Alternative Pathway is Involved in Nitric Oxide-Enhanced Tolerance to Cadmium Stress in Barley Roots
Abstract
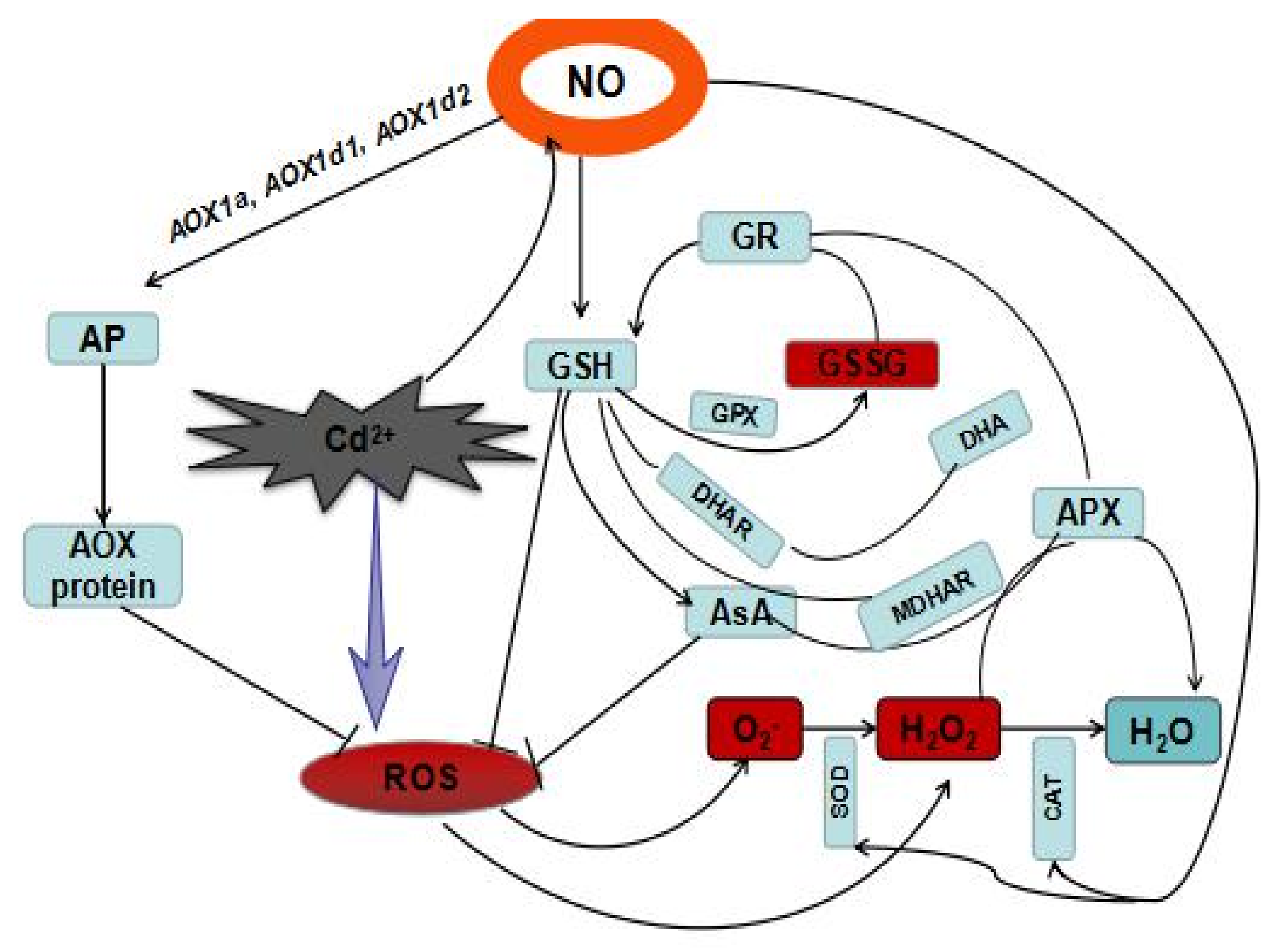
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Root Elongation Measurement
2.3. Root Electrolyte Leakage Determination
2.4. Malonaldehyde Content Determination
2.5. Measurements of Respiration Rate
2.6. Determination of NO Content
2.7. Determination of Nitrate Reductase (NR) Activity
2.8. Determination of Nitric Oxide Synthase (NOS) Activity
2.9. Cloning, Sequencing, and Bioinformatics Analyses
2.10. RNA Isolation and qRT-PCR
2.11. Western Blot Analysis
2.12. H2O2 and O2− Staining
2.13. Extraction and Estimation of Antioxidants
2.14. Antioxidant Enzyme Activity Assay
2.15. Statistical Analysis
3. Results
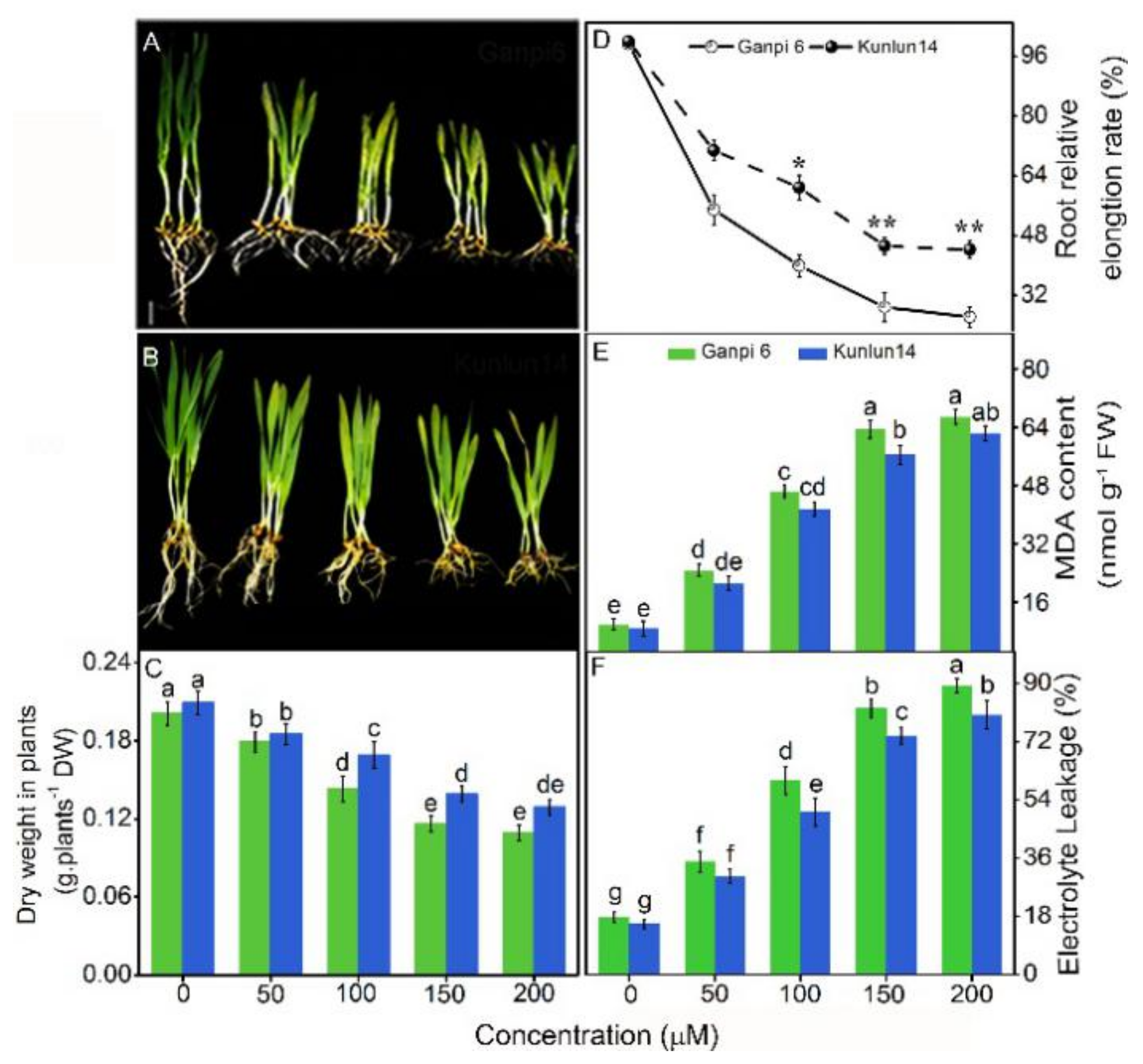
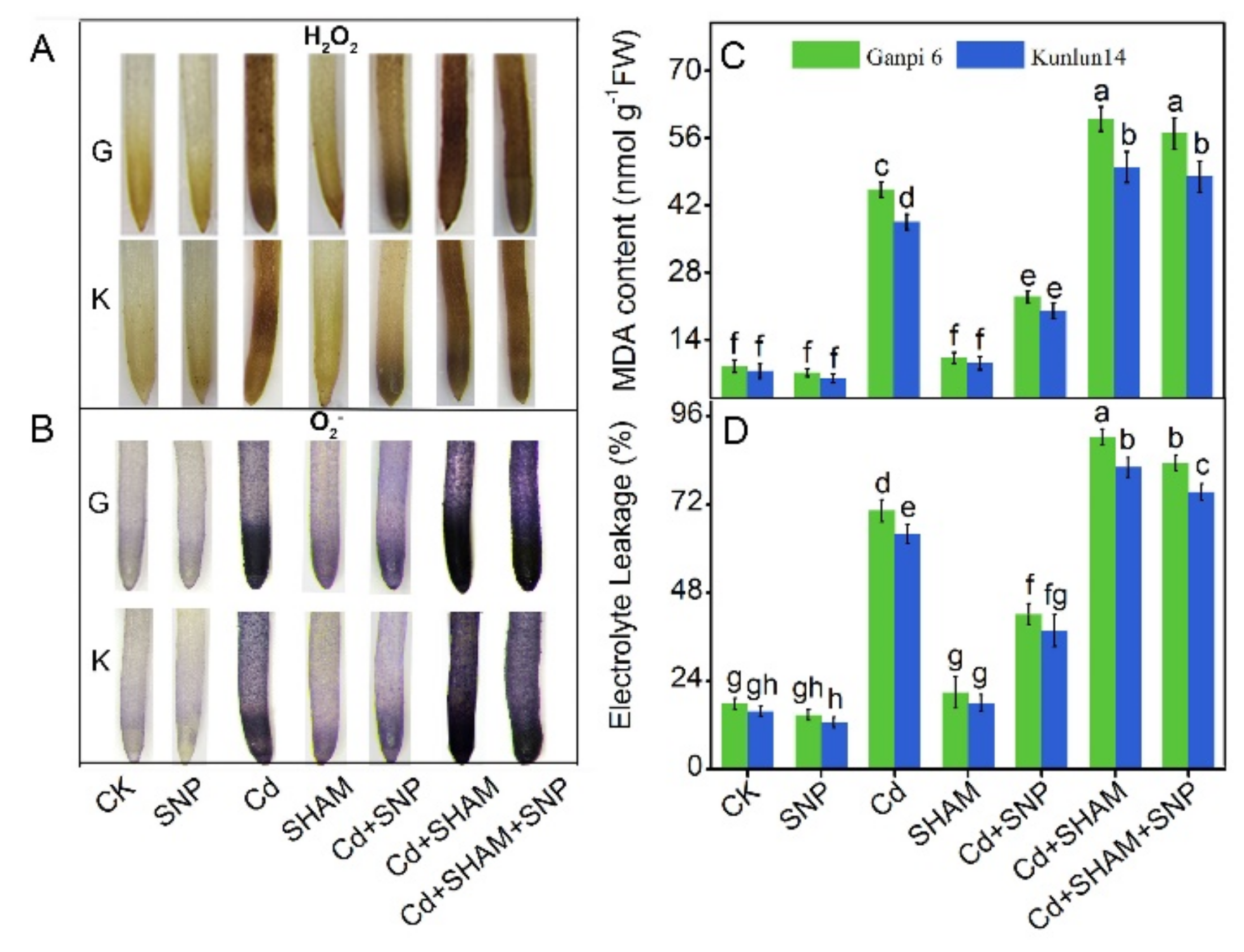
3.1. Effects of Cd Stress on Dry Weight, Root Elongation, MDA Content, and EL
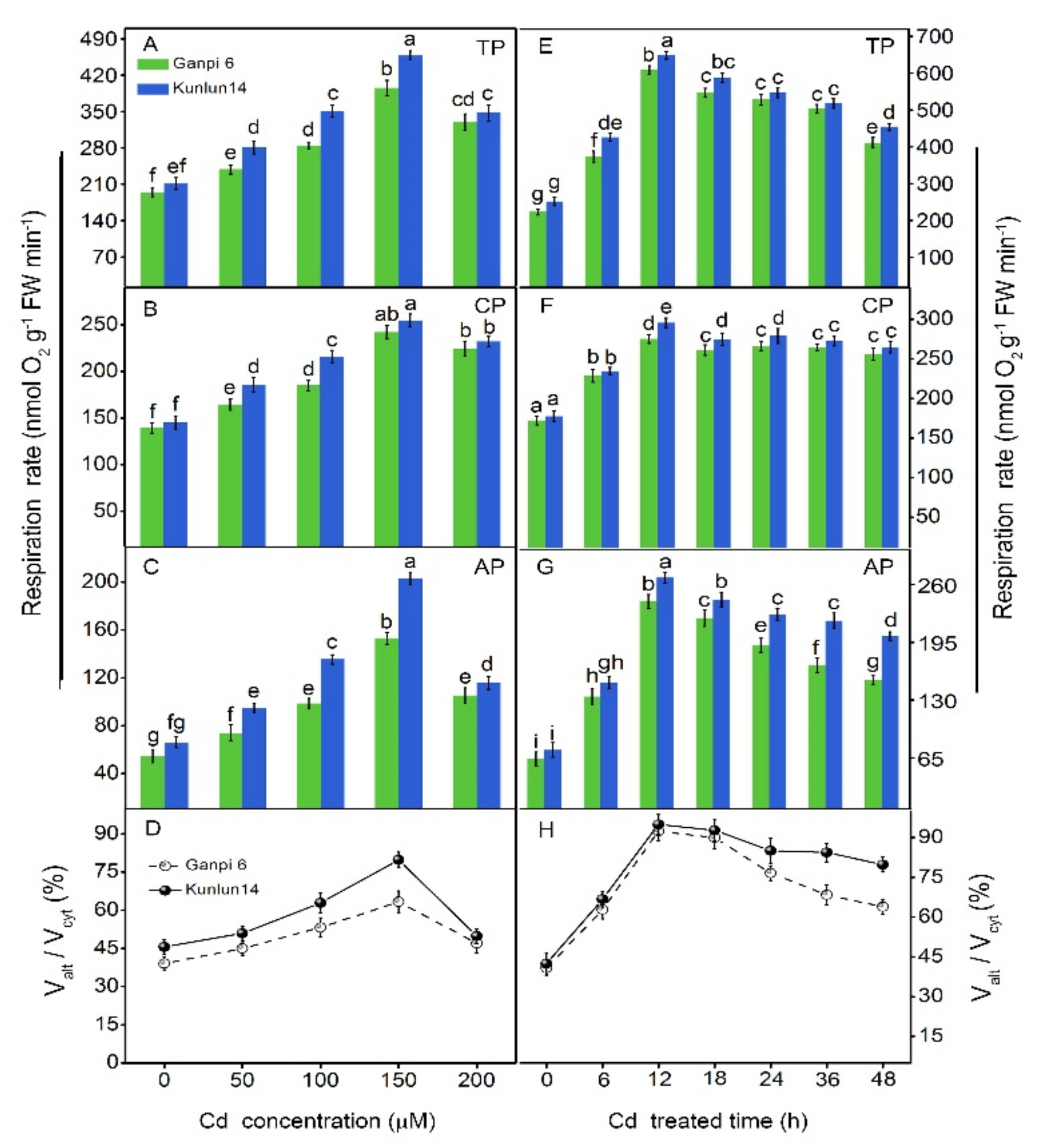
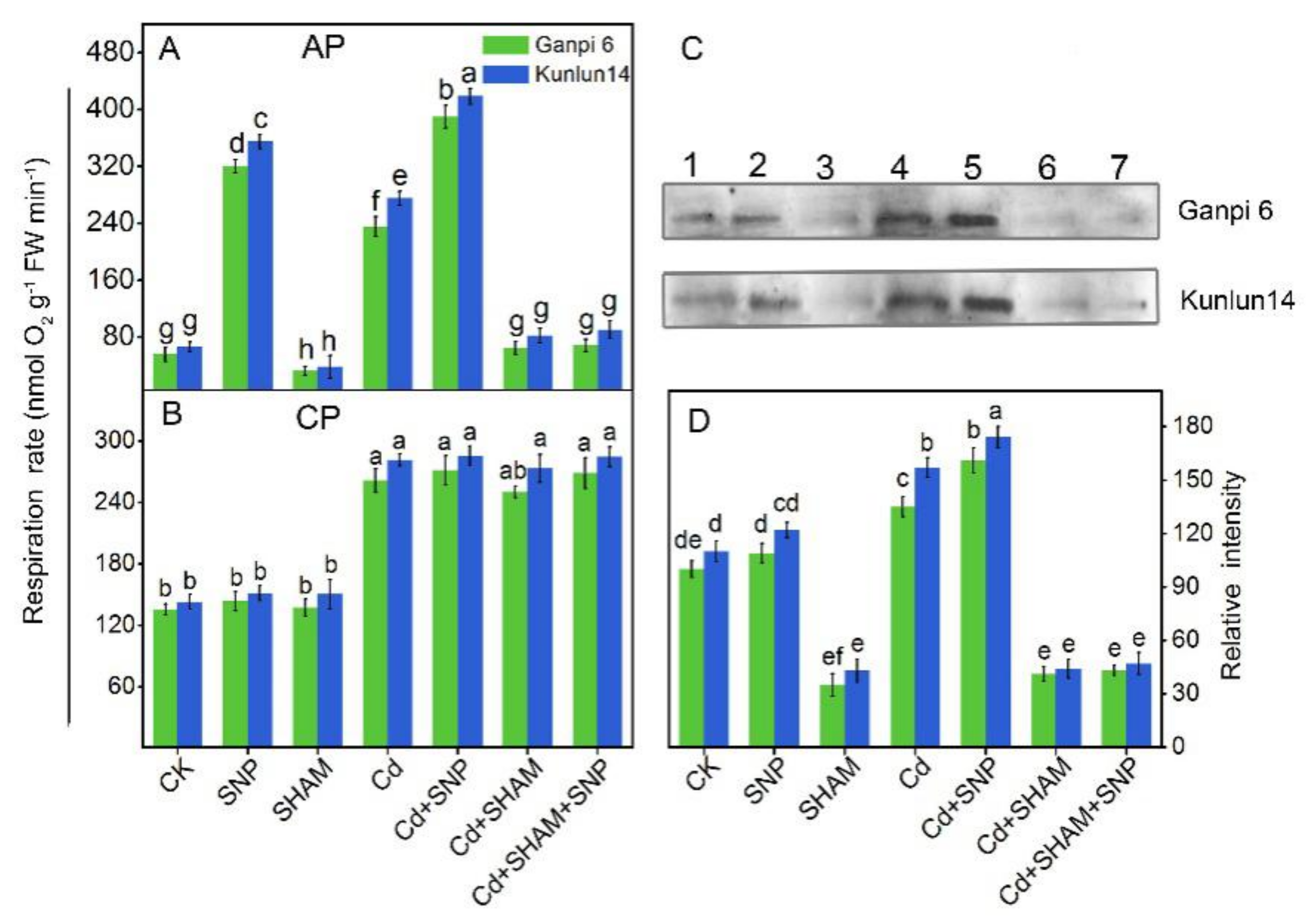
3.2. Effects of Cd Stress on the Respiratory Pathways
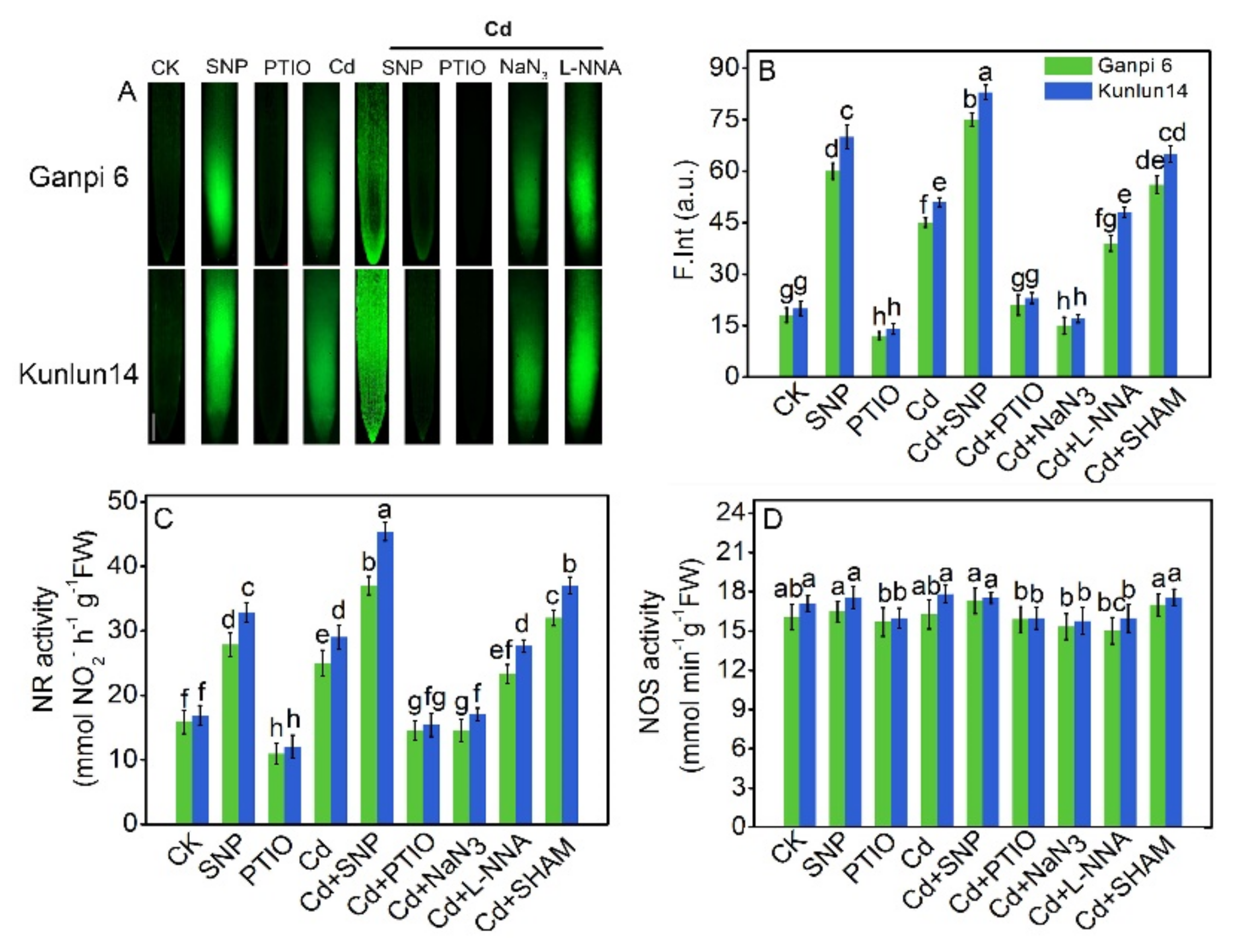
3.3. Cd Stress Induced NO Production from NR Pathway
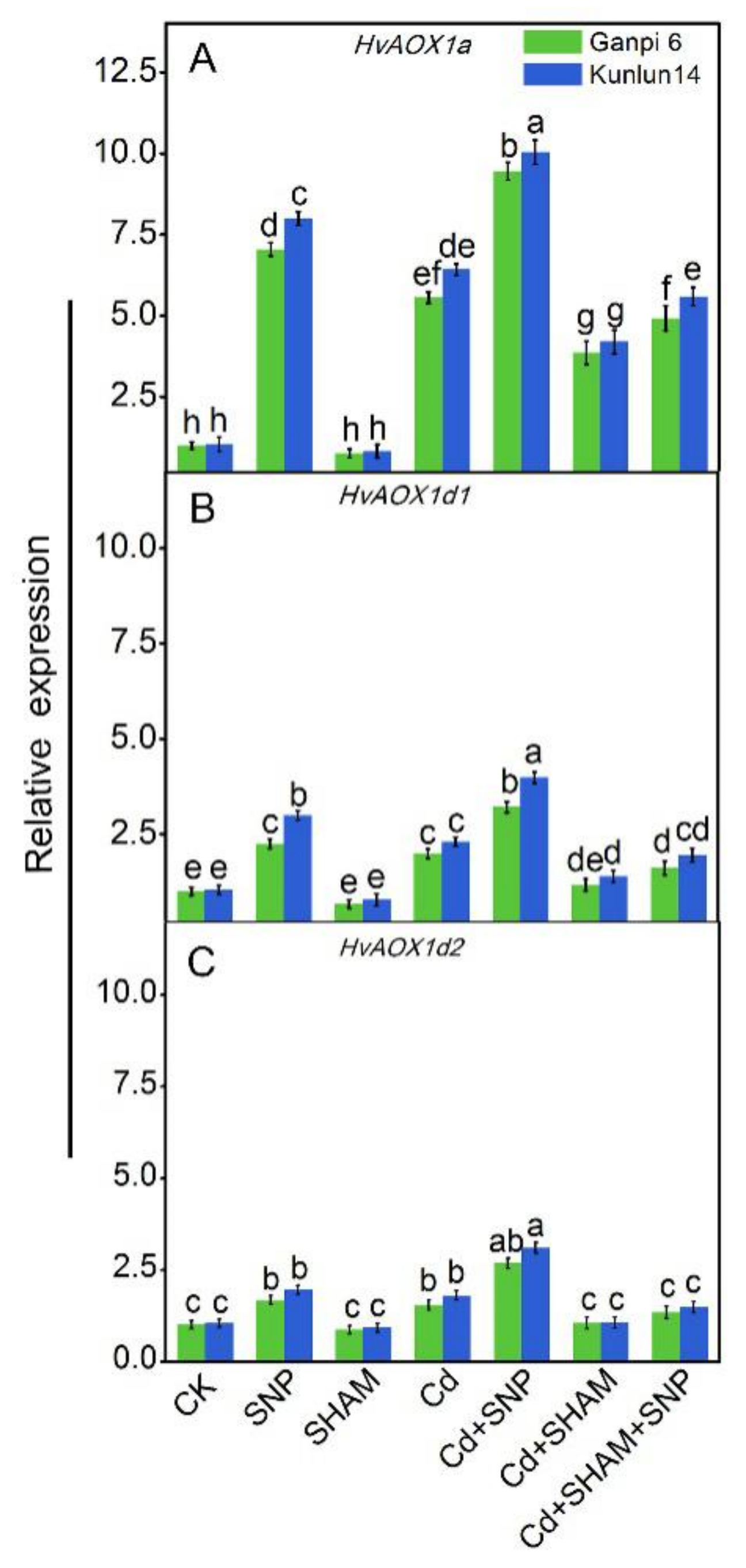
3.4. Expression Patterns of AOX Genes in Highland Barley
3.5. Exogenous NO Enhanced HvAOX Expression in Ganpi6 and Kunlun14 Roots under Cd Stress
3.6. Exogenous NO Enhanced Valt and AOX Protein Level under Cd Stress
3.7. Exogenous NO Did Not Relieve Cd-Induced Oxidative Stress under SHAM Treatment
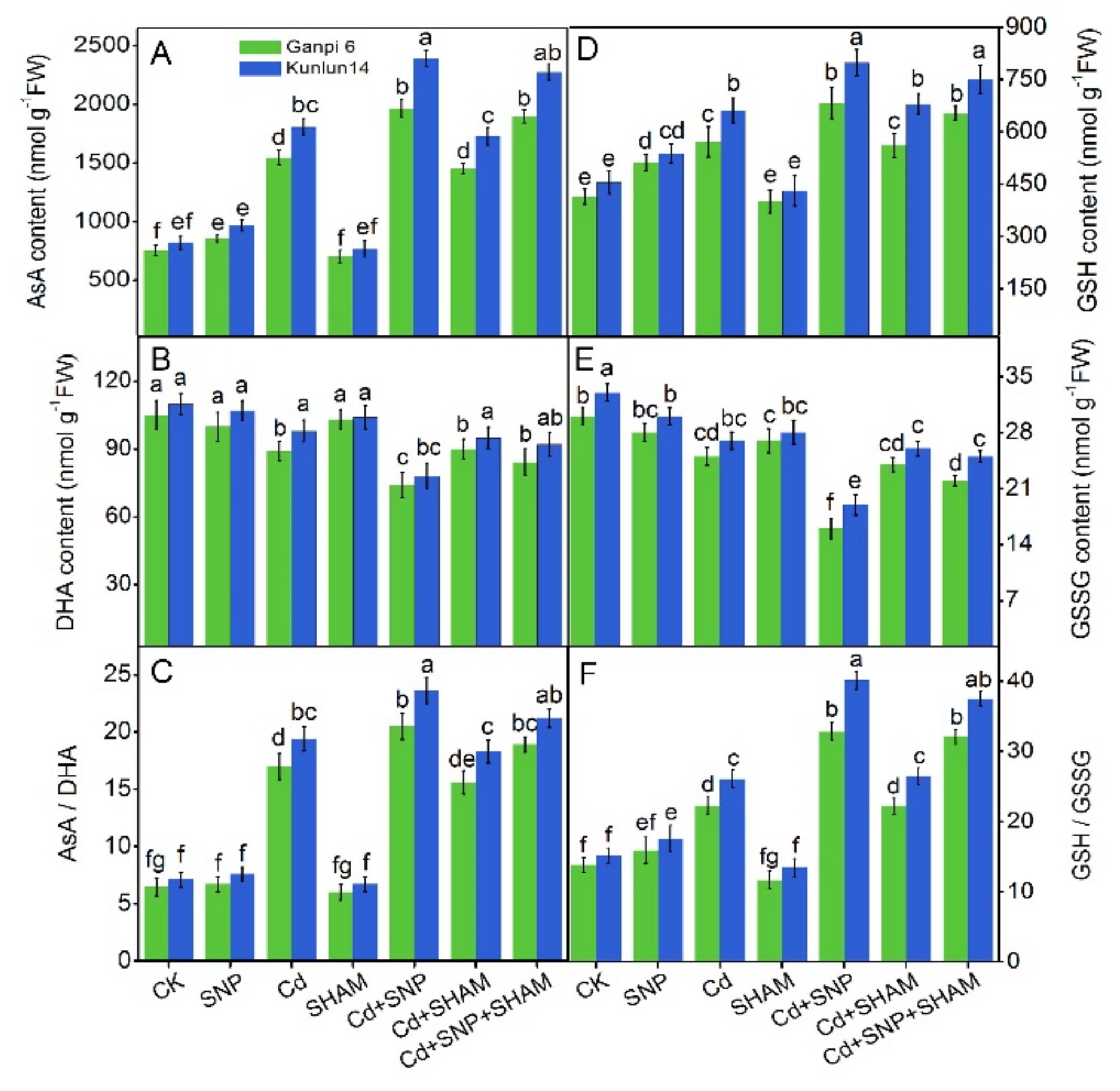
3.8. Effects of Exogenous NO on AsA and GSH Levels in the Presence of SHAM under Cd Stress
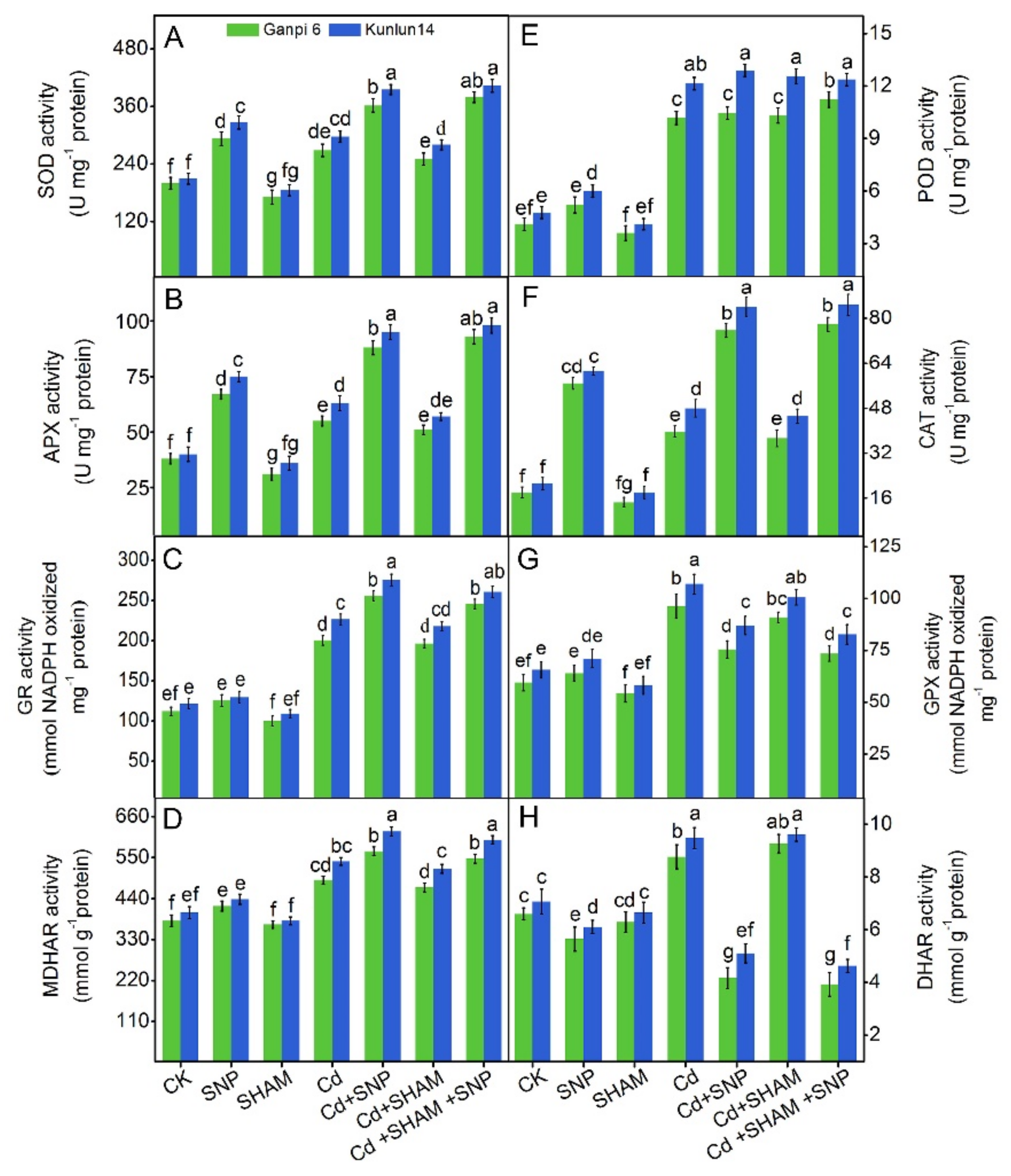
3.9. Effects of Exogenous NO on Antioxidant Enzymes in the Presence of SHAM under Cd Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, S.; Yang, R.; Pan, Y.; Ma, M.; Pan, J.; Zhao, Y.; Cheng, Q.; Wu, M.; Wang, M.; Zhang, L. Nitric oxide contributes to minerals absorption, proton pumps and hormone equilibrium under cadmium excess in Trifolium repens L. plants. Ecotoxicol. Environ. Saf. 2015, 119, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Li, S.W.; Sun, H.J.; Li, H.B.; Luo, J.; Ma, L.Q. Assessment of cadmium bioaccessibility to predict its bioavailability in contaminated soils. Environ. Int. 2016, 94, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Villasante, C.; Rellán-Álvarez, R.; Del Campo, F.; Carpena-Ruiz, O.; Hernández, L.E. Cellular damage induced by cadmium and mercury in Medicago sativa. J. Exp. Bot. 2005, 56, 2239–2251. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J.; Mimura, T. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ. 2016, 39, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Li, T.; Liu, G.Y.; Smith, J.M.; Zhao, Z.W. Unraveling the role of dark septate endophyte (DSE) colonizing maize (Zea mays L.) under cadmium stress: Physiological, cytological and genic aspects. Sci. Rep. 2016, 6, 22028. [Google Scholar] [CrossRef] [PubMed]
- Elobeid, M.; Gobel, C.; Feussner, I.; Polle, A. Cadmium interferes with auxin physiology and lignification in poplar. J. Exp. Bot. 2012, 63, 1413–1421. [Google Scholar] [CrossRef]
- Qin, W.; Bazeille, N.; Henry, E.; Zhang, B.; Deprez, E. Mechanistic insight into cadmium–induced inactivation of the bloom protein. Sci. Rep. 2016, 6, 26225. [Google Scholar] [CrossRef]
- Kopyra, M.; Stachoń-Wilk, M.; Gwóźdź, E.A. Effects of exogenous nitric oxide on the antioxidant capacity of cadmium-treated soybean cell suspension. Acta Physiol. Plant. 2006, 28, 525–536. [Google Scholar] [CrossRef]
- Semane, B.; Cuypers, A.; Smeets, K.; Van Belleghem, F.; Horemans, N.; Schat, H.; Vangronsveld, J. Cadmium responses in Arabidopsis thaliana: Glutathione metabolism and antioxidative defence system. Physiol. Plant. 2007, 129, 519–528. [Google Scholar] [CrossRef]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Basalah, M.O. Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma 2011, 248, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Mur, L.; Mandon, J.; Persijn, S.; Cristescu, S.M.; Moshkov, I.E.; Novikova, G.V.; Hall, M.A.; Harren, F.J.M.; Hebelstrup, K.H.; Gupta, K.J. Nitric oxide in plants: An assessment of the current state of knowledge. AoB Plants 2013, 5, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Freschi, L.; Corpas, F.J.; Khan, N.A. Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ. Sci. Pollut. Res. 2017, 24, 2273–2285. [Google Scholar] [CrossRef] [PubMed]
- Domingos, P.; Prado, A.M.; Wong, A.; Gehring, C.; Feijo, J.A. Nitric oxide: Amultitasked signaling gas in plants. Mol. Plant 2015, 8, 506–520. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Tan, X.; Liu, H.; Zeng, G.; Hu, X.; Jian, H.; Gu, Y. Effect of exogenous nitric oxide on antioxidative system and S-nitrosylation in leaves of Boehmeria nivea (L.) Gaud under cadmium stress. Environ. Sci. Pollut. Res. Int. 2015, 22, 3489–3497. [Google Scholar] [CrossRef]
- Barroso, J.B.; Corpas, F.J.; Carreras, A.; Rodriguez-Serrano, M.; Esteban, F.J.; Fernandez-Ocana, A.; Chaki, M.; Romero, M.C.; Valderrama, R.; Sandalio, L.M. Localization of S-nitrosoglutathione and expression of S-nitrosoglutathione reductase in pea plants under cadmium stress. J. Exp. Bot. 2006, 57, 1785–1793. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B.; Palma, J.M.; Rodriguez-Ruiz, M. Plant peroxisomes: A nitro-oxidative cocktail. Redox Biol. 2017, 11, 535–542. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Wang, L.N.; Yang, L.M.; Yang, G.J.; Li, X.G.; Song, Y.P.; Wang, X.F.; Hu, X.Y. Involvements of H2O2 and metallothionein in NO-mediated tomato tolerance to copper toxicity. J. Plant Physiol. 2010, 167, 1298–1306. [Google Scholar] [CrossRef]
- Panda, P.; Nath, S.; Chanu, T.T.; Sharma, G.D.; Panda, S.K. Cadmium stress-induced oxidative stress and role of nitric oxide in rice (Oryza sativa L.). Acta Physiol. Plant. 2011, 33, 1737–1747. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Yin, H.; Liu, X.; Sun, H.; Mi, Q. Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots of Medicago truncatula seedlings under cadmium stress. Plant Soil 2010, 326, 321–330. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Sun, J.; Zhang, Y.; Ge, Q.; Du, L.; Yin, H.; Liu, X. Involvement of auxin and nitric oxide in plant Cd-stress responses. Plant Soil 2011, 346, 107–119. [Google Scholar] [CrossRef]
- Fan, S.K.; Fang, X.Z.; Guan, M.Y.; Ye, Y.Q.; Lin, X.Y.; Du, S.T.; Jin, C.W. Exogenous abscisic acid application decreases cadmium accumulation in Arabidopsis plants, which is associated with the inhibition of IRT1-mediated cadmium uptake. Front. Plant Sci. 2014, 5, 721. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liang, X.; Dong, Y.; Xu, L.; Zhang, X.; Kong, J.; Liu, J. Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of perennial ryegrass under cadmium stress. J. Plant Growth Regul. 2013, 32, 721–731. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Z.; Zhu, C. Endogenous nitric oxide mediates alleviation of cadmium toxicity induced by calcium in rice seedlings. J. Environ. Sci. 2012, 24, 940–948. [Google Scholar] [CrossRef]
- Shi, H.; Ye, T.; Chan, Z. Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol. Biochem. 2014, 74, 99–107. [Google Scholar] [CrossRef]
- Gill, M.; Hasanuzzaman, K.; Nahar, A.; Macovei, N.; Tuteja, N. Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol. Biochem. 2012, 63, 254–261. [Google Scholar] [CrossRef]
- Vanlerberghe, G.C.; Day, D.A.; Wiskich, J.T.; Vanlerberghe, A.E.; McIntosh, L. AOX activity in tobacco leaf mitochondria: Dependence on tricarboxylic acid cycle-mediated redox regulation and pyruvate activation. Plant Physiol. 1995, 109, 353–361. [Google Scholar] [CrossRef]
- Király, L.; Hafez, Y.; Fodor, J.; Király, Z. Suppression of tobacco mosaic virus-induced hypersensitive-type necrotization in tobacco at high temperature is associated with downregulation of NADPH oxidase and superoxide and stimulation of dehydroascorbate reductase. J. Gen. Virol. 2008, 89, 799–808. [Google Scholar] [CrossRef]
- Watanabe, C.K.; Hachiya, T.; Terashima, I.; Noguchi, K. The lack of alternative oxidase at low temperature leads to a disruption of the balance in carbon and nitrogen metabolism, and to an up-regulation of antioxidant defence systems in Arabidopsis thaliana leaves. Plant Cell Environ. 2008, 31, 1190–1202. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.M.; Zhao, C.Z.; Wang, J.F.; Li, P.; Dou, Y.Q.; Bi, Y.R. Alternative pathway is involved in the tolerance of highland barley to the low-nitrogen stress by maintaining the cellular redox homeostasis. Plant Cell Rep. 2016, 35, 317–328. [Google Scholar] [CrossRef]
- Uraguchi, S.; Fujiwara, T. Cadmium transport and tolerance in rice: Perspectives for reducing grain cadmium accumulation. Rice 2012, 27, 5. [Google Scholar] [CrossRef]
- Vanlerberghe, G.C. Alternative oxidase: A mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int. J. Mol. Sci. 2013, 14, 6805–6847. [Google Scholar] [CrossRef]
- Jia, H.L.; Wang, X.F.; Dou, Y.H.; Liu, D.; Si, W.T.; Fang, H.; Zhao, C.; Chen, S.L.; Xi, J.J.; Li, J.S. Hydrogen sulfide-cysteine cycle system enhances cadmium tolerance through alleviating cadmium-induced oxidative stress and ion toxicity in Arabidopsis roots. Sci. Rep. 2016, 6, 39702. [Google Scholar] [CrossRef]
- Fu, L.J.; Shi, K.; Gu, M.; Zhou, Y.H.; Dong, D.K.; Liang, W.S.; Song, F.M.; Yu, J.Q. Systemic induction and role of mitochondrial alternative oxidase and nitric oxide in a compatible tomato-Tobacco mosaic virus interaction. Mol. Plant Microbe Interact. 2010, 23, 39–48. [Google Scholar] [CrossRef]
- Jian, W.; Zhang, D.W.; Zhu, F.; Wang, S.X.; Pu, X.J.; Deng, X.G.; Luo, S.S.; Lin, H.H. Alternative oxidase pathway is involved in the exogenous SNP-elevated tolerance of Medicago truncatula to salt stress. J. Plant Physiol. 2016, 193, 79–87. [Google Scholar] [CrossRef]
- Aftab, T.; Khan, M.M.A.; Naeem, M.; Idrees, M.; da Silva, J.A.T.; Ram, M. Exogenous nitric oxide donor protec ts Artemisia annua from oxidative stress generated by boron and aluminium toxicity. Ecotoxicol. Environ. Saf. 2012, 80, 60–68. [Google Scholar] [CrossRef]
- Huang, X.; Rad, U.; Durner, J. Nitric oxide induces transcriptional activation of the nitric oxide-tolerant alternative oxidase in Arabidopsis suspension cells. Planta 2002, 215, 914–923. [Google Scholar] [CrossRef]
- Ederli, L.; Morettini, R.; Borgogni, A.; Wasternack, C.; Miersch, O.; Reale, L.; Ferranti, F.; Tosti, N.; Pasqualini, S. Interaction between nitric oxide and ethylene in the induction of alternative oxidase in ozone-treated tobacco Plants. Plant Physiol. 2006, 142, 595–608. [Google Scholar] [CrossRef]
- Siddiqi, M.Y.; Glass, A.D.M.; Ruth, T.J.; Fernando, M. Studies of the regulation of nitrate influx by barley seedlings using (No3-1)-N-13. Plant Physiol. 1989, 90, 806–813. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, D.W.; Yuan, S.; Zhu, F.; Xu, F.; Fu, F.Q.; Wang, S.X.; Lin, H.H. Plastid signals induce alternative oxidase expression to enhance the cold stress tolerance in Arabidopsis thaliana. Plant Growth Regul. 2014, 74, 275–283. [Google Scholar] [CrossRef]
- Wang, H.H.; Huang, J.J.; Liang, X.L.; Bi, Y.R. Involvement of hydrogen peroxide, calcium, and ethylene in the induction of the alternative pathway in chilling-stressed Arabidopsis callus. Planta 2012, 235, 53–67. [Google Scholar] [CrossRef]
- Wang, X.M.; Chang, N.; Bi, Y.R.; Tan, B.C. Measurement of mitochondrial respiration rate in maize (Zea mays L.) leaves. Bio Protocol 2015, 5, e1483. [Google Scholar] [CrossRef]
- Mackintosh, C.; Douglas, P.; Lillo, C. Identification of protein that inhibits the phosphorylated form of nitrate reductase from spinach (Spinacia oleracea) leaves. Plant Physiol. 1995, 107, 451–457. [Google Scholar] [CrossRef]
- Lin, A.H.; Wang, Y.Q.; Tang, J.Y.; Xue, P.; Li, C.L.; Liu, L.C.; Hu, B.; Yang, F.Q.; Loake, G.J.; Chu, C.C. Nitric oxide and protein S-nitrosylation are integral to hydrogen peroxide-induced leaf cell death in rice. Plant Physiol. 2012, 158, 451–464. [Google Scholar] [CrossRef]
- Zhao, C.Z.; Wang, X.M.; Wang, X.Y.; Wu, K.L.; Li, P.; Chang, N.; Wang, J.F.; Wang, F.; Li, J.L.; Bi, Y.R. Glucose-6-phosphate dehydrogenase and alternative oxidase are involved in the cross tolerance of highland barley to salt stress and UV-B radiation. J. Plant Physiol. 2015, 181, 83–95. [Google Scholar] [CrossRef]
- Wang, H.H.; Hou, J.J.; Li, Y.; Zhang, Y.Y.; Huang, J.J.; Liang, W.H. Nitric oxide-mediated cytosolic glucose-6-phosphate dehydrogenase is involved in aluminum toxicity of soybean under high aluminum concentration. Plant Soil 2017, 416, 39–52. [Google Scholar] [CrossRef]
- Giraud, E.; Ho, L.H.M.; Clifton, R.; Carroll, A.; Estavillo, G.; Tan, Y.F.; Howell, K.A.; Ivanova, A.; Pogson, B.J.; Millar, A.H.; et al. The absence of alternative oxidase1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol. 2008, 147, 595–610. [Google Scholar] [CrossRef]
- Zhang, F.Q.; Zhang, H.X.; Wang, G.P.; Xu, L.L.; Shen, Z.G. Cadmium-induced accumulation of hydrogen peroxide in the leaf apoplast of Phaseolus aureus and Vicia sativa and the roles of different antioxidant enzymes. J. Hazard. Mater. 2009, 168, 76–84. [Google Scholar] [CrossRef]
- Perez-Chaca, M.V.; Rodriguez-Serrano, M.; Molina, A.S.; Pedranzani, H.E.; Zirulnik, F.; Sandalio, L.M.; Romero-Puertas, M.C. Cadmium induces two waves of reactive oxygen species in Glycine max (L.) roots. Plant Cell Environ. 2014, 37, 1672–1681. [Google Scholar] [CrossRef]
- Liu, S.L.; Yang, R.J.; Tripathi, D.K.; Li, X.; Jiang, M.Y.; Lv, B.Y.; Ma, M.D.; Chen, Q.B. Signalling cross-talk between nitric oxide and active oxygen in Trifolium repens L. plants responses to cadmium stress. Environ. Pollut. 2018, 239, 53–68. [Google Scholar] [CrossRef]
- Planchet, E.; Gupta, K.J.; Sonoda, M.; Kaiser, W.M. Nitric oxide emission from tobacco leaves and cell suspensions: Rate limiting factors and evidence for the involvement of mitochondrial electron transport. Plant J. 2005, 41, 732–743. [Google Scholar] [CrossRef]
- Gupta, K.J.; Fernie, A.R.; Kaiser, W.M.; Dongen, J.T. On the origins of nitric oxide. Trends Plant Sci. 2010, 16, 160–168. [Google Scholar] [CrossRef]
- Cvetkovska, M.; Vanlerberghe, G.C. Alternative oxidase impacts the plant response to biotic stress by influencing the mitochondrial generation of reactive oxygen species. Plant Cell Environ. 2013, 36, 721–732. [Google Scholar] [CrossRef]
- Wanniarachchi, V.; Dametto, L.; Sweetman, C.; Shavrukov, Y.; Day, D.; Jenkins, C.; Soole, K. Alternative respiratory pathway component genes(AOX and ND) in rice and barley and their response to stress. Int. J. Mol. Sci. 2018, 19, 915. [Google Scholar] [CrossRef]
- Feng, H.Q.; Li, X.; Duan, J.G.; Li, H.Y.; Liang, H.G. Chilling tolerance of wheat seedlings is related to an enhanced alternative respiratory pathway. Crop Sci. 2008, 48, 2381–2388. [Google Scholar] [CrossRef]
- Cvetkovska, M.; Vanlerberghe, G.C. Alternative oxidase modulates leaf mitochondrial concentrations of superoxide and nitric oxide. New Phytol. 2012, 195, 32–39. [Google Scholar] [CrossRef]
- Shukla, P.; Singh, S.; Dubey, P.; Singh, A.; Singh, A.K. Nitric oxide mediated amelioration of arsenic toxicity which alters the alternative oxidase (Aox1) gene expression in Hordeum vulgare L. Ecotoxicol. Environ. Saf. 2015, 120, 59–65. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, R.; Wan, Q.; Xie, G.; Bi, Y. Glucose-6-phosphate dehydrogenase plays a pivotal role in nitric oxide-involved defense against oxidative stress under salt stress in red kidney bean roots. Plant Cell Physiol. 2007, 48, 511–522. [Google Scholar] [CrossRef]
- Zhao, M.G.; Chen, L.; Zhang, L.L.; Zhang, W.H. Nitric reductase dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol. 2009, 151, 755–767. [Google Scholar] [CrossRef]
- Hu, Y.; You, J.; Liang, X. Nitrate reductase-mediated nitric oxide production is involved in copper tolerance in shoots of hulless barley. Plant Cell Rep. 2015, 34, 367–379. [Google Scholar] [CrossRef]
- Zhao, M.G.; Tian, Q.Y.; Zhang, W.H. Nitric oxide synthase dependent nitric oxide production is associated with salt tolerance in Arabidopsis. Plant Physiol. 2007, 144, 206–217. [Google Scholar] [CrossRef]
- Peksen, S.; Sanal, F. Arsenic effects on lipid peroxidation and antioxidative enzyme activities in barley cultivars roots. Fresenius Environ. Bull. 2018, 27, 5871–5881. [Google Scholar]
- Chen, F.; Wang, F.; Sun, H.Y.; Cai, Y.; Mao, W.H.; Zhang, G.P.; Vincze, E. Genotype-dependent effect of exogenous nitric oxide on Cd-induced changes in antioxidative metabolism, ultrastructure, and photosynthetic performance in barley seedlings (Hordeum vulgare). J. Plant Growth Regul. 2010, 29, 394–408. [Google Scholar] [CrossRef]
- Wang, X.J.; Ma, R.N.; Cui, D.J.; Cao, Q.; Shan, Z.; Jiao, Z. Physio-biochemical and molecular mechanism underlying the enhanced heavy metal tolerance in highland barley seedlings pretreated with low-dose gamma irradiation. Sci. Rep. 2017, 7, 14233. [Google Scholar] [CrossRef]
- Wang, Z.F.; Li, Q.; Wu, W.G.; Guo, J.; Yang, Y.L. Cadmium stress tolerance in wheat seedlings induced by ascorbic acid was mediated by NO signaling pathways. Ecotoxicol. Environ. Saf. 2017, 135, 75–81. [Google Scholar] [CrossRef]
- Qian, H.F.; Chen, W.; Li, J.J.; Wang, J.; Zhou, Z.; Liu, W.P.; Fu, Z.W. The effect of exogenous nitric oxide on alleviating herbicide damage in Chlorella vulgaris. Aquat. Toxicol. 2009, 92, 250–257. [Google Scholar] [CrossRef]
- Amirsadeghi, S.; Robson, C.A.; McDonald, A.E.; Vanlerberghe, G.C. Changes in plant mitochondrial electron transport alter cellular levels of reactive oxygen species and susceptibility to cell death signaling molecules. Plant Cell Physiol. 2006, 47, 1509–1519. [Google Scholar] [CrossRef]
- Millenaar, F.F.; Gonzalez-Meler, M.A.; Fiorani, F.; Welschen, R.; Ribas-Carbo, M.; Siedow, J.N.; Anneke, M.; Wagner, A.M.; Lambers, H. Regulation of alternative oxidase activity in six wild monocotyledonous species. An in vivo study at the whole root level. Plant Physiol. 2001, 126, 376–387. [Google Scholar] [CrossRef]
- Ribas-Carbo, M.; Berry, J.A.; Yakir, D.; Giles, L.; Robinson, S.A.; Lennon, A.M.; Siedow, J.N. Electron Partitioning between the Cytochrome and Alternative Pathways in Plant Mitochondria. Plant Physiol. 1995, 109, 829–837. [Google Scholar] [CrossRef]

| Primer Name | Primer Sequence (5′—3′) |
|---|---|
| Hvactin-F | GTGGTCGTACAACWGGTATTGTG |
| Hvactin-R | GCTCATCAAATCCAAACACTG |
| ORFHvAOX1a-F | GCAACGAACCTACAAGCGTG |
| ORFHvAOX1a-R | GCTAAAGAGCCCTCATTTCCTC |
| ORFHvAOX1d1-F | CCTCCCATTAGCTTTTCGACCAG |
| ORFHvAOX1d1-R | CGGTAGCACGTAACAGCGTGGACT |
| ORFHvAOX1d2-F | TACGACCACGAGTTTCGCGAGCA |
| ORFHvAOX1d2-R | GCTAAAGAGCCCTCATTTCCTC |
| qHvAOX1a-F | GCAACGAACCTACAAGCGTG |
| qHvAOX1a-R | AAGAGCCCAGCACCAACAA |
| qHvAOX1d1-F | CCTCCCATTAGCTTTTCGACCAG |
| qHvAOX1d1-R | CGGTAGCACGTAACAGCGTGGACT |
| qHvAOX1d2-F | TACGACCACGAGTTTCGCGAGCA |
| qHvAOX1d2-R | GCTAAAGAGCCCTCATTTCCTC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Wang, X.; Feng, R.; He, Q.; Wang, S.; Liang, C.; Yan, L.; Bi, Y. Alternative Pathway is Involved in Nitric Oxide-Enhanced Tolerance to Cadmium Stress in Barley Roots. Plants 2019, 8, 557. https://doi.org/10.3390/plants8120557
He L, Wang X, Feng R, He Q, Wang S, Liang C, Yan L, Bi Y. Alternative Pathway is Involved in Nitric Oxide-Enhanced Tolerance to Cadmium Stress in Barley Roots. Plants. 2019; 8(12):557. https://doi.org/10.3390/plants8120557
Chicago/Turabian StyleHe, Li, Xiaomin Wang, Ruijun Feng, Qiang He, Shengwang Wang, Cuifang Liang, Lili Yan, and Yurong Bi. 2019. "Alternative Pathway is Involved in Nitric Oxide-Enhanced Tolerance to Cadmium Stress in Barley Roots" Plants 8, no. 12: 557. https://doi.org/10.3390/plants8120557
APA StyleHe, L., Wang, X., Feng, R., He, Q., Wang, S., Liang, C., Yan, L., & Bi, Y. (2019). Alternative Pathway is Involved in Nitric Oxide-Enhanced Tolerance to Cadmium Stress in Barley Roots. Plants, 8(12), 557. https://doi.org/10.3390/plants8120557





