Effects of Bactericera cockerelli Herbivory on Volatile Emissions of Three Varieties of Solanum lycopersicum
Abstract
1. Introduction
2. Results
2.1. Volatile Organic Compounds Profile in Healthy Plants
2.2. Volatile Organic Compounds Released in Infested Plants with Bactericera cockerelli
3. Discussion
4. Materials and Methods
4.1. Plant Growth
4.2. Insect Rearing
4.3. Headspace Collection and Analysis of Plant Volatiles
4.4. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Vos, M.; Van Oosten, V.R.; Van Poecke, R.M.P.; Van Pelt, J.A.; Pozo, M.J.; Mueller, M.J.; Buchala, A.J.; Metraux, J.P.; Van Loon, L.C.; Dicke, M.; et al. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol. Plant Microbe Interact. 2005, 18, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Zarate, S.I.; Kempema, L.A.; Walling, L.L. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007, 143, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Baldwin, I.T. Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 2002, 53, 299–328. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Erb, M. Volatiles as inducers and suppressors of plant defense and immunity-origins, specificity, perception and signaling. Curr. Opin. Plant Biol. 2018, 44, 117–121. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, J.; Sun, H.; Salse, J.; Lucas, W.J.; Zhang, H.; Zheng, Y.; Mao, L.; Ren, Y.; Wang, Z.; et al. The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nat. Genet. 2013, 45, 51–58. [Google Scholar] [CrossRef]
- Lundgren, L.; Norelius, C.; Stenhagen, G. Leaf volatiles from some wild tomato species. Nord. J. Bot. 1985, 5, 315–320. [Google Scholar] [CrossRef]
- Li, X.; Garvey, M.; Kaplan, I.; Li, B.; Carrillo, J. Domestication of tomato has reduced the attraction of herbivore natural enemies to pest-damaged plants. Agric For. Entomol. 2017, 20, 390–401. [Google Scholar] [CrossRef]
- Bleeker, P.M.; Diergaarde, P.J.; Ament, K.; Guerra, J.; Weidner, M.; Schütz, S.; de Both, M.T.J.; Haring, M.A.; Schuurink, R.C. The role of specific tomato volatiles in tomato-whitefly interaction. Plant Physiol. 2009, 151, 925–935. [Google Scholar] [CrossRef]
- Peralta, I.E.; Spooner, D. History, origin and early cultivation of tomato (Solanaceae). In Genetic Improvement of Solanaceous Crops; Razdan, M.K., Mattoo, A.K., Eds.; Science Publishers: Enfield, NH, USA, 2007; pp. 1–27. [Google Scholar]
- Larry, R.; Joanne, L.; Razdan, M.K.; Mattoo, A.K. Genetic Resources of Tomato (Lycopersicon esculentum Mill) and Wild Relatives. In Genetic Improvement of Solanaceous Crops; Science Publishers: Enfield, NH, USA, 2007. [Google Scholar]
- Dan, Y.; Fei, Z.; Rothan, C. MicroTom-A new model system for plant genomics. 3G-Genes Genom. Genet. 2007, 1, 167–179. [Google Scholar]
- Shikata, M.; Hoshikawa, K.; Ariizumi, T.; Fukuda, N.; Yamazaki, Y.; Ezura, H. TOMATOMA update: Phenotypic and metabolite information in the Micro-Tom mutant resource. Plant Cell Physiol. 2016, 57, e11. [Google Scholar] [CrossRef] [PubMed]
- Aflitos, S.; Schijlen, E.; de Jong, H.; de Ridder, D.; Smit, S.; Finkers, R.; Wang, J.; Zhang, G.; Li, N.; Mao, L.; et al. E×ploring genetic variation in the tomato (Solanum section Lycopersicon) clade by whole-genome sequencing. Plant J. 2014, 80, 136–148. [Google Scholar] [PubMed]
- Foolad, M.R. Genome mapping and molecular breeding of tomato. Int. J. Plant Genom. 2007, 2007, 64358. [Google Scholar] [CrossRef] [PubMed]
- List, G.M. The potato and tomato psyllid and its control on tomatoes. In Bulletin–Colorado Agricultural Experimental Station; Russell, M.J., Ed.; Colorado State University: Fort Collins, CO, USA, 1939; pp. 3–33. [Google Scholar]
- Pletsch, D.J. The Potato Psyllid Paratrioza Cockerelli (Sulc.) its Biology and Control; Montana Agricultural Experiment Station Bulletin: Bozeman, MT, USA, 1947; p. 95. [Google Scholar]
- Wallis, R.L. Ecological Studies on the Potato Psyllid as a Pest of Potatoes; USDA Technical Bulletin: Washington, DC, USA, 1955; p. 25. [Google Scholar]
- Munyaneza, J.E. Zebra chip disease of potato: Biology, epidemiology, and management. Am. J. Potato Res. 2012, 89, 329–350. [Google Scholar] [CrossRef]
- CNAS. Economic Impacts of Zebra Chip on the Texas Potato Industry; Texas A and M University, Center for North American Studies: College Station, TX, USA, 2006. [Google Scholar]
- Huot, O.B.; Levy, J.G.; Tamborindeguy, C. Global gene regulation in tomato plant (Solanum lycopersicum) responding to vector (Bactericera cockerelli) feeding and pathogen (‘Candidatus Liberibacter solanacearum’) infection. Plant Mol. Biol. 2018, 97, 57–72. [Google Scholar] [CrossRef]
- Casteel, C.L.; Hansen, A.K.; Walling, L.L.; Paine, T.D. Manipulation of plant defense responses by the tomato psyllid (Bactericera cockerelli) and its associated endosymbiont Candidatus Liberibacter psyllaurous. PLoS ONE 2012, 7, e35191. [Google Scholar] [CrossRef]
- Prager, S.M.; Esquivel, I.; Trumble, J.T. Factors influencing host plant choice and larval performance in Bactericera cockerelli. PLoS ONE 2014, 9, e94047. [Google Scholar] [CrossRef]
- Bautista-Lozada, A.; Espinosa-García, F.J. Odor uniformity among tomato individuals in response to herbivore depends on insect species. PLoS ONE 2013, 8, e77199. [Google Scholar] [CrossRef]
- Mayo-Hernández, J.; Flores-Olivas, A.; Valenzuela-Soto, J.H.; Rodríguez-Pagaza, Y.; Vega-Chávez, J.; Hernández-Castillo, F.; Aguirre-Uribe, L. Bactericera cockerelli Sulc oviposition preference and development on three tomato varieties. Southwest Entomol. 2018, 43, 905–910. [Google Scholar] [CrossRef]
- Thinakaran, J.; Pierson, E.A.; Longnecker, M.; Tamborindeguy, C.; Munyaneza, J.E.; Rush, C.M.; Henne, D.C. Settling and ovipositional behavior of Bactericera cockerelli (Hemiptera: Triozidae) on solanaceous hosts under field and laboratory conditions. J. Econ. Entomol. 2015, 108, 904–916. [Google Scholar] [CrossRef]
- Casteel, C.L.; Walling, L.L.; Paine, T.D. Behavior and biology of the tomato psyllid, Bactericera cockerelli, in response to the Mi-12 gene. Entomol. Exp. Appl. 2006, 121, 67–72. [Google Scholar] [CrossRef]
- Yarou, B.B.; Bawin, T.; Boullis, A.; Heukin, S.; Lognay, G.; Verheggen, F.J.; Francis, F. Oviposition deterrent activity of basil plants and their essentials oils against Tuta absoluta (Lepidoptera: Gelechiidae). Environ. Sci. Pollut. Res. 2018, 25, 29880–29888. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Liu, G.; Shi, F.; Jones, A.D.; Beaudry, R.M.; Howe, G.A. Trichome-based production of diverse specialized metabolites and broad-spectrum resistance to insect herbivores. Plant Physiol. 2010, 154, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Prager, S.M.; Wallis, C.M.; Jones, M.; Novy, R.; Trumble, J.T. E×amining the potential role of foliar chemistry in imparting potato germplasm tolerance to potato psyllid, green peach aphid, and zebra chip disease. J. Econ. Entomol. 2018, 111, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Sengoda, V.G.; Cooper, W.R.; Swisher, K.D.; Henne, D.C.; Munyaneza, J.E. Latent period and transmission of “Candidatus Liberibacter solanacearum” by the potato psyllid Bactericera cockerelli (Hemiptera: Triozidae). PLoS ONE 2014, 9, e93475. [Google Scholar] [CrossRef] [PubMed]
- Mas, F.; Vereijssen, J.; Suckling, D.M. Influence of the pathogen Candidatus liberibacter solanacearum on tomato host plant volatiles and psyllid vector settlement. J. Chem. Ecol. 2014, 40, 1197–1202. [Google Scholar] [CrossRef]
- Mustafa, T.; Horton, D.R.; Swisher, K.D.; Zack, R.S.; Munyaneza, J.E. Effects of host plant on development and body size of three haplotypes of Bactericera cockerelli (Hemiptera: Triozidae). Environ. Entomol. 2015, 44, 593–600. [Google Scholar] [CrossRef]

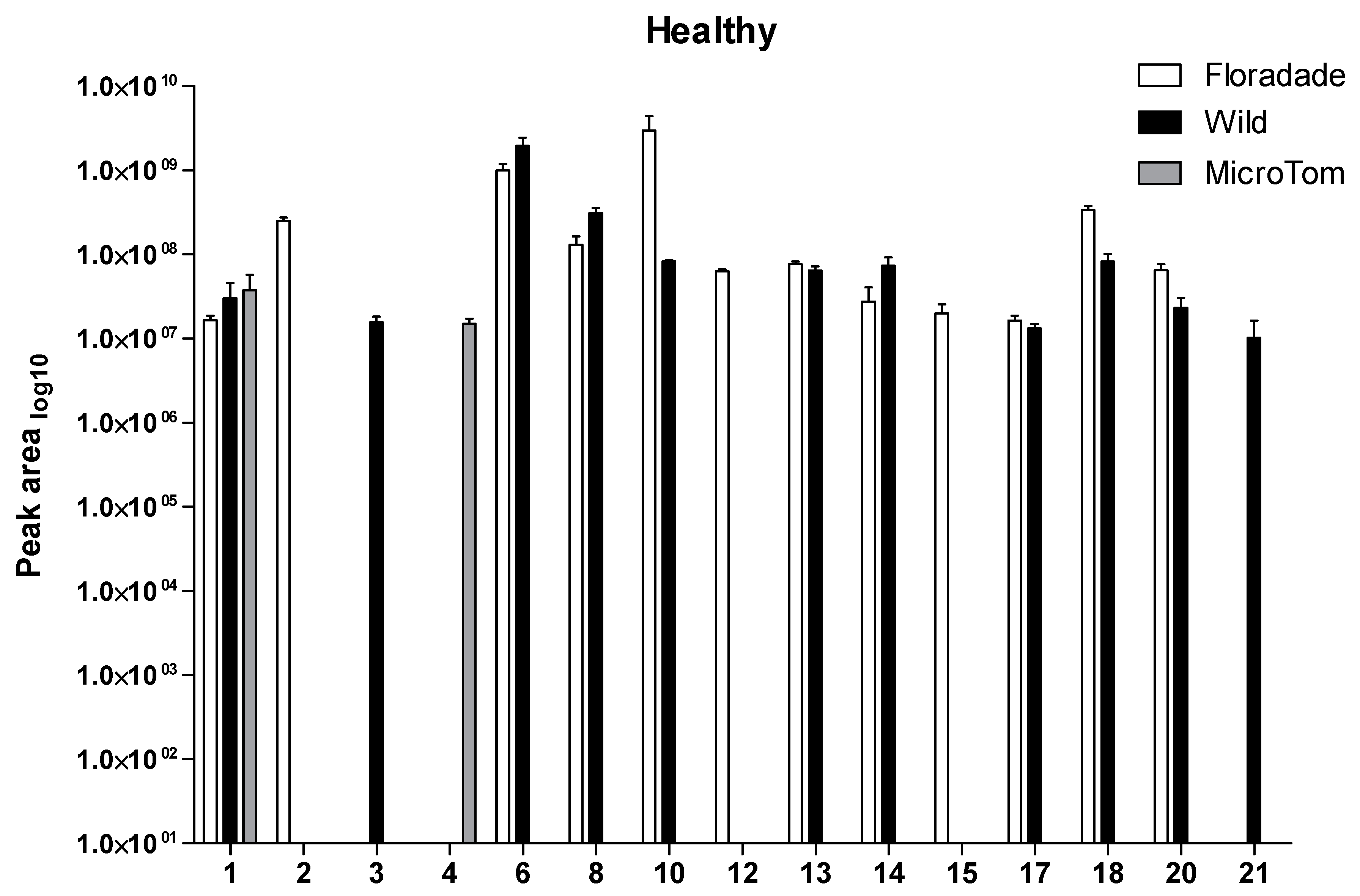
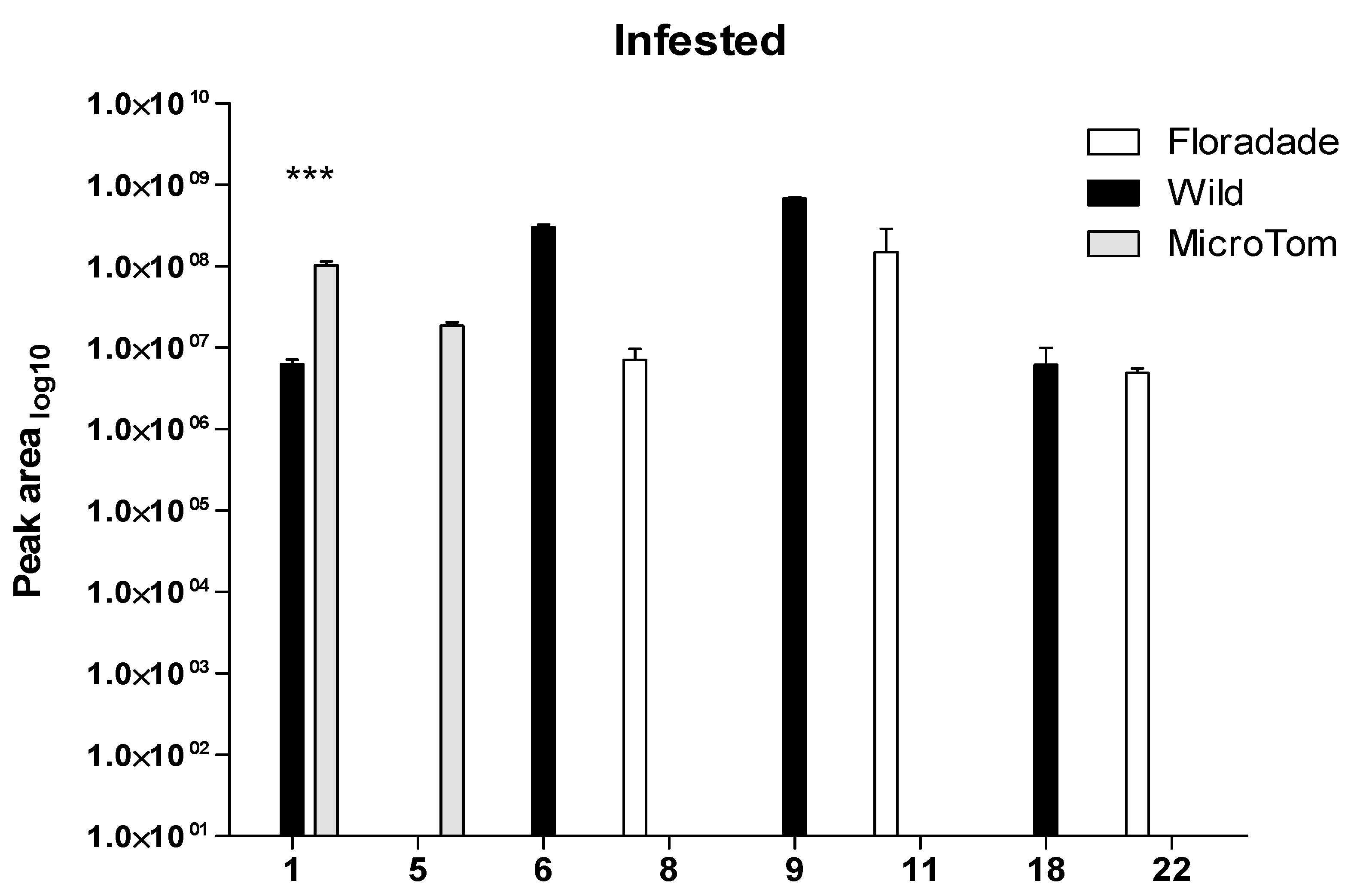

| RT | Wild | Floradade | Micro-Tom | |||||
|---|---|---|---|---|---|---|---|---|
| Compound | (min) | Healthy | Infested | Healthy | Infested | Healthy | Infested | |
| 1 | α-Pinene | 4.568 | 7.28 ± 0.46 | 6.78 ± 0.09 | 7.21 ± 0.09 | - | 7.43 ± 0.36 | 8.00 ± 0.07 |
| 2 | o-Cymene | 5.157 | - | - | 8.39 ± 0.06 | 6.96 | - | - |
| 3 | 1,3,5-Cycloheptatriene, 3,7,7-trimethyl- | 5.232 | 7.18 ± 0.11 | 6.47 | - | - | - | - |
| 4 | l-β-Pinene | 5.275 | - | - | - | - | 7.17 ± 0.07 | - |
| 5 | β-Pinene | 5.344 | - | - | - | - | 6.53 | 7.26 ± 0.06 |
| 6 | (+)-4-Carene | 5.615 | 9.26 ± 0.18 | 8.48 ± 0.03 | 8.97 ± 0.13 | 7.66 | 6.98 | - |
| 7 | α-Thujene | 5.692 | - | - | 8.78 | 8.46 | - | - |
| 8 | (+)-2-Carene | 5.771 | 8.49 ± 0.06 | 7.25 | 8.10 ± 0.11 | 6.81 ± 0.17 | - | - |
| 9 | β-Phellandrene | 6.166 | 9.55 | 8.83 ± 0.01 | - | - | - | - |
| 10 | ɣ-Terpinene | 6.193 | 7.92 ± 0.02 | 7.63 | 9.02 ± 0.88 | 6.48 | - | - |
| 11 | (+)-Sabinene | 6.413 | - | 7.94 | 6.89 | 7.67 ± 0.79 | - | - |
| 12 | α-Terpinolene | 7.224 | - | 7.69 | 7.79 ± 0.03 | - | - | - |
| 13 | Ascaridole | 10.463 | 7.80 ± 0.05 | - | 7.88 ± 0.03 | - | - | - |
| 14 | δ-Elemene | 11.523 | 7.83 ± 0.16 | 7.18 | 7.29 ± 0.39 | 6.78 | - | - |
| 15 | Copaene | 12.207 | - | - | 7.26 ± 0.17 | - | - | - |
| 16 | (−)-cis-β-Elemene | 12.452 | 7.07 | - | 7.41 | - | - | - |
| 17 | Isocaryophyllene | 12.715 | 7.12 ± 0.05 | - | 7.21 ± 0.06 | - | - | - |
| 18 | Caryophyllene | 12.914 | 7.89 ± 0.13 | 6.61 ± 0.37 | 8.52 ± 0.06 | 7.50 | - | 6.63 |
| 19 | Bicyclo[7.2.0]undecane, 10,10-dimethyl-2,6-bis(methylene)-, [1S-(1R*,9S*)]- | 13.065 | 7.25 | - | 7.30 | - | - | - |
| 20 | Humulene | 13.468 | 7.32 ± 0.19 | 6.60 | 7.79 ± 0.12 | 6.85 | - | - |
| 21 | Hexadecane | 15.827 | 6.91 ± 0.30 | - | - | - | - | - |
| 22 | Phenol, 2,6-bis(1,1-dimethylethyl)-4-(1-ethylpropyl)- | 16.142 | - | - | 6.48 | 6.68 ± 0.06 | - | - |
| Number of compounds | 14 | 11 | 17 | 10 | 4 | 3 | ||
| # | Compound | Wild | Floradade | Micro-Tom | P Value | F Value | Df |
|---|---|---|---|---|---|---|---|
| 1 | α-Pinene | 7.287 ± 0.46 | 7.21 ± 0.09 | 7.437 ± 0.36 | 0.8021 (ns) | 0.2288 | 2 |
| 6 | (+)-4-Carene | 9.260 ± 0.18 | 8.979 ± 0.13 | - | 0.1501 (ns) | 3.1594 | 1 |
| 8 | (+)-2-Carene | 8.490 ± 0.06 | 8.100 ± 0.11 | - | 0.0955 (ns) | 8.9951 | 1 |
| 10 | ɣ-Terpinene | 7.922 ± 0.02 | 9.026 ± 0.88 | - | 0.1493 (ns) | 3.1757 | 1 |
| 13 | Ascaridole | 7.807 ± 0.05 | 7.883 ± 0.03 | - | 0.3418 (ns) | 1.5289 | 1 |
| 14 | δ-Elemene | 7.838 ± 0.16 | 7.296 ± 0.39 | - | 0.1420 (ns) | 3.331 | 1 |
| 17 | Isocaryophyllene | 7.121 ± 0.05 | 7.212 ± 0.06 | - | 0.3764 (ns) | 1.2728 | 1 |
| 18 | Caryophyllene | 7.893 ± 0.13 | 8.529 ± 0.06 | - | 0.0037 ** | 36.783 | 1 |
| 20 | Humulene | 7.323 ± 0.19 | 7.797 ± 0.12 | - | 0.0386 * | 9.1983 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayo-Hernández, J.; Ramírez-Chávez, E.; Molina-Torres, J.; Guillén-Cisneros, M.d.L.; Rodríguez-Herrera, R.; Hernández-Castillo, F.; Flores-Olivas, A.; Valenzuela-Soto, J.H. Effects of Bactericera cockerelli Herbivory on Volatile Emissions of Three Varieties of Solanum lycopersicum. Plants 2019, 8, 509. https://doi.org/10.3390/plants8110509
Mayo-Hernández J, Ramírez-Chávez E, Molina-Torres J, Guillén-Cisneros MdL, Rodríguez-Herrera R, Hernández-Castillo F, Flores-Olivas A, Valenzuela-Soto JH. Effects of Bactericera cockerelli Herbivory on Volatile Emissions of Three Varieties of Solanum lycopersicum. Plants. 2019; 8(11):509. https://doi.org/10.3390/plants8110509
Chicago/Turabian StyleMayo-Hernández, Juan, Enrique Ramírez-Chávez, Jorge Molina-Torres, María de Lourdes Guillén-Cisneros, Raúl Rodríguez-Herrera, Francisco Hernández-Castillo, Alberto Flores-Olivas, and José Humberto Valenzuela-Soto. 2019. "Effects of Bactericera cockerelli Herbivory on Volatile Emissions of Three Varieties of Solanum lycopersicum" Plants 8, no. 11: 509. https://doi.org/10.3390/plants8110509
APA StyleMayo-Hernández, J., Ramírez-Chávez, E., Molina-Torres, J., Guillén-Cisneros, M. d. L., Rodríguez-Herrera, R., Hernández-Castillo, F., Flores-Olivas, A., & Valenzuela-Soto, J. H. (2019). Effects of Bactericera cockerelli Herbivory on Volatile Emissions of Three Varieties of Solanum lycopersicum. Plants, 8(11), 509. https://doi.org/10.3390/plants8110509





