Exogenous Nitric Oxide Mitigates Nickel-Induced Oxidative Damage in Eggplant by Upregulating Antioxidants, Osmolyte Metabolism, and Glyoxalase Systems
Abstract
1. Introduction
2. Results
2.1. Exogenous SNP Improves Growth by Enhancing Mineral Uptake and Reducing Ni Accumulation
2.2. Photosynthetic Pigments, Photosynthesis, and Gas Exchange Parameters Increased Due to Exogenous SNP
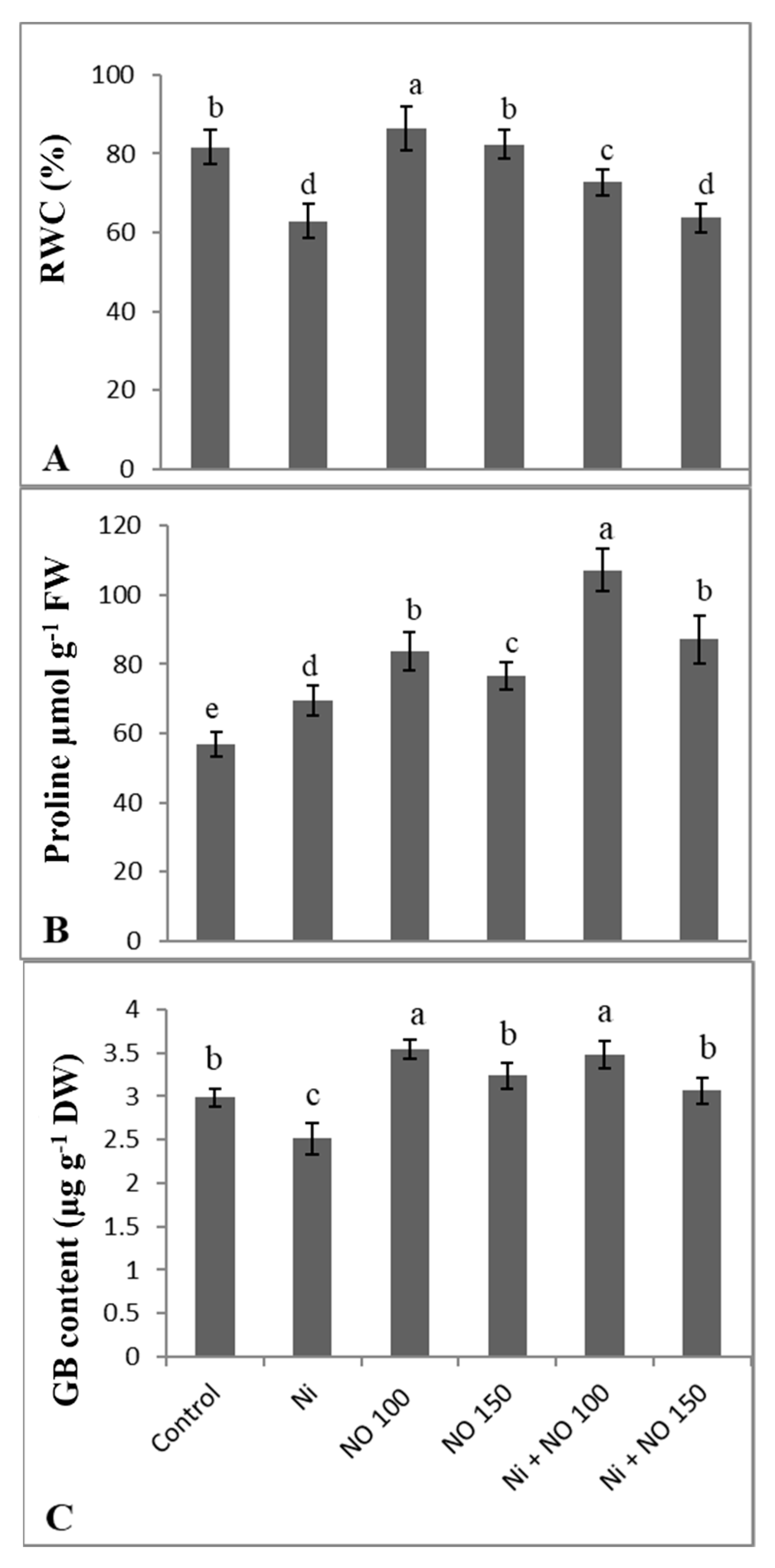
2.3. NO Supplemented Seedlings Exhibit Higher RWC, Proline, and Glycine Betaine (GB)
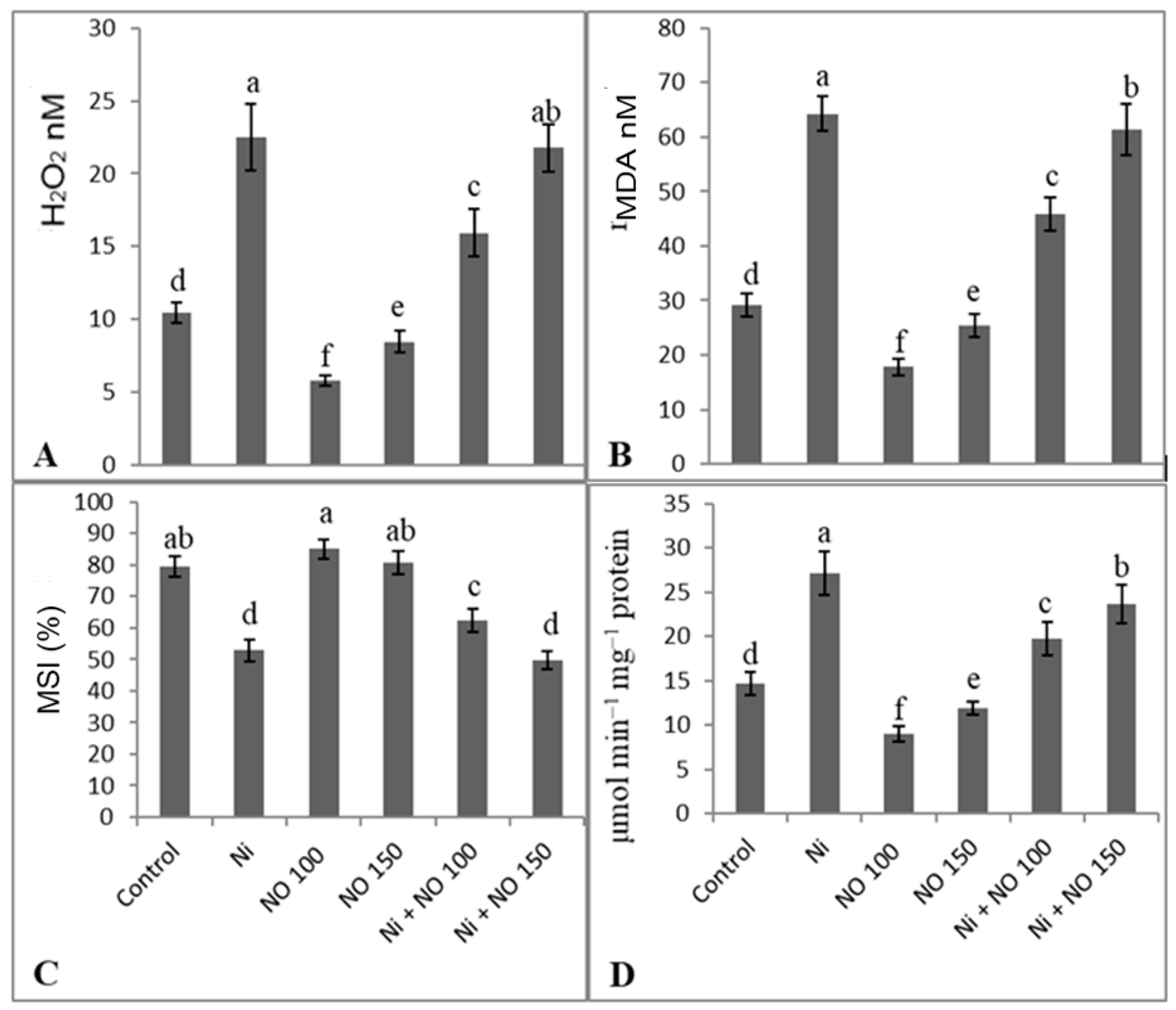
2.4. Application of SNP Reduces Oxidative Damage by Declining H2O2, Lipid Peroxidation, and Lipoxygenase
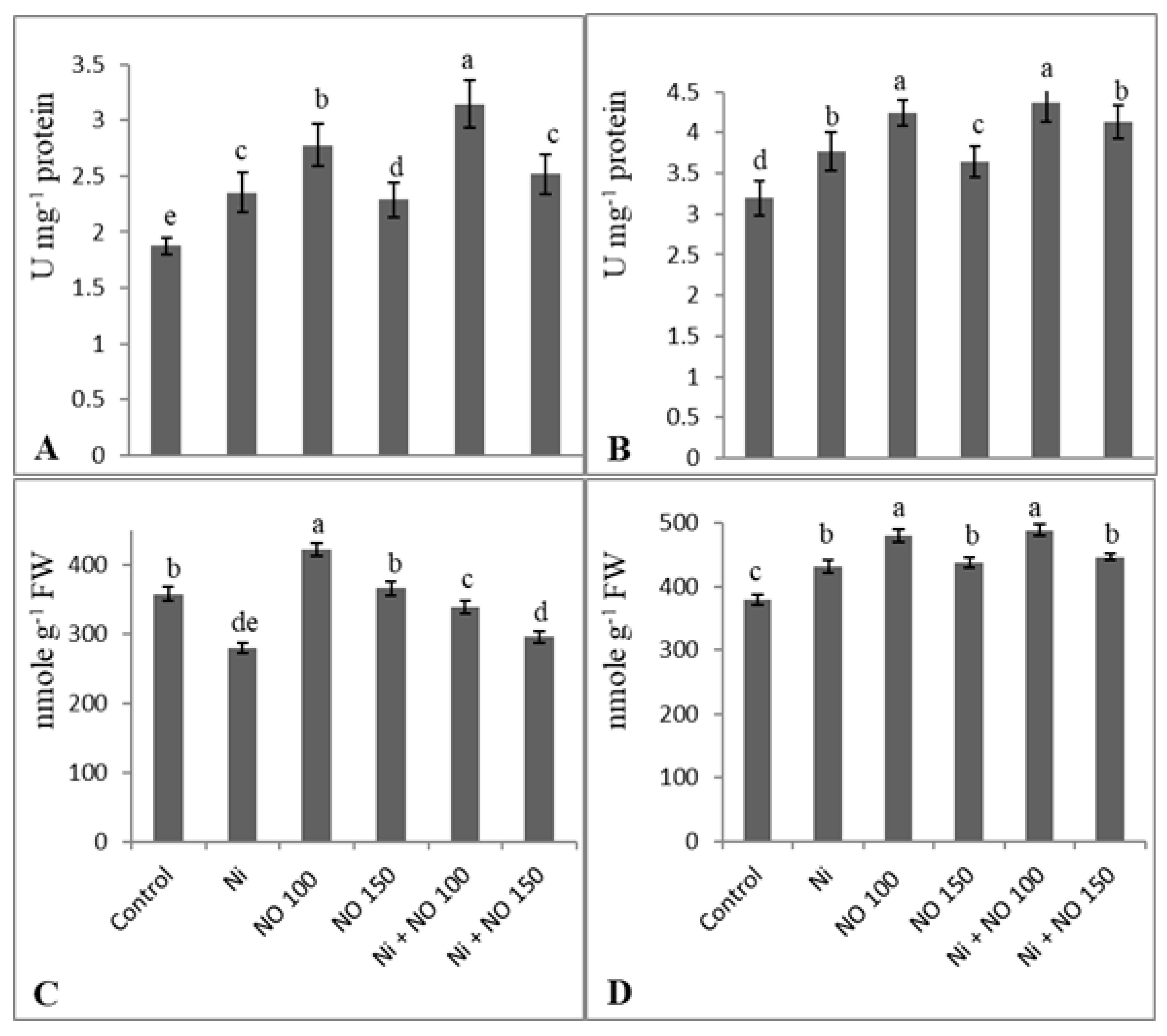
2.5. NO Upregulates Antioxidant System under Ni Stress
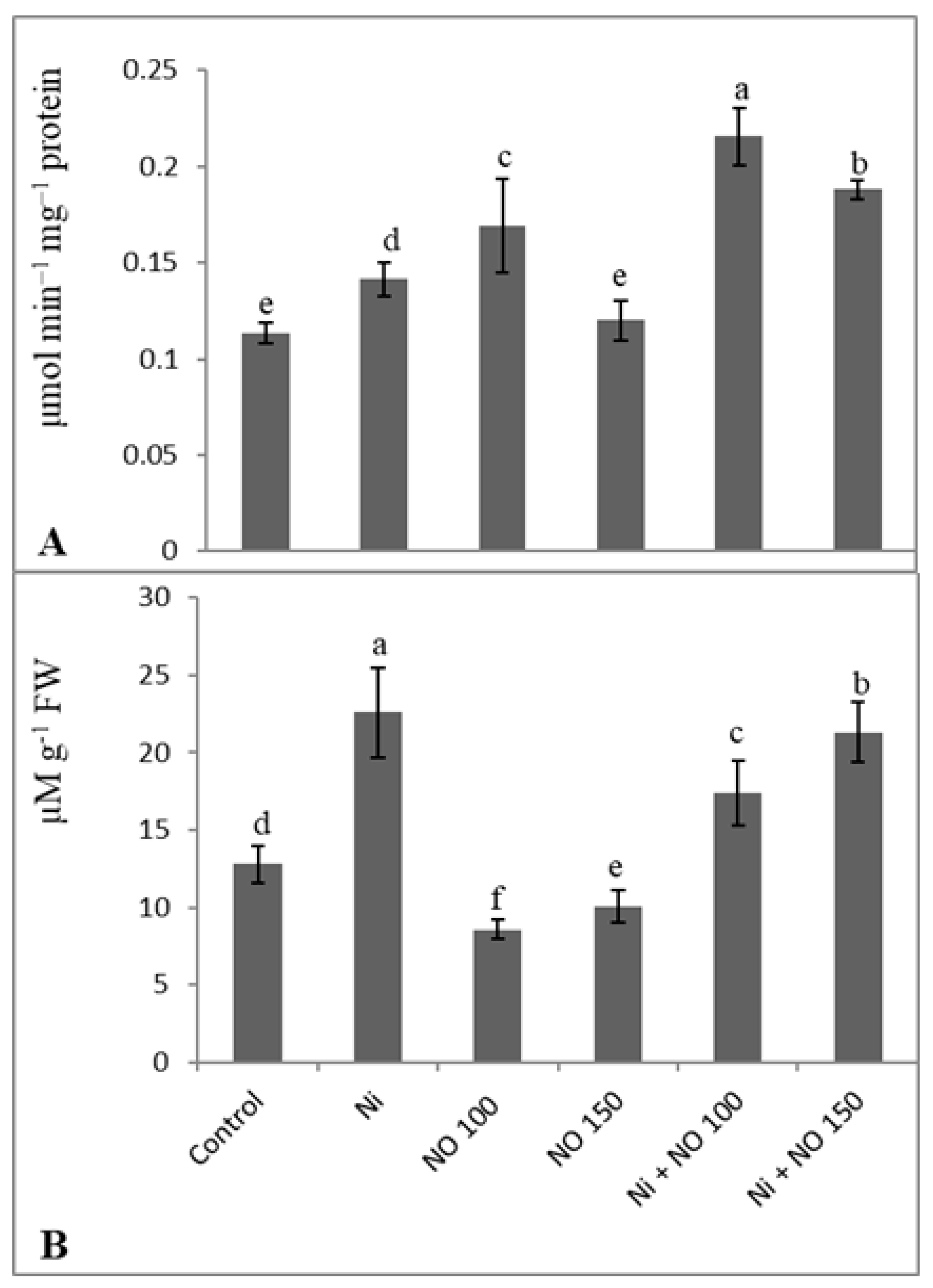
2.6. Exogenous Application of NO Reduces Methylglyoxal by Upregulation Glyoxalase I Activity
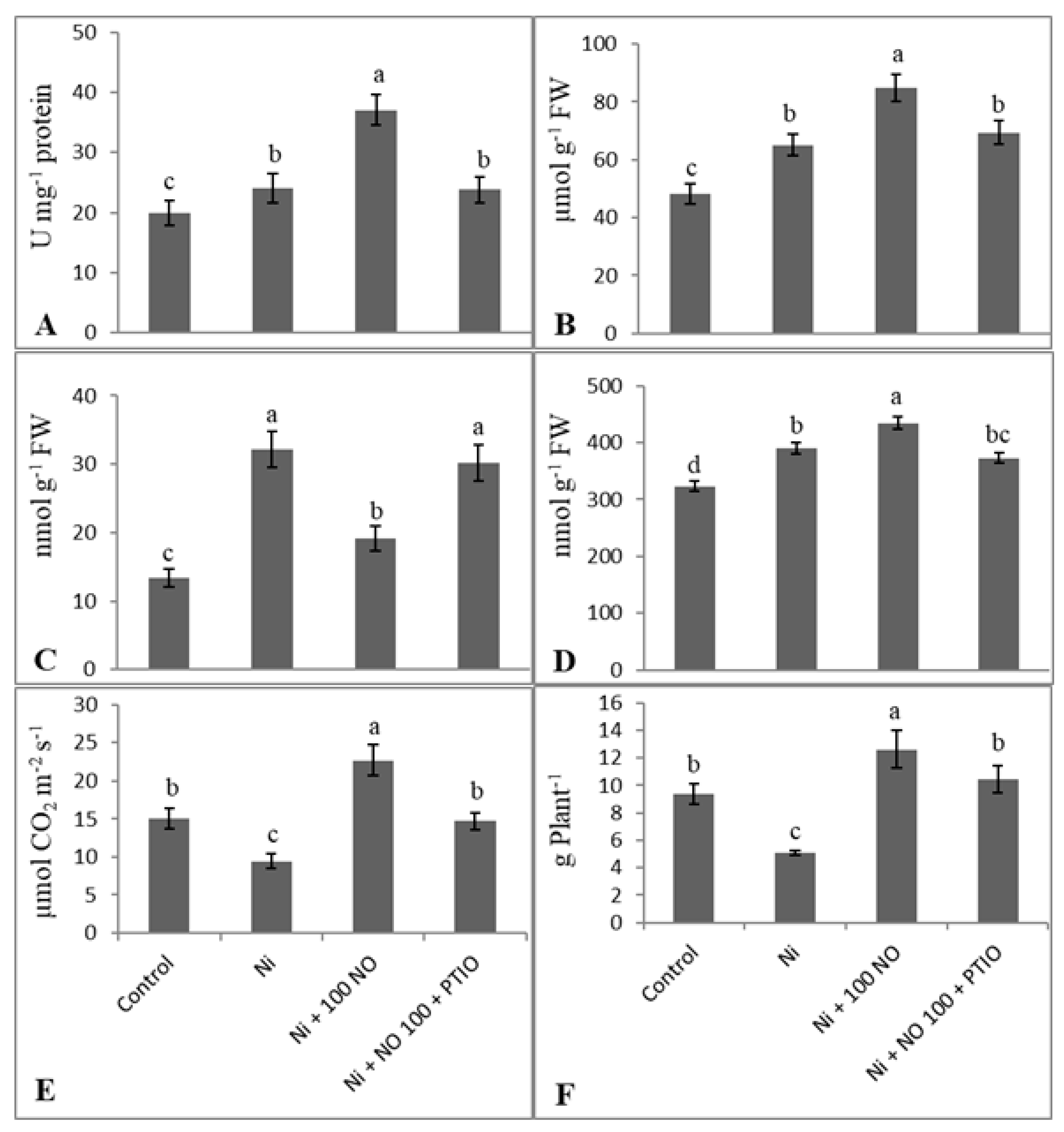
2.7. Effect of Exogenous NO and NO Scavenger (PTIO) on Alleviation of Ni Stress
3. Discussion
4. Materials and Methods
4.1. Plant Growth and Stress Treatments
4.2. Estimation of Photosynthetic Pigments, Photosynthesis and Gas Exchange Parameters
4.3. Estimation of Leaf Water Content, Proline, and Glycine Betaine
4.4. Measurement of Membrane Stability Index, Hydrogen Peroxide, Lipid Peroxidation, and Lipoxygenase
4.5. Assay of Glyoxalase I and Content of Methylglyoxal
4.6. Assay of Antioxidant Enzymes
4.7. Ascorbate and Reduced Glutathione Estimation
4.8. Estimation of Ions
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; whether toxic or essential for plants and environment—A review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, D.; Liu, J. Functions and toxicity of nickel in plants: Recent advances and future prospects. CLEAN–Soil Air Water 2009, 37, 304–313. [Google Scholar] [CrossRef]
- Mousavi, H.Z.; Seyedi, S.R. Nettle ash as a low cost adsorbent for the removal of nickel and cadmium from wastewater. Int. J. Environ. Sci. Technol. 2011, 8, 195–202. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D. Physiological role of nickel and its toxic effects on higher plants. Russ. J. Plant Physiol. 2006, 53, 257–277. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Khan, N.A. Ethylene reverses photosynthetic inhibition by nickel and zinc in mustard through changes in PS II activity, photosynthetic nitrogen use efficiency, and antioxidant metabolism. Protoplasma 2014, 251, 1007–1019. [Google Scholar] [CrossRef]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzalka, K.; Prasad, M.N.V. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol. Plant. 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Ventrella, A.; Catucci, L.; Piletska, E.; Piletsky, S.; Agostiano, A. Interactions between heavy metals and photosynthetic materials studied by optical techniques. Bioelectrochemistry 2009, 77, 19–25. [Google Scholar] [CrossRef][Green Version]
- Per, T.S.; Khan, N.A.; Masood, A.; Fatma, M. Methyl Jasmonate Alleviates Cadmium-Induced Photosynthetic Damages through Increased S-Assimilation and Glutathione Production in Mustard. Front. Plant Sci. 2016, 7, 1933. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Polyamine and nitric oxide crosstalk: Antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol. Environ. Saf. 2016, 126, 245–255. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Elkelish, A.; Elansary, H.O.; Ali, H.M.; Elshikh, M.; Witczak, J.; Ahmad, M. Genetic transformation and hairy root induction enhance the antioxidant potential of Lactuca serriola L. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tyagi, S.R.; Wani, M.R.; Ahmad, P. Drought tolerance: Role of organic osmolytes, growth regulators, and mineral nutrients. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Springer: New York, NY, USA, 2014; pp. 25–55. [Google Scholar]
- Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alamri, S.A.; Alam, P.; Ashraf, M.; Ahmad, P. Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE 2018, 13, e0202175. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.H.; Alayafi, A.A.M.; El Kelish, A.A.; Abu-Elsaoud, A.M. Acetylsalicylic acid enhance tolerance of Phaseolus vulgaris L. to chilling stress, improving photosynthesis, antioxidants and expression of cold stress responsive genes. Bot. Stud. 2018, 59, 6. [Google Scholar] [CrossRef]
- Elkeilsh, A.; Awad, Y.M.; Soliman, M.H.; Abu-Elsaoud, A.; Abdelhamid, M.T.; El-Metwally, I.M. Exogenous application of β-sitosterol mediated growth and yield improvement in water-stressed wheat (Triticum aestivum) involves up-regulated antioxidant system. J. Plant Res. 2019, 132, 881–901. [Google Scholar] [CrossRef]
- Arasimowicz, M.; Floryszak-Wieczorek, J. Nitric oxide as a bioactive signalling molecule in plant stress responses. Plant Sci. 2007, 172, 876–887. [Google Scholar] [CrossRef]
- Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Freschi, L.; Corpas, F.J.; Khan, N.A. Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ. Sci. Pollut. Res. 2017, 24, 2273–2285. [Google Scholar] [CrossRef]
- Fatma, M.; Masood, A.; Per, T.S.; Khan, N.A. Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front. Plant Sci. 2016, 7, 521. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 2018, 255, 79–93. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Tzvetkov, N.T.; El-Tawil, O.S.; Bungǎu, S.G.; Abdel-Daim, M.M.; Atanasov, A.G. Antioxidants: Scientific Literature Landscape Analysis. Available online: https://www.hindawi.com/journals/omcl/2019/8278454/ (accessed on 16 June 2019).
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Naeem, M. Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide 2012, 27, 210–218. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Zakhary, N.I.; Aleya, L.; Bungǎu, S.G.; Bohara, R.A.; Siddiqi, N.J. Aging, Metabolic, and Degenerative Disorders: Biomedical Value of Antioxidants. Oxidative Med. Cell. Longev. 2018, 2018, 1–2. [Google Scholar] [CrossRef]
- Mahmoud, E.K.; Ghoneim, A.M. Effect of polluted water on soil and plant contamination by heavy metals in El-Mahla El-Kobra, Egypt. Solid Earth 2016, 7, 703–711. [Google Scholar] [CrossRef]
- Demchenko, N.P.; Kalimova, I.B.; Demchenko, K.N. Effect of nickel on growth, proliferation, and differentiation of root cells in Triticum aestivum seedlings. Russ. J. Plant Physiol. 2005, 52, 220–228. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Signal transduction and biotechnology in response to environmental stresses. Biol. Plant. 2017, 61, 401–416. [Google Scholar] [CrossRef]
- Kotapati, K.V.; Palaka, B.K.; Ampasala, D.R. Alleviation of nickel toxicity in finger millet (Eleusinecoracana L.) germinating seedlings by exogenous application of salicylic acid and nitric oxide. Crop J. 2017, 5, 240–250. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Agarwal, R.M. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L) as influenced by potassium supplementation. Plant Physiol. Biochem. 2017, 115, 449–460. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–91. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Alnusaire, T.S.; Soliman, M.H.; Gowayed, S.; Senousy, H.H.; Fahad, S. Calcium availability regulates antioxidant system, physio-biochemical activities and alleviates salinity stress mediated oxidative damage in soybean seedlings. J. Appl. Bot. Food Qual. 2019, 92, 258–266. [Google Scholar]
- Dalal, V.K.; Tripathy, B.C. Modulation of chlorophyll biosynthesis by water stress in rice seedlings during chloroplast biogenesis. Plant Cell Environ. 2012, 35, 1685–1703. [Google Scholar] [CrossRef]
- Agurla, S.; Gayatri, G.; Raghavendra, A.S. Polyamines increase nitric oxide and reactive oxygen species in guard cells of Arabidopsis thaliana during stomatal closure. Protoplasma 2018, 255, 153–162. [Google Scholar] [CrossRef]
- Kazemi, N.; Khavari-Nejad, R.A.; Fahimi, H.; Saadatmand, S.; Nejad-Sattari, T. Effects of exogenous salicylic acid and nitric oxide on lipid peroxidation and antioxidant enzyme activities in leaves of Brassica napus L. under nickel stress. Sci. Hortic. 2010, 126, 402–407. [Google Scholar] [CrossRef]
- Liu, F.; Guo, F.-Q. Nitric Oxide Deficiency Accelerates Chlorophyll Breakdown and Stability Loss of Thylakoid Membranes during Dark-Induced Leaf Senescence in Arabidopsis. PLoS ONE 2013, 8, e56345. [Google Scholar] [CrossRef]
- Díaz, J.; Bernal, A.; Pomar, F.; Merino, F. Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annuum L.) seedlings in response to copper stress and its relation to lignification. Plant Sci. 2001, 161, 179–188. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Ahmad, M. Overexpression of AtWRKY30 Transcription Factor Enhances Heat and Drought Stress Tolerance in Wheat (Triticum aestivum L.). Genes 2019, 10, 163. [Google Scholar] [CrossRef]
- Rizwan, M.; Mostofa, M.G.; Ahmad, M.Z.; Imtiaz, M.; Mehmood, S.; Adeel, M.; Dai, Z.; Li, Z.; Aziz, O.; Zhang, Y. Nitric oxide induces rice tolerance to excessive nickel by regulating nickel uptake, reactive oxygen species detoxification and defense-related gene expression. Chemosphere 2018, 191, 23–35. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Li, Z.-G. Methylglyoxal and glyoxalase system in plants: Old players, new concepts. Bot. Rev. 2016, 82, 183–203. [Google Scholar] [CrossRef]
- Martins, A.M.T.; Cordeiro, C.A.A.; Ponces Freire, A.M.J. In situ analysis of methylglyoxal metabolism in Saccharomyces cerevisiae. FEBS Lett. 2001, 499, 41–44. [Google Scholar] [CrossRef]
- Yadav, S.K.; Singla-Pareek, S.L.; Ray, M.; Reddy, M.K.; Sopory, S.K. Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem. Biophys. Res. Commun. 2005, 337, 61–67. [Google Scholar] [CrossRef]
- Yadav, S.K.; Singla-Pareek, S.L.; Sopory, S.K. An overview on the role of methylglyoxal and glyoxalases in plants. Drug Metab. Drug Interact. 2008, 23, 51–68. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol. Biochem. 2014, 80, 67–74. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Nazir, F.; Asgher, M.; Per, T.S.; Khan, N.A. Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J. Plant Physiol. 2015, 173, 9–18. [Google Scholar] [CrossRef]
- Sivakumar, P.; Sharmila, P.; PardhaSaradhi, P. Proline Alleviates Salt-Stress-Induced Enhancement in Ribulose-1,5-Bisphosphate Oxygenase Activity. Biochem. Biophys. Res. Commun. 2000, 279, 512–515. [Google Scholar] [CrossRef]
- Goldstein, S.; Russo, A.; Samuni, A. Reactions of PTIO and Carboxy-PTIO with·NO,·NO2, and. J. Biol. Chem. 2003, 278, 50949–50955. [Google Scholar] [CrossRef]
- He, H.; Oo, T.L.; Huang, W.; He, L.-F.; Gu, M. Nitric oxide acts as an antioxidant and inhibits programmed cell death induced by aluminum in the root tips of peanut (Arachis hypogaea L.). Sci. Rep. 2019, 9, 1. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Smart, R.E.; Bingham, G.E. Rapid estimates of relative water content. Plant Physiol. 1974, 53, 258–260. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Doderer, A.; Kokkelink, I.; van der Veen, S.; Valk, B.E.; Schram, A.; Douma, A.C. Purification and characterization of two lipoxygenase isoenzymes from germinating barley. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1992, 1120, 97–104. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353. [Google Scholar] [CrossRef]
- Wild, R.; Ooi, L.; Srikanth, V.; Münch, G. A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: The N-acetyl-l-cysteine assay. Anal. Bioanal. Chem. 2012, 403, 2577–2581. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Luck, H. Catalase in Methods of Enzymatic Analysis; Bergmeyer, J., Grabi, M., Eds.; Academic Press: New York, NY, USA, 1974; Volume II. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Foyer, C.H.; Fletcher, J.M. Plant antioxidants: Colour me healthy. Biologist 2001, 48, 115–120. [Google Scholar]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Subbaiah, B.V. A rapid procedure for estimation of available nitrogen in soil. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Sagner, S.; Kneer, R.; Wanner, G.; Cosson, J.-P.; Deus-Neumann, B.; Zenk, M.H. Hyperaccumulation, complexation and distribution of nickel in Sebertiaacuminata. Phytochemistry 1998, 47, 339–347. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Ali, H.M.; Elshikh, M.S.; Abdel-Salam, E.M.; El-Esawi, M.; El-Ansary, D.O. Bioactivities of traditional medicinal plants in Alexandria. Evid. Based. Complement. Alternat. Med. 2018, 2018. [Google Scholar] [CrossRef]

| Control | Ni | 100 µM SNP | 150 µM SNP | Ni+100µM SNP | Ni+150µM SNP | |
|---|---|---|---|---|---|---|
| Shoot height (cm) | 43.8 ± 3.01c | 31.0 ± 2.87e | 52.0 ± 4.46a | 48.2 ± 4.6ab | 37.8 ± 3.2d | 33.20 ± 3.40e |
| Shoot dry weight (g /plant) | 7.3 ± 1.02c | 4.9 ± 0.35e | 12.6 ± 1.37a | 10.4 ± 1.0b | 9.1 ± 0.8b | 6.73 ± 0.92d |
| Leaf Nitrogen (mg /g DW) | 20.7 ± 2.05c | 11.5 ± 1.69ef | 27.7 ± 2.05a | 23.3 ± 2.3b | 16.3 ± 1.2d | 13.53 ± 1.26e |
| Leaf potassium (mg /g DW) | 22.5 ± 2.50b | 12.5 ± 1.50e | 29.7 ± 3.05a | 28.2 ± 2.8a | 17.1 ± 1.3c | 15.03 ± 2.04d |
| Leaf calcium (mg /g DW) | 5.8 ± 0.630b | 3.0 ± 0.061d | 7.1 ± 0.351a | 6.2 ± 0.26ab | 4.5 ± 0.3c | 3.43 ± 0.294d |
| Leaf Nickel (mg /g DW) | 0.0047 ± 0.0004d | 3.09 ± 0.26a | 0.0043 ± 0.0004d | 0.0047 ± 0.0007d | 2.15 ± 0.17c | 2.84 ± 0.15b |
| Control | Ni | 100 µM SNP | 150 µM SNP | Ni+100µM SNP | Ni+150µM SNP | |
|---|---|---|---|---|---|---|
| Total chlorophyll (mg /g FW) | 1.3 ± 0.088b | 0.7 ± 0.025de | 1.8 ± 0.065a | 1.4 ± 0.07b | 1.1 ± 0.04c | 0.8 ± 0.018d |
| Carotenoids (mg /g FW) | 0.3211 ± 0.01bc | 0.2074 ± 0.01f | 0.3945 ± 0.0064a | 0.3348 ± 0.006b | 0.3000 ± 0.002d | 0.2279 ± 0.0042e |
| Net Photosynthesis (µmol CO2 m−2S−1) | 16.3 ± 0.55c | 8.8 ± 0.20f | 25.0 ± 1.68a | 17.9 ± 0.9b | 13.3 ± 0.81d | 10.3 ± 0.98e |
| Stomatal conductance (mmol m−2 S−1) | 307.6 ± 12.42c | 229.6 ± 8.50e | 410.3 ± 11.1a | 356.0 ± 10.5b | 307.0 ± 9.5c | 286.3 ± 8.62d |
| Intercellular CO2 concentration (µmol mol−1) | 220.6 ± 7.37c | 162.6 ± 5.85e | 318.6 ± 7.02a | 271.3 ± 6.5b | 213.0 ± 6.2c | 196.6 ± 7.09d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, M.; Alhaithloul, H.A.; Hakeem, K.R.; Alharbi, B.M.; El-Esawi, M.; Elkelish, A. Exogenous Nitric Oxide Mitigates Nickel-Induced Oxidative Damage in Eggplant by Upregulating Antioxidants, Osmolyte Metabolism, and Glyoxalase Systems. Plants 2019, 8, 562. https://doi.org/10.3390/plants8120562
Soliman M, Alhaithloul HA, Hakeem KR, Alharbi BM, El-Esawi M, Elkelish A. Exogenous Nitric Oxide Mitigates Nickel-Induced Oxidative Damage in Eggplant by Upregulating Antioxidants, Osmolyte Metabolism, and Glyoxalase Systems. Plants. 2019; 8(12):562. https://doi.org/10.3390/plants8120562
Chicago/Turabian StyleSoliman, Mona, Haifa A. Alhaithloul, Khalid Rehman Hakeem, Basmah M. Alharbi, Mohamed El-Esawi, and Amr Elkelish. 2019. "Exogenous Nitric Oxide Mitigates Nickel-Induced Oxidative Damage in Eggplant by Upregulating Antioxidants, Osmolyte Metabolism, and Glyoxalase Systems" Plants 8, no. 12: 562. https://doi.org/10.3390/plants8120562
APA StyleSoliman, M., Alhaithloul, H. A., Hakeem, K. R., Alharbi, B. M., El-Esawi, M., & Elkelish, A. (2019). Exogenous Nitric Oxide Mitigates Nickel-Induced Oxidative Damage in Eggplant by Upregulating Antioxidants, Osmolyte Metabolism, and Glyoxalase Systems. Plants, 8(12), 562. https://doi.org/10.3390/plants8120562








