Recombinant Human Dentin Matrix Protein 1 (hDMP1) Expressed in Nicotiana benthamiana Potentially Induces Osteogenic Differentiation
Abstract
1. Introduction
2. Results
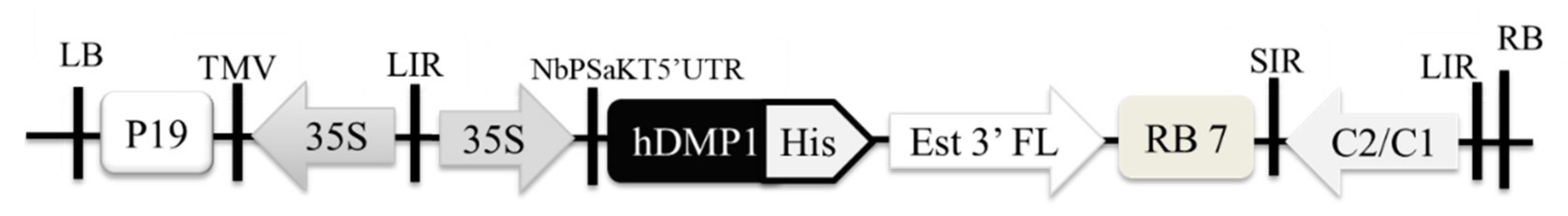
2.1. Optimized Expression of Human Dentin Matrix Protein 1 (hDMP1) in N. benthamiana
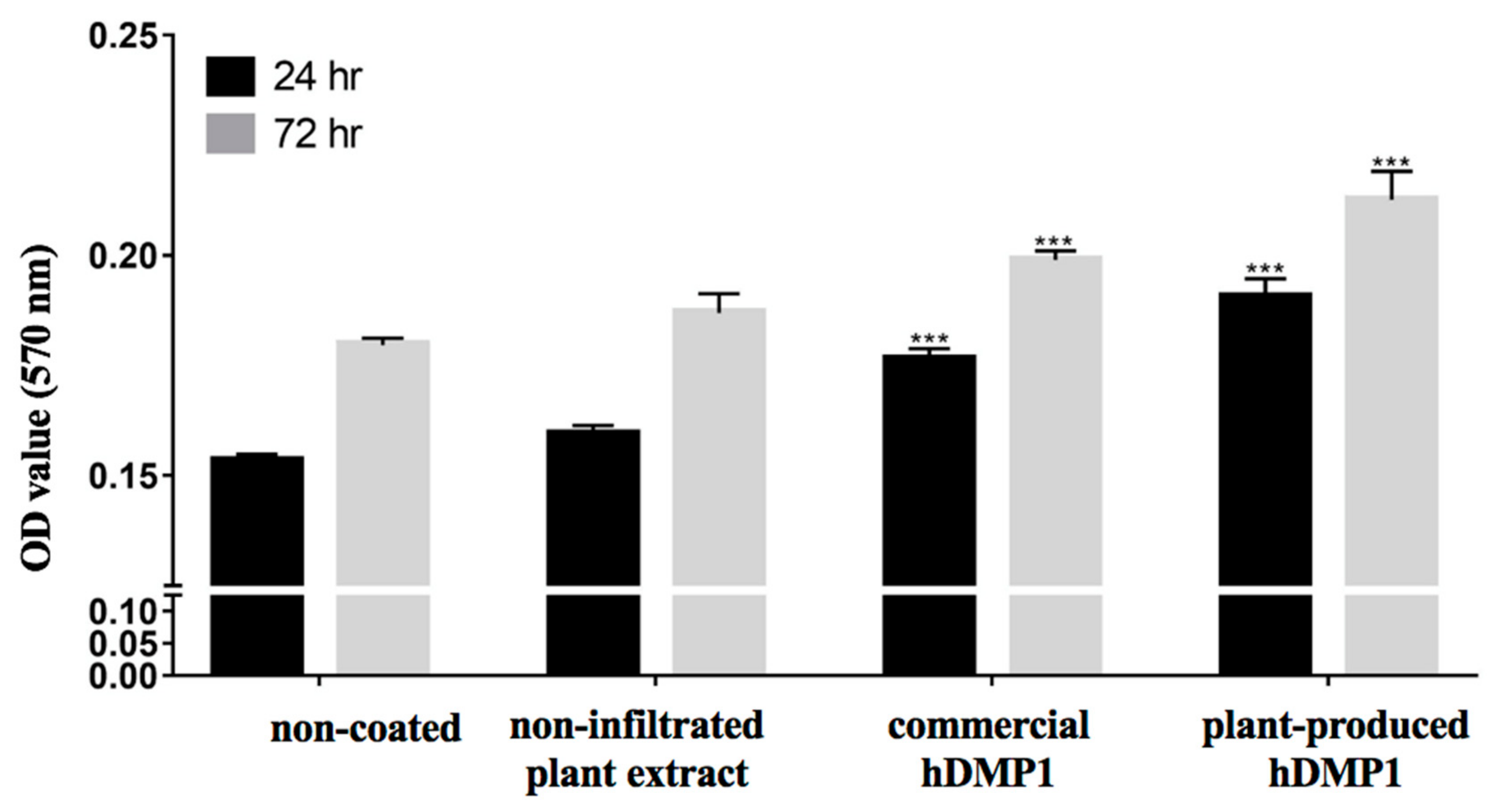
2.2. Effect of hDMP1 on Cell Proliferation
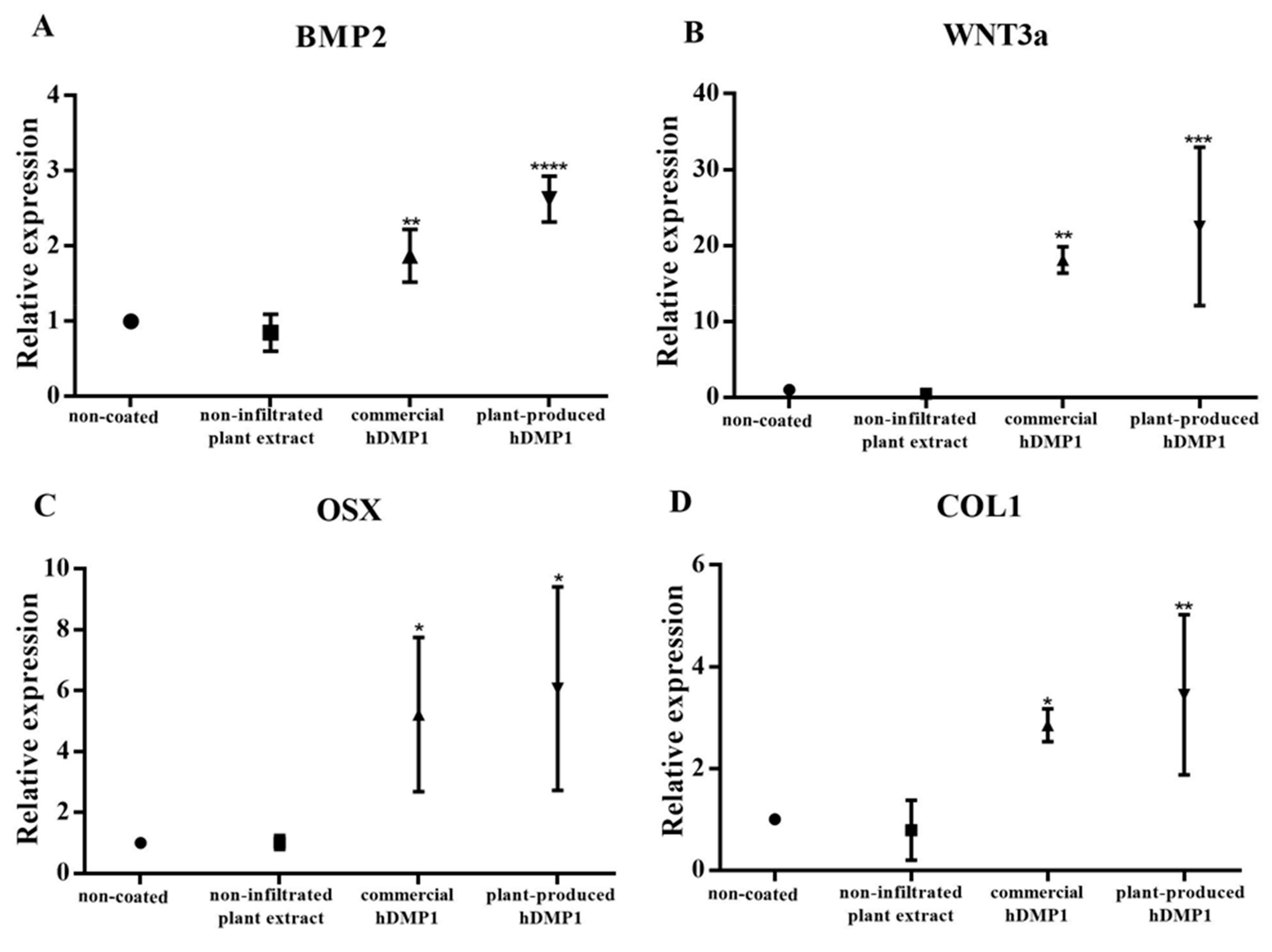
2.3. Plant-Produced hDMP1 Activates Osteogenesis-Related Genes
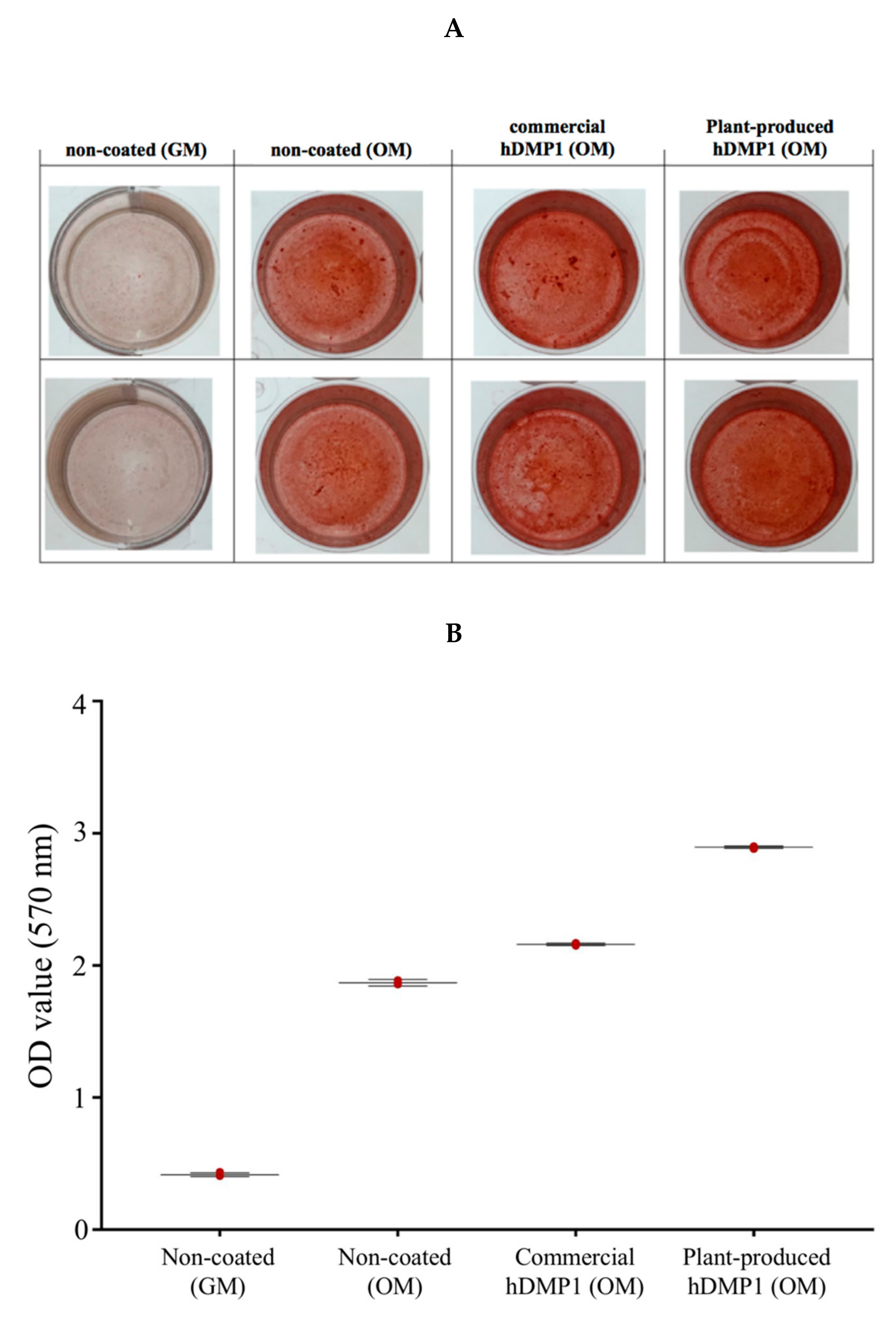
2.4. Plant-Produced hDMP1 Activates Calcification
3. Discussion
4. Materials and Methods
4.1. Construction of pBYR2e-hDMP1
4.2. Expression of hDMP1 in N. benthamiana
4.3. Purification of hDMP1
4.4. SDS PAGE and Western Blot
4.5. DMP1 Quantification by Enzyme-Linked Immunosorbent Assays
4.6. Cell Culture
4.7. The DMP1 Coating Protocol
4.8. Cell Proliferation Assay
4.9. Real-Time PCR (RT-PCR) Analysis
4.10. Calcium Deposition
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, F.M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- Kanczler, J.M.; Oreffo, R.O. Osteogenesis and angiogenesis: The potential for engineering bone. Eur. Cells Mater. 2008, 15, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Klees, R.F.; Salasznyk, R.M.; Kingsley, K.; Williams, W.A.; Boskey, A.; Plopper, G.E. Laminin-5 induces osteogenic gene expression in human mesenchymal stem cells through an ERK-dependent pathway. Mol. Biol. Cell 2005, 16, 881–890. [Google Scholar] [CrossRef]
- Kundu, A.K.; Putnam, A.J. Vitronectin and collagen I differentially regulate osteogenesis in mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2006, 347, 347–357. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, B.; Qin, C.; Cao, Z.; Xie, Y.; Dallas, S.L.; McKee, M.D.; Drezner, M.K.; Bonewald, L.F.; Feng, J.Q. The biological function of DMP-1 in osteocyte maturation is mediated by its 57-kDa C-terminal fragment. J. Bone Miner. Res. 2011, 26, 331–340. [Google Scholar] [CrossRef]
- Jain, A.; Karadag, A.; Fohr, B.; Fisher, L.W.; Fedarko, N.S. Three SIBLINGs (small integrin-binding ligand, N-linked glycoproteins) enhance factor H’s cofactor activity enabling MCP-like cellular evasion of complement-mediated attack. J. Biol. Chem. 2002, 277, 13700–13708. [Google Scholar] [CrossRef]
- Feng, J.Q.; Ward, L.M.; Liu, S.; Lu, Y.; Xie, Y.; Yuan, B.; Yu, X.; Rauch, F.; Davis, S.I.; Zhang, S.; et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 2006, 38, 1310–1315. [Google Scholar] [CrossRef]
- Kulkarni, G.V.; Chen, B.; Malone, J.P.; Narayanan, A.S.; George, A. Promotion of selective cell attachment by the RGD sequence in dentine matrix protein 1. Arch. Oral Biol. 2000, 45, 475–484. [Google Scholar] [CrossRef]
- Qin, C.; D’Souza, R.; Feng, J.Q. Dentin matrix protein 1 (DMP1): New and important roles for biomineralization and phosphate homeostasis. J. Dent. Res. 2007, 86, 1134–1141. [Google Scholar] [CrossRef]
- Zhang, S.; Wan, H.; Wang, P.; Liu, M.; Li, G.; Zhang, C.; Sun, Y. Extracellular matrix protein DMP1 suppresses osteogenic differentiation of Mesenchymal Stem Cells. Biochem. Biophys. Res. Commun. 2018, 501, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.B.; Abranches, R.; Fischer, R.; Sack, M.; Holland, T. Putting the Spotlight Back on Plant Suspension Cultures. Front. Plant Sci. 2016, 7, 297. [Google Scholar] [CrossRef] [PubMed]
- Gerasimova, S.V.; Smirnova, O.G.; Kochetov, A.V.; Shumnyi, V.K. Production of recombinant proteins in plant cells. Russ. J. Plant Physiol. 2016, 63, 26–37. [Google Scholar] [CrossRef]
- Cao, D.V.; Pamplona, R.S.; Kim, J.; Oh, Y.K.; Cho, S.K.; Ahn, J.; Yang, S.W.; Riu, K.Z.; Boo, K.H. Optimization of Agrobacterium-mediated transient expression of heterologous genes in spinach. Plant Biotechnol. Rep. 2017, 11, 397–405. [Google Scholar] [CrossRef]
- Chen, Q.; Dent, M.; Hurtado, J.; Stahnke, J.; McNulty, A.; Leuzinger, K.; Lai, H. Transient protein expression by agroinfiltration in lettuce. In Recombinant Proteins from Plants; Humana Press: New York, NY, USA, 2016; pp. 55–67. [Google Scholar]
- Sun, Y.; Weng, Y.; Zhang, C.; Liu, Y.; Kang, C.; Liu, Z.; Jing, B.; Zhang, Q.; Wang, Z. Glycosylation of Dentin Matrix Protein 1 is critical for osteogenesis. Sci. Rep. 2015, 5, 17518. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Production of recombinant proteins by microbes and higher organisms. Biotechnol. Adv. 2009, 27, 297–306. [Google Scholar] [CrossRef]
- Cooper, C.A.; Maga, E.A.; Murray, J.D. Production of human lactoferrin and lysozyme in the milk of transgenic dairy animals: Past, present, and future. Transgenic Res. 2015, 24, 605–614. [Google Scholar] [CrossRef]
- Phoolcharoen, W.; Prehaud, C.; van Dolleweerd, C.J.; Both, L.; da Costa, A.; Lafon, M.; Ma, J.K.C. Enhanced transport of plant-produced rabies single-chain antibody-RVG peptide fusion protein across an in cellulo blood-brain barrier device. Plant Biotechnol. J. 2017, 15, 1331–1339. [Google Scholar] [CrossRef]
- Rattanapisit, K.; Abdulheem, S.; Chaikeawkaew, D.; Kubera, A.; Mason, H.S.; Ma, J.K.; Pavasant, P.; Phoolcharoen, W. Recombinant human osteopontin expressed in Nicotiana benthamiana stimulates osteogenesis related genes in human periodontal ligament cells. Sci. Rep. 2017, 7, 17358. [Google Scholar] [CrossRef]
- Rattanapisit, K.; Srijangwad, A.; Chuanasa, T.; Sukrong, S.; Tantituvanont, A.; Mason, H.S.; Nilubol, D.; Phoolcharoen, W. Rapid Transient Production of a Monoclonal Antibody Neutralizing the Porcine Epidemic Diarrhea Virus (PEDV) in Nicotiana benthamiana and Lactuca sativa. Planta Med. 2017, 83, 1412–1419. [Google Scholar] [CrossRef]
- Ahmad, A.R.; Kaewpungsup, P.; Khorattanakulchai, N.; Rattanapisit, K.; Pavasant, P.; Phoolcharoen, W. Recombinant human dentin matrix protein 1 (DMP1) induces the osteogenic differentiation of human periodontal ligament cells. Biotechnol. Rep. 2019, 23, e00348. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Chen, B.; Gorski, J.P.; George, A. Recombinant expression and characterization of dentin matrix protein 1. Connect. Tissue Res. 1999, 40, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Terasawa, M.; Shimokawa, R.; Terashima, T.; Ohya, K.; Takagi, Y.; Shimokawa, H. Expression of dentin matrix protein 1 (DMP1) in nonmineralized tissues. J. Bone Miner. Metab. 2004, 22, 430–438. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Sabsay, B.; Simonian, P.A.; Veis, A. Characterization of a novel dentin matrix acidic phosphoprotein. Implications for induction of biomineralization. J. Biol. Chem. 1993, 268, 12624–12630. [Google Scholar] [PubMed]
- Butler, W.T.; Brunn, J.C.; Qin, C. Dentin Extracellular Matrix (ECM) Proteins: Comparison to Bone ECM and Contribution to Dynamics of Dentinogenesis. Connect. Tissue Res. 2003, 44, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Eapen, A.; Sundivakkam, P.; Song, Y.; Ravindran, S.; Ramachandran, A.; Tiruppathi, C.; George, A. Calcium-mediated stress kinase activation by DMP1 promotes osteoblast differentiation. J. Biol. Chem. 2010, 285, 36339–36351. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, F.S.; Ai, J.; Kashi, T.S.J.; Khazaei, M.; Kajbafzadeh, A.M.; Ghanbari, Z. Effect of dentine matrix proteins on human endometrial adult stem-like cells: In vitro regeneration of odontoblasts cells. Arch. Oral Biol. 2013, 58, 871–879. [Google Scholar] [CrossRef]
- Carter, S.S.D.; Costa, P.F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.W.; Malda, J. Additive Biomanufacturing: An Advanced Approach for Periodontal Tissue Regeneration. Ann. Biomed. Eng. 2017, 45, 12–22. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, C.M.; An, S.; Cheng, Q.; Huang, Y.F.; Wang, Y.T.; Gou, Y.C.; Xiao, L.; Yu, W.J.; Wang, J. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells. Oral Dis. 2014, 20, 25–34. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Q.; Hjelm, B.; Arntzen, C.; Mason, H. A DNA replicon system for rapid high-level production of virus-like particles in plants. Biotechnol. Bioeng. 2009, 103, 706–714. [Google Scholar] [CrossRef]
- Osathanon, T.; Ritprajak, P.; Nowwarote, N.; Manokawinchoke, J.; Giachelli, C.; Pavasant, P. Surface-bound orientated Jagged-1 enhances osteogenic differentiation of human periodontal ligament-derived mesenchymal stem cells. J. Biomed. Mater. Res. A 2013, 101, 358–367. [Google Scholar] [CrossRef] [PubMed]

| Gene | Sequence | Reference |
|---|---|---|
| OSX | F; 5′ GCCAGAAGCTGTGAAACCTC 3′ R; 5′ GCTGCAAGCTCTCCATAACC 3′ | NM-001300837.1 |
| OPN | F; 5′ AGGAGGAGGCAGAGCACA 3′ R; 5′ CTGGTATGGCACAGGTGATG 3′ | NM-001040060.1 |
| BMP2 | F; 5′ CTCAGCGAGTTTGAGTTGAGG 3′ R; 5′ GGTACAGGTCGAGCATATAGGG 3′ | NM-017178.1 |
| WNT3a | F; 5′ CTGTTGGGCCACAGTATTCC 3′ R; 5′ GGGCATGATCTCCACGTAGT 3′ | NM-033131.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, A.R.; Kaewpungsup, P.; Khorattanakulchai, N.; Rattanapisit, K.; Pavasant, P.; Phoolcharoen, W. Recombinant Human Dentin Matrix Protein 1 (hDMP1) Expressed in Nicotiana benthamiana Potentially Induces Osteogenic Differentiation. Plants 2019, 8, 566. https://doi.org/10.3390/plants8120566
Ahmad AR, Kaewpungsup P, Khorattanakulchai N, Rattanapisit K, Pavasant P, Phoolcharoen W. Recombinant Human Dentin Matrix Protein 1 (hDMP1) Expressed in Nicotiana benthamiana Potentially Induces Osteogenic Differentiation. Plants. 2019; 8(12):566. https://doi.org/10.3390/plants8120566
Chicago/Turabian StyleAhmad, Aktsar Roskiana, Pornjira Kaewpungsup, Narach Khorattanakulchai, Kaewta Rattanapisit, Prasit Pavasant, and Waranyoo Phoolcharoen. 2019. "Recombinant Human Dentin Matrix Protein 1 (hDMP1) Expressed in Nicotiana benthamiana Potentially Induces Osteogenic Differentiation" Plants 8, no. 12: 566. https://doi.org/10.3390/plants8120566
APA StyleAhmad, A. R., Kaewpungsup, P., Khorattanakulchai, N., Rattanapisit, K., Pavasant, P., & Phoolcharoen, W. (2019). Recombinant Human Dentin Matrix Protein 1 (hDMP1) Expressed in Nicotiana benthamiana Potentially Induces Osteogenic Differentiation. Plants, 8(12), 566. https://doi.org/10.3390/plants8120566





