, A.; Nuzillard, J.-M.; Renault, J.-H.; Clément, C.; Martiny, L.; Delmas, D.; et al. Anti-cancer activity of resveratrol and derivatives produced by grapevine cell suspensions in a 14 L stirred bioreactor. Molecules 2017, 22, 474. [Google Scholar] [CrossRef]
- Nivelle, L.; Hubert, J.; Courot, E.; Borie, N.; Renault, J.H.; Nuzillard, J.M.; Harakat, D.; Clément, C.; Martiny, L.; Delmas, D.; et al. Characterization of labruscol, a new resveratrol dimer produced by grapevine cell suspensions on Human Skin Melanoma Cancer Cell Line HT-144. Molecules 2017, 22, 1940. [Google Scholar] [CrossRef]
- He, S.; Yan, X. From resveratrol to its derivatives: New sources of natural antioxidant. Curr. Med. Chem. 2013, 20, 1005–1017. [Google Scholar] [CrossRef]
- Ohyama, M.; Tanaka, T.; Ito, T.; Iinuma, M.; Bastow, K.F.; Lee, K.-H. Antitumor agents 200. Cytotoxicity of naturally occurring resveratrol oligomers and their acetate derivatives. Bioorg. Med. Chem. Lett. 1999, 9, 3057–3060. [Google Scholar] [CrossRef]
- Yim, N.; Ha, D.T.; Trung, T.N.; Kim, J.P.; Lee, S.; Na, M.; Jung, H.; Kim, H.S.; Kim, Y.H.; Bae, K. The antimicrobial activity of compounds from the leaf and stem of Vitis amurensis against two oral pathogens. Bioorg. Med. Chem. Lett. 2010, 20, 1165–1168. [Google Scholar] [CrossRef]
- Colin, D.; Lancon, A.; Delmas, D.; Lizard, G.; Abrossinow, J.; Kahn, E.; Jannin, B.; Latruffe, N. Antiproliferative activities of resveratrol and related compounds in human hepatocyte derived HepG2 cells are associated with biochemical cell disturbance revealed by fluorescence analyses. Biochimie 2008, 90, 1674–1684. [Google Scholar] [CrossRef]
- Kim, H.J.; Chang, E.J.; Bae, S.J.; Shim, S.M.; Park, H.D.; Rhee, C.H.; Park, J.H.; Choi, S.W. Cytotoxic and antimutagenic stilbenes from seeds of Paeonia lactiflora. Arch. Pharm. Res. 2002, 25, 293–299. [Google Scholar] [CrossRef]
- Richard, T.; Poupard, P.; Nassra, M.; Papastamoulis, Y.; Iglésias, M.-L.; Krisa, S.; Waffo-Teguo, P.; Mérillon, J.-M.; Monti, J.-P. Protective effect of ε-viniferin on β-amyloid peptide aggregation investigated by electrospray ionization mass spectrometry. Bioorg. Med. Chem. 2011, 19, 3152–3155. [Google Scholar] [CrossRef] [PubMed]
- Zghonda, N.; Yoshida, S.; Ezaki, S.; Otake, Y.; Murakami, C.; Mliki, A.; Ghorbel, A.; Miyazaki, H. ε-Viniferin is more effective than its monomer resveratrol in improving the functions of vascular endothelial cells and the heart. Biosci. Biotechnol. Biochem. 2012, 76, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Donnez, D.; Jeandet, P.; Clément, C.; Courot, E. Bioproduction of resveratrol and stilbene derivatives by plant cells and microorganisms. Trends Biotechnol. 2009, 27, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Richard, T.; Renouf, E.; Bisson, J.; Waffo-Téguo, P.; Bordenave, L.; Ollat, N.; Mérillon, J.-M.; Cluzet, S. Comparative analyses of stilbenoids in canes of major Vitis vinifera L. cultivars. J. Agric. Food Chem. 2013, 61, 11392–11399. [Google Scholar] [CrossRef]
- Pawlus, A.D.; Sahli, R.; Bisson, J.; Rivière, C.; Delaunay, J.-C.; Richard, T.; Gomès, E.; Bordenave, L.; Waffo-Téguo, P.; Mérillon, J.-M. Stilbenoid profiles of canes from Vitis and Muscadinia species. J. Agric. Food Chem. 2013, 61, 501–511. [Google Scholar] [CrossRef]
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Huhn, T.; Eibl, D. Plant cell culture technology in the cosmetics and food industries current state and future trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675. [Google Scholar] [CrossRef]
- Melchior, F.; Hohmann, F.; Schwer, B.; Kindl, H. Induction of stilbene synthase by Botrytis cinerea in cultured grapevine cells. Planta 1991, 183, 307–314. [Google Scholar] [CrossRef]
- Jeandet, P.; Clément, C.; Courot, E. Resveratrol production at large scale using plant cell suspensions. Eng. Life Sci. 2014, 14, 622–632. [Google Scholar] [CrossRef]
- Donnez, D.; Kim, K.-H.; Antoine, S.; Conreux, A.; De Luca, V.; Jeandet, P.; Clément, C.; Courot, E. Bioproduction of resveratrol and viniferins by an elicited grapevine cell culture in a 2L stirred bioreactor. Process Biochem. 2011, 46, 1056–1062. [Google Scholar] [CrossRef]
- Krisa, S.; Larronde, F.; Budzinski, H.; Decendit, A.; Deffieux, G.; Mérillon, J.-M. Stilbene production by Vitis vinifera cell suspension cultures: Methyl jasmonate induction and 13C Biolabeling. J. Nat. Prod. 1999, 62, 1688–1690. [Google Scholar] [CrossRef]
- Vitrac, X.; Krisa, S.; Decendit, A.; Vercauteren, J.; Nuhrich, A.; Monti, J.-P.; Deffieux, G.; Mérillon, J.-M. Carbon14 biolabelling of wine polyphenols in Vitis vinifera cell suspension cultures. J. Biotechnol. 2002, 95, 49–56. [Google Scholar] [CrossRef]
- Belchí-Navarro, S.; Almagro, L.; Lijavetzky, D.; Bru, R.; Pedreño, M.A. Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and methyljasmonate. Plant Cell Rep. 2012, 31, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Lijavetzky, D.; Almagro, L.; Belchi-Navarro, S.; Martínez-Zapater, J.M.; Bru, R.; Pedreño, M.A. Synergistic effect of methyljasmonate and cyclodextrin on stilbene biosynthesis pathway gene expression and resveratrol production in Monastrell grapevine cell cultures. BMC Res. Notes 2008, 1, 132. [Google Scholar] [CrossRef] [PubMed]
- Belchí-Navarro, S.; Abellan, R.M.; Pedreño, M.A.; Almagro, L. Production and localization of hydrogen peroxide and nitric oxide in grapevine cells elicited with cyclodextrins and methyl jasmonate. J. Plant Physiol. 2019, 237, 80–86. [Google Scholar] [CrossRef]
- Vuong, T.V.; Franco, C.; Zhang, W. Treatment strategies for high resveratrol induction in Vitis vinifera L. cell suspension culture. Biotechnol. Rep. 2014, 1, 15–21. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, W.; Deng, M. Hyper-production of 13C-labeled trans-resveratrol in Vitis vinifera suspension cell culture by elicitation and in situ adsorption. Biochem. Eng. J. 2011, 53, 292–296. [Google Scholar] [CrossRef]
- Lucas-Abellán, C.; Fortea, I.; López-Nicolás, J.M.; Núñez-Delicado, E. Cyclodextrins as resveratrol carrier system. Food Chem. 2007, 104, 39–44. [Google Scholar] [CrossRef]
- Larronde, F.; Richard, T.; Delaunay, J.-C.; Decendit, A.; Monti, J.-P.; Krisa, S.; Mérillon, J.M. New stilbenoid glucosides isolated from Vitis vinifera cell suspension cultures (cv. Cabernet Sauvignon). Planta Med. 2005, 71, 888–890. [Google Scholar] [CrossRef]
- Mérillon, J.M.; Fauconneau, B.; Waffo-Téguo, P.; Barrier, L.; Vercauteren, J.; Huguet, F. Antioxidant activity of the stilbene astringin, newly extracted from Vitis vinifera cell cultures. Clin. Chem. 1997, 43, 1092–1093. [Google Scholar]
- Mulinacci, N.; Innocenti, M.; Santamaria, A.R.; La Marca, G.; Pasqua, G. High-performance liquid chromatography/electrospray ionization tandem mass spectrometric investigation of stilbenoids in cell cultures of Vitis vinifera L., cv. Malvasia. Rapid Commun. Mass Spectrom. 2010, 24, 2065–2073. [Google Scholar] [CrossRef]
- Santamaria, A.R.; Antonacci, D.; Caruso, G.; Cavaliere, C.; Gubbiotti, R.; Laganà, A.; Valletta, A.; Pasqua, G. Stilbene production in cell cultures of Vitis vinifera L. cvs Red Globe and Michele Palieri elicited by methyl jasmonate. Nat. Prod. Res. 2010, 24, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Waffo-Téguo, P.; Hawthorne, M.E.; Cuendet, M.; Mérillon, J.M.; Kinghorn, A.D.; Pezzuto, J.M.; Mehta, R.G. Potential cancer-chemopreventive activities of wine stilbenoids and flavans extracted from grape (Vitis vinifera) cell cultures. Nutr. Cancer 2001, 40, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Waffo-Téguo, P.; Fauconneau, B.; Deffieux, G.; Huguet, F.; Vercauteren, J.; Mérillon, J.M. Isolation, identification, and antioxidant activity of three stilbene glucosides newly extracted from Vitis vinifera cell cultures. J. Nat. Prod. 1998, 61, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Taurino, M.; Ingrosso, I.; D’amico, L.; De Domenico, S.; Nicoletti, I.; Corradini, D.; Santino, A.; Giovinazzo, G. Jasmonates elicit different sets of stilbenes in Vitis vinifera cv. Negramaro cell cultures. SpringerPlus 2015, 4. [Google Scholar] [CrossRef]
- Chastang, T.; Pozzobon, V.; Taidi, B.; Courot, E.; Clément, C.; Pareau, D. Resveratrol production by grapevine cells in fed-batch bioreactor: Experiments and modelling. Bioch. Eng. J. 2018, 131, 9–16. [Google Scholar] [CrossRef]
- Belhadj, A.; Telef, N.; Saigne, C.; Cluzet, S.; Barrieu, F.; Hamdi, S.; Mérillon, J.M. Effect of methyl jasmonate in combination with carbohydrates on gene expression of PR proteins, stilbene and anthocyanin accumulation in grapevine cell cultures. Plant Physiol. Biochem. 2008, 46, 493–499. [Google Scholar] [CrossRef]
- Ferri, M.; Righetti, L.; Tassoni, A. Increasing sucrose concentrations promote phenylpropanoid biosynthesis in grapevine cell cultures. J. Plant Physiol. 2011, 168, 189–195. [Google Scholar] [CrossRef]
- Cruz, E.F.; Cerezo, A.B.; Cantos-Villar, E.; Richard, E.; Troncoso, A.M.; Garcia-Parilla, M.C. Inhibition of VEGFR-2 phosphorylation and effects on downstream signaling pathways in cultivated human endothelial cells by stilbenes from Vitis spp. J. Agric. Food Chem. 2019, 67, 3909–3918. [Google Scholar] [CrossRef]
- Li, C.; Xu, X.; Tao, Z.; Wang, X.J.; Pan, Y. Resveratrol dimers, nutritional components in grape wine, are selective ROS scavengers and weak Nrf2 activators. Food Chem. 2015, 173, 218–223. [Google Scholar] [CrossRef]
- Morales, M.; Bru, R.; García-Carmona, F.; Barceló, A.R.; Pedreño, M.A. Effect of dimethyl-β-cyclodextrins on resveratrol metabolism in Gamay grapevine cell cultures before and after inoculation with shape Xylophilus ampelinus. Plant Cell Tissue Organ Cult. 1998, 53, 179–187. [Google Scholar] [CrossRef]
- Oliva, E.; Mathiron, D.; Bertaut, E.; Landy, D.; Cailleu, D.; Pilard, S.; Clément, C.; Courot, E.; Bonnet, V.; Djedaini-Pilard, F. Physico-chemical studies of resveratrol, methyl-jasmonate and cyclodextrins interactions: An approach to resveratrol bioproduction optimization. RSC Adv. 2018, 8, 1528–1538. Available online: http://xlink.rsc.org/? (accessed on 16 October 2019). [CrossRef]
- Chang, X.; Heene, E.; Qiao, F.; Nick, P. The Phytoalexin Resveratrol Regulates the Initiation of hypersensitive cell death in Vitis cell. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Bru, R.; Sellés, S.; Casado-Vela, J.; Belchí-Navarro, S.; Pedreño, M.A. Modified cyclodextrins are chemically defined glucan inducers of defense responses in grapevine cell cultures. J. Agric. Food Chem. 2006, 54, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, A.R.; Mulinacci, N.; Valetta, A.; Innocenti, M.; Pasqua, G. Effects of elicitors on the production of resveratrol and viniferins in cell culture of Vitis vinfera L. cv Italia. J. Agric. Food Chem. 2011, 59, 9094–9101. [Google Scholar] [CrossRef]
- Gabaston, J.; Leborgne, C.; Valls, J.; Renouf, E.; Richard, T.; Waffo-Teguo, P.; Mérillon, J.-M. Subcritical water extraction of stilbenes from grapevine by-products: A new green chemistry approach. Ind. Crop. Prod. 2018, 126, 272–279. [Google Scholar] [CrossRef]
- Barceló, A.R.; Pomar, F.; López-Serrano, M.; Pedreño, M.A. Peroxidase: A multifunctional enzyme in grapevines. Funct. Plant Biol. 2003, 30, 577–591. [Google Scholar] [CrossRef]
- Vera-Urbina, J.C.; Sellès-Marchart, S.; Martinez-Esteso, M.J.; Pedreno, M.A.; Bru-Martinez, R. Production of grapevine cell biomass (Vitis vinifera L. Gamay) and resveratrol in custom and commercial bioreactors using cyclodextrins and methyl jasmonate elicitors. In Resveratrol: Source, Production, and Health Benefits; Delmas, D., Ed.; Nova Science Publisher Inc.: Hauppauge, NY, USA, 2013; pp. 19–39. [Google Scholar]
- Bru, M.R.; Pedreno, G.M. Method for the Production of Resveratrol in Cell Cultures. U.S. Patent 7309591, 18 December 2007. [Google Scholar]
- Chen, J.; Hall, D.E.; Murata, J.; De Luca, V. L-Alanine induces programmed cell death in V. labrusca cell suspension cultures. Plant Sci. 2006, 171, 734–744. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Plant cell cultures 1. Nutrients requirements of suspension cultures of soybean root cells. Exp. Cell. Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]


| Factor a | Coefficient Estimate | Standard Error | t-Student | p-Value |
|---|---|---|---|---|
| Intercept | 11.56 | 0.91 | 12.6 | <0.0001 * |
| B | −7.41 | 1.02 | −7.3 | <0.0001 * |
| M | 5.12 | 1.02 | 5.0 | <0.0001 * |
| S | 0.20 | 0.97 | 0.2 | 0.84 |
| B × M | −2.84 | 1.02 | −2.8 | 0.011 * |
| B × S | 4.97 | 1.02 | 4.9 | <0.0001 * |
| M × S | −0.77 | 1.02 | −0.8 | 0.46 |
| B × M × S | 2.91 | 1.02 | 2.9 | 0.0095 * |
| Factor a | Coefficient Estimate | Standard Error | t-Student | p-Value |
|---|---|---|---|---|
| Intercept | 11.71 | 0.46 | 25.3 | <0.0001 * |
| B | −5.14 | 0.51 | −10.0 | <0.0001 * |
| M | 0.50 | 0.51 | 1.0 | 0.34 |
| S | 1.75 | 0.49 | 3.6 | 0.0019 * |
| B × M | 0.40 | 0.51 | 0.8 | 0.45 |
| B × S | 3.34 | 0.51 | 6.5 | <0.0001 * |
| M × S | −2.19 | 0.51 | −4.3 | <0.0001 * |
| B × M × S | 1.86 | 0.51 | 3.6 | 0.0017 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambert, C.; Lemaire, J.; Auger, H.; Guilleret, A.; Reynaud, R.; Clément, C.; Courot, E.; Taidi, B. Optimize, Modulate, and Scale-up Resveratrol and Resveratrol Dimers Bioproduction in Vitis labrusca L. Cell Suspension from Flasks to 20 L Bioreactor. Plants 2019, 8, 567. https://doi.org/10.3390/plants8120567
Lambert C, Lemaire J, Auger H, Guilleret A, Reynaud R, Clément C, Courot E, Taidi B. Optimize, Modulate, and Scale-up Resveratrol and Resveratrol Dimers Bioproduction in Vitis labrusca L. Cell Suspension from Flasks to 20 L Bioreactor. Plants. 2019; 8(12):567. https://doi.org/10.3390/plants8120567
Chicago/Turabian StyleLambert, Carole, Julien Lemaire, Hélène Auger, Arnaud Guilleret, Romain Reynaud, Christophe Clément, Eric Courot, and Behnam Taidi. 2019. "Optimize, Modulate, and Scale-up Resveratrol and Resveratrol Dimers Bioproduction in Vitis labrusca L. Cell Suspension from Flasks to 20 L Bioreactor" Plants 8, no. 12: 567. https://doi.org/10.3390/plants8120567
APA StyleLambert, C., Lemaire, J., Auger, H., Guilleret, A., Reynaud, R., Clément, C., Courot, E., & Taidi, B. (2019). Optimize, Modulate, and Scale-up Resveratrol and Resveratrol Dimers Bioproduction in Vitis labrusca L. Cell Suspension from Flasks to 20 L Bioreactor. Plants, 8(12), 567. https://doi.org/10.3390/plants8120567







