Floral Organogenesis in Three Members of the Tribe Delphinieae (Ranunculaceae)
Abstract
1. Introduction
2. Results
2.1. Aconitum Taipeicum
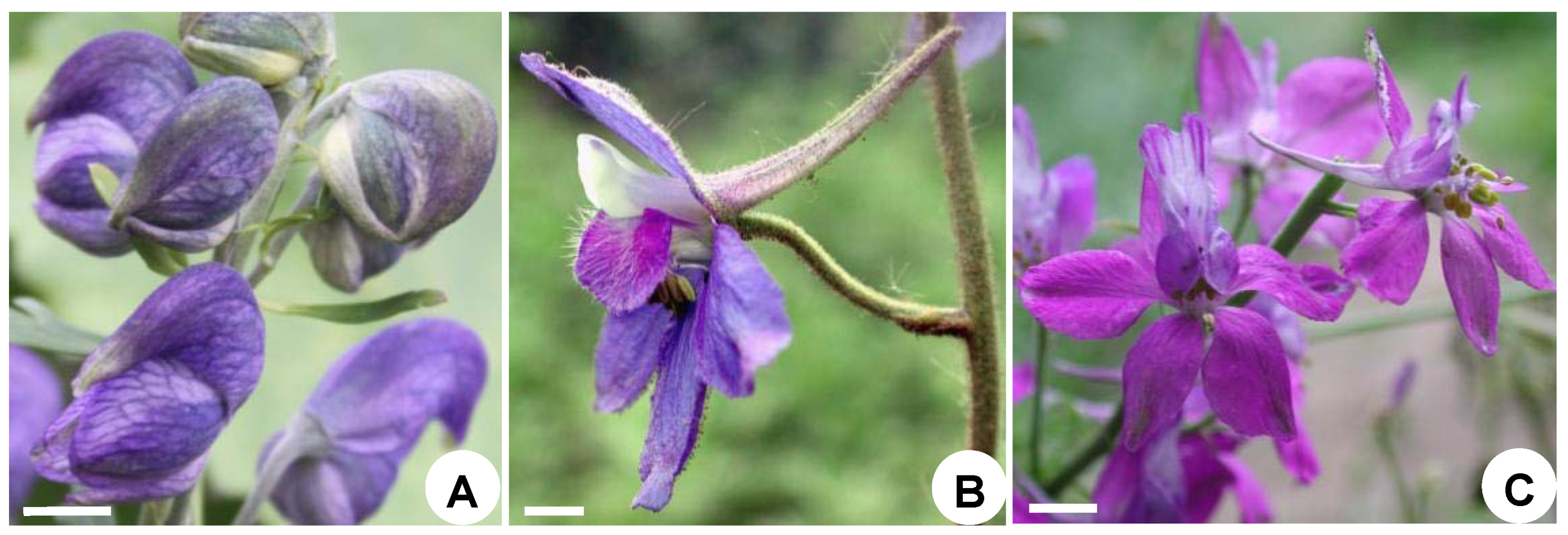
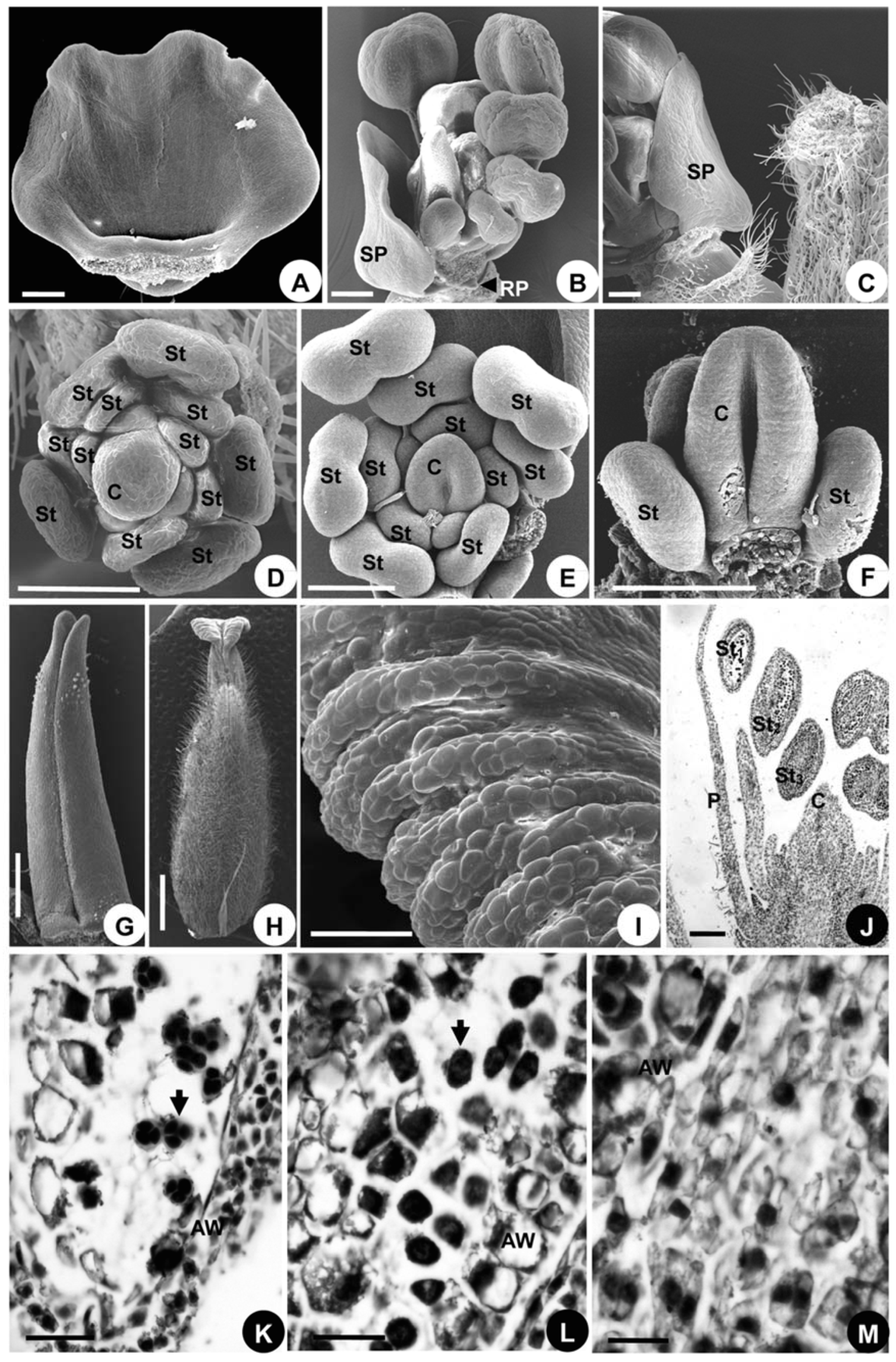
2.1.1. Flowers at Anthesis
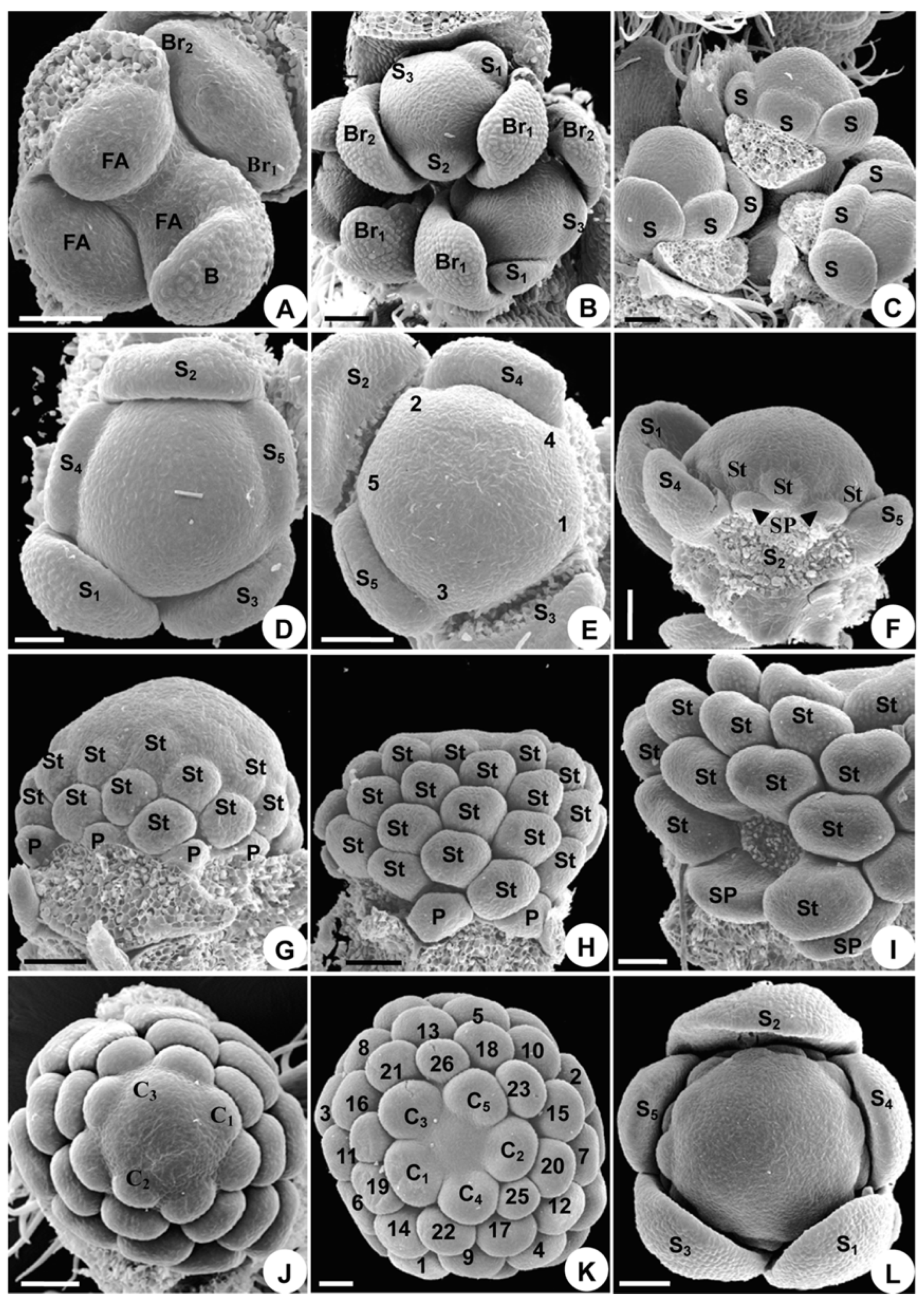
2.1.2. Organ Initiation
2.1.3. Organ Development
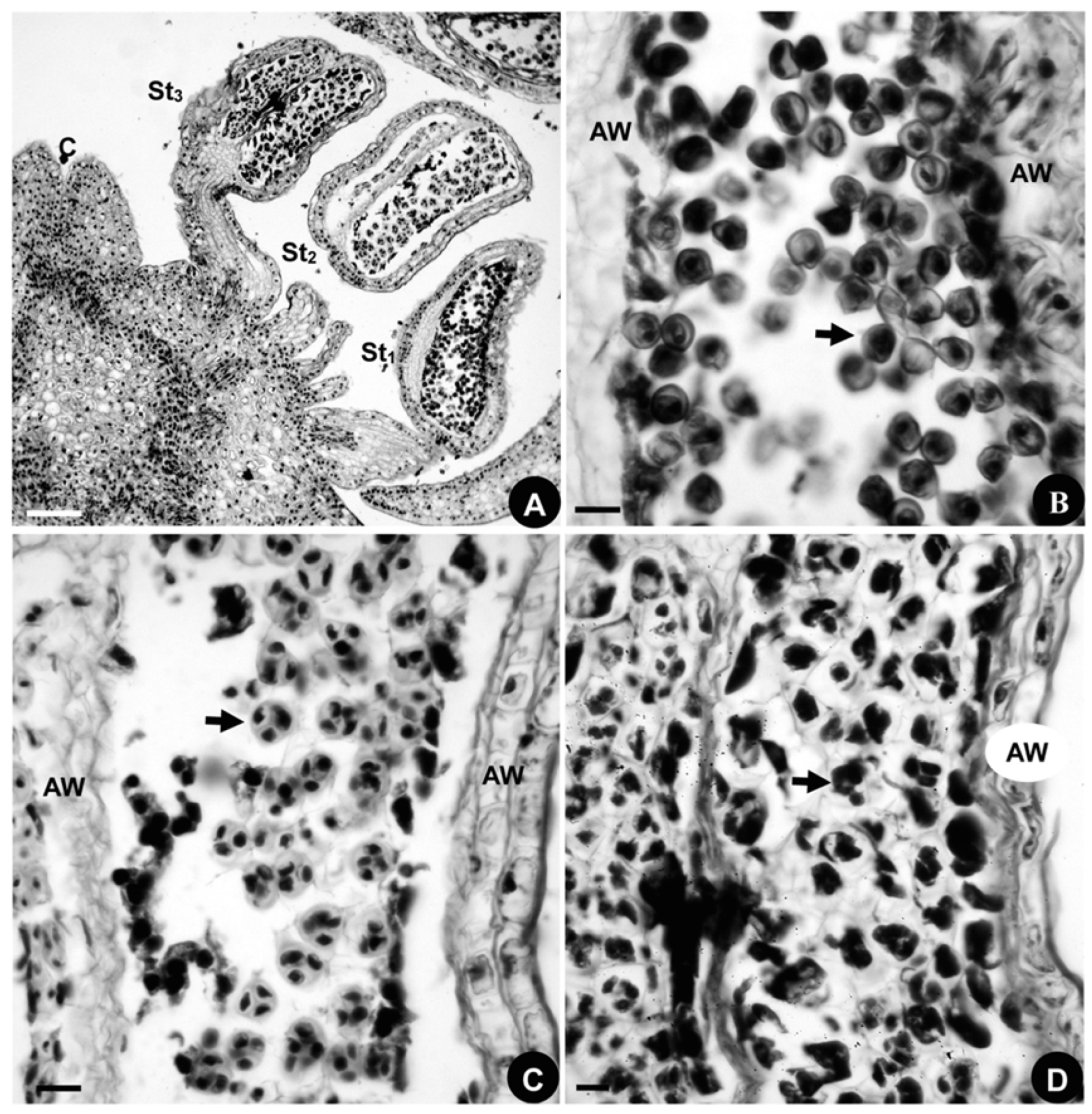
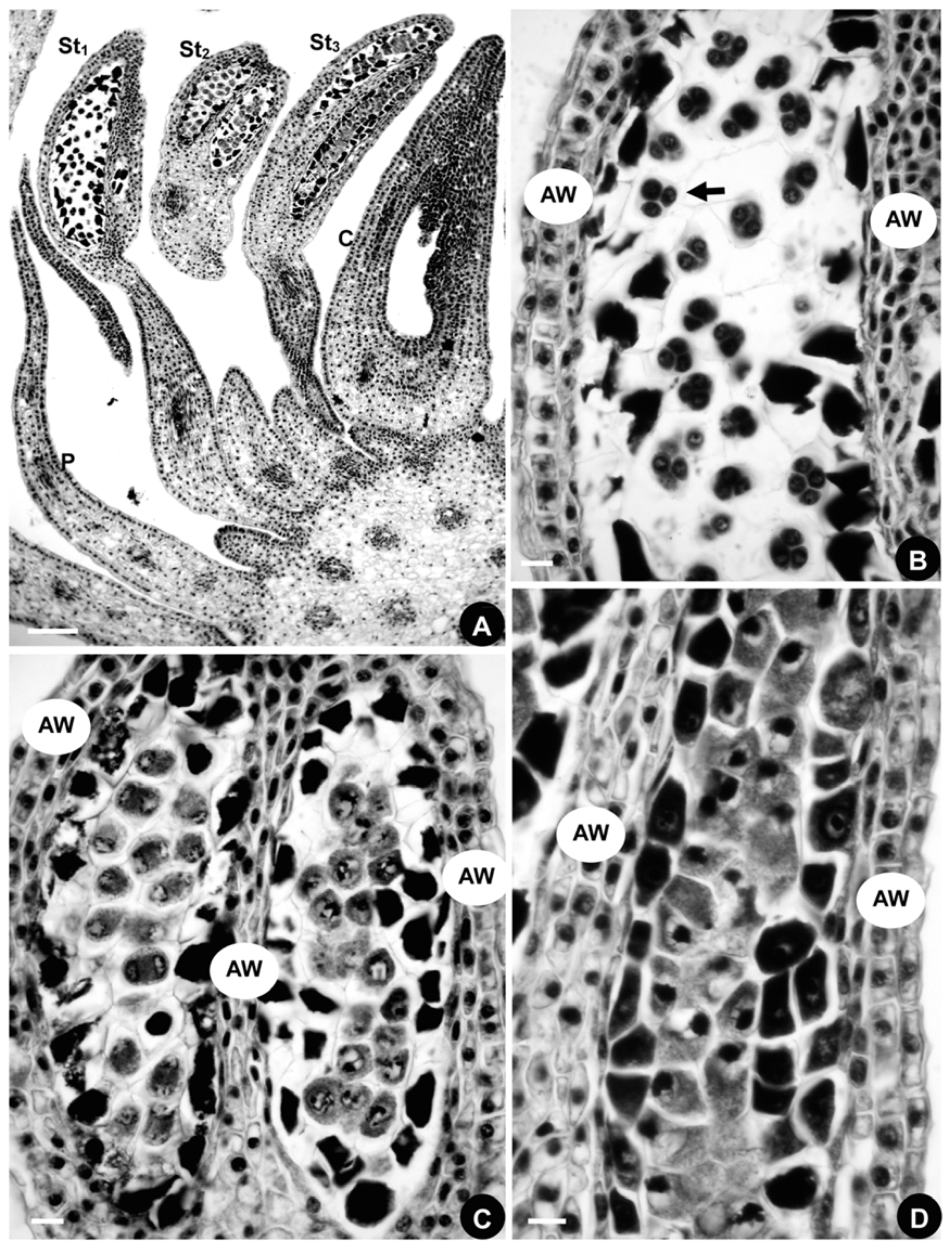
2.1.4. Developmental Sequence of Microspores
2.2. Delphinium Giraldii
2.2.1. Flowers at Anthesis
2.2.2. Organ Initiation
2.2.3. Organ Development
2.2.4. Developmental Sequence of Microspores
2.3. Consolida Ajacis
2.3.1. Flowers at Anthesis
2.3.2. Organ Initiation
2.3.3. Organ Development
2.3.4. Developmental Sequence of Microspores
3. Discussion
3.1. Floral Ontogenesis Comparisons
3.2. Comparison of Tribe Delphinieae with Other Genera of Ranunculaceae
3.3. Comparison of the Tribe Delphinieae with Nigella
3.4. Developmental Sequences of Stamens and Microspores
3.5. Floral Symmetry
4. Materials and Methods
4.1. Species Examined
4.2. Floral Organogenesis and Histological Studies
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Staedler, Y.M.; Weston, P.H.; Endress, P.K. Floral phyllotaxis and floral architecture in Calycanthaceae (Laurales). Int. J. Plant Sci. 2007, 168, 285–306. [Google Scholar] [CrossRef]
- Rasmussen, D.A.; Kramer, E.M.; Zimmer, E.A. One size fits all? Molecular evidence for a commonly inherited petal identity program in Ranunculaceae. Am. J. Bot. 2009, 96, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Kosuge, K.; Tamura, M. Morphology of the petal in Aconitum. Bot. Mag. Tokyo 1988, 101, 223–237. [Google Scholar] [CrossRef]
- Kosuge, K.; Tamura, M. Ontogenetic studies on petals of the Ranunculaceae. J. Jpn. Bot. 1989, 64, 65–74. [Google Scholar]
- Jabbour, F.; Ronse De Craene, L.P.; Nadot, S.; Damerval, C. Establishment of zygomorphy on an ontogenic spiral and evolution of perianth in the tribe Delphinieae (Ranunculaceae). Ann. Bot. 2009, 104, 809–822. [Google Scholar] [CrossRef]
- Leppik, E.E. Floral evolution in the Ranunculaceae. IOWA State Coll. J. Sci. 1964, 39, 1–101. [Google Scholar]
- Hoot, S.B. Phylogeny of the Ranunculaceae based on epidermal microcharacters and macromorphology. Syst. Bot. 1991, 16, 741–755. [Google Scholar] [CrossRef]
- Erbar, C.; Kusma, S.; Leins, P. Development and interpretation of nectary organs in Ranunculaceae. Flora 1998, 194, 317–332. [Google Scholar] [CrossRef]
- Prantl, K. Beiträge zur Morphologie und Systematik der Ranunculaceen. Bot. Jahrb. Syst. 1887, 9, 225–273. [Google Scholar]
- Weberling, F. Morphology of Flowers and Inflorescences; Cambridge University Press: London, UK, 1989. [Google Scholar]
- Tamura, M. Ranunculaceae. In The Families and Genera of Vascular Plants Flowering Plants—Dicotyledons; Kubitzki, K., Rohwer, J.G., Bittrich, V., Eds.; Springer: Berlin, Germany, 1993; Volume 2, pp. 563–583. [Google Scholar]
- Ronse De Craene, L.P.; Smets, E.F. The distribution and systematc relevance of the androecial character oligomery. Bot. J. Linn. Soc. 1995, 118, 193–247. [Google Scholar]
- Endress, P.K. Floral structure and evolution in Ranunculanae. Plant Syst. Evol. 1995, 9, 47–61. [Google Scholar]
- Hiepko, P. Vergleichend-morphologische und entwicklungsgeschichtliche Untersuchungen über das Perianth bei den Polycarpicae. Bot. Jahrb. Syst. 1965, 84, 427–508. [Google Scholar]
- Tamura, M. Morphology, ecology and phylogeny of the Ranunculaceae IV. Sci. Rep. Osaka Univ. 1965, 14, 53–71. [Google Scholar]
- Jabbour, F.; Renner, S.S. Resurrection of the genus Staphisagria J. Hill, sister to all the other Delphinieae (Ranunculaceae). Phytokeys 2011, 7, 21–26. [Google Scholar] [CrossRef]
- Jabbour, F.; Renner, S.S. Spurs in a spur: Perianth evolution in the Delphinieae (Ranunculaceae). Int. J. Plant Sci. 2012, 173, 1036–1054. [Google Scholar] [CrossRef]
- Jabbour, F.; Cossard, G.; Le Guilloux, M.; Sannier, J.; Nadot, S.; Damerval, C. Specific duplication and dorsoventrally asymmetric expression patterns of Cycloidea-like genes in zygomorphic species of Ranunculaceae. PLoS ONE 2014, 9, e95727. [Google Scholar] [CrossRef]
- Liangqian, L.; Kadota, Y. Aconitum L. In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden: St. Louis, MO, USA, 2001; Volume 6, pp. 149–222. [Google Scholar]
- Wang, W.T.; Warnock, M.J. Delphinium L. In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden: St. Louis, MO, USA, 2001; Volume 6, pp. 223–237. [Google Scholar]
- Ronse De Craene, L.P.; Soltis, P.S.; Soltis, D.E. Evolution of floral structures in basal angiosperms. Int. J. Plant Sci. 2003, 164, S329–S363. [Google Scholar] [CrossRef]
- Damerval, C.; Nadot, S. Evolution of perianth and stamen characteristics with respect to floral symmetry in Ranunculales. Ann. Bot. 2007, 100, 631–640. [Google Scholar] [CrossRef]
- Citerne, H.; Jabbour, F.; Nadot, S.; Damerval, C. The evolution of floral symmetry. Adv. Bot. Res. 2010, 54, 85–137. [Google Scholar]
- Endress, P.K. Evolutionary diversification of the flowers in angiosperms. Am. J. Bot. 2011, 98, 370–396. [Google Scholar] [CrossRef]
- Tamura, M. Morphology, ecology and phylogeny of the Ranunculaceae. VI. Sci. Rep. Osaka Univ. 1966, 15, 13–35. [Google Scholar]
- Jensen, U.; Hoot, S.B.; Johansson, J.T.; Kosuge, K. Systematics and phylogeny of the Ranunculaceae—A revised family concept on the basis of molecular data. Plant Syst. Evol. 1995, 9, 273–280. [Google Scholar]
- Ro, K.E.; Keener, C.S.; McPheron, B.A. Molecular phylogenetic study of the Ranunculaceae: Utility of the nuclear 26S ribosomal DNA in inferring intrafamilial relationships. Mol. Phylogenet. Evol. 1997, 8, 117–127. [Google Scholar] [CrossRef]
- Hoot, S.B. Phylogeny of the Ranunculaceae based on preliminary atpB, rbcL, and 18S nuclear ribosomal DNA sequence data. Plant Syst. Evol. 1995, 9, 241–251. [Google Scholar]
- Johansson, J.T. A revised chloroplast DNA phylogeny of the Ranunculaceae. Plant Syst. Evol. 1995, 9, 253–261. [Google Scholar]
- Wang, L.; Abbott, R.J.; Zheng, W.; Chen, P.; Wang, Y.; Liu, J. History and evolution of alpine plants endemic to the Qinghai-Tibetan Plateau: Aconitum gymnandrum (Ranunculaceae). Mol. Ecol. 2009, 18, 709–721. [Google Scholar] [CrossRef]
- Jabbour, F.; Renner, S.S. Consolida and Aconitella are an annual clade of Delphinium (Ranunculaceae) that diversified in the Mediterranean basin and the Irano-Turanian region. Taxon 2011, 60, 1029–1040. [Google Scholar] [CrossRef]
- Jabbour, F.; Renner, S.S. A phylogeny of Delphinieae (Ranunculaceae) shows that Aconitum is nested within Delphinium and that Late Miocene transitions to long life cycles in the Himalayas and southwest China coincide with bursts in diversification. Mol. Phylogenet. Evol. 2012, 62, 928–942. [Google Scholar] [CrossRef]
- Song, P.; Tian, X.H.; Ren, Y. Floral morphogenesis of Caltha and Trollius (Ranunculaceae) and the systematic significance. Acta Phytotaxon. Sin. 2007, 45, 769–782. [Google Scholar] [CrossRef]
- Ren, Y.; Chang, H.L.; Tian, X.H.; Song, P.; Endress, P.K. Floral development in Adonideae (Ranunculaceae). Flora 2009, 204, 506–517. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, P.; Che, X.F.; Wang, W.; Ren, Y. Floral organogenesis of Helleborus thibetanus and Nigella damascena (Ranunculaceae) and its systematic significance. Bot. J. Linn. Soc. 2011, 166, 431–443. [Google Scholar] [CrossRef]
- Ren, Y.; Gu, T.Q.; Chang, H.L. Floral development of Dichocarpum, Thalictrum, and Aquilegia (Thalictroideae, Ranunculaceae). Plant Syst. Evol. 2011, 292, 203–213. [Google Scholar] [CrossRef]
- Ronse De Craene, L.P.; Smets, E.F. Floral developmental evidence for the systematic relationships of Tropaeolum (Tropaeolaceae). Ann. Bot. 2001, 8, 879–892. [Google Scholar] [CrossRef][Green Version]
- Paulino, J.V.; Groppo, M.; Teixeira, S.P. Floral developmental morphology of three Indigofera species (Leguminosae) and its systematic significance within Papilionoideae. Plant Syst. Evol. 2011, 292, 165–176. [Google Scholar] [CrossRef]
- Khodaverdi, M.; Movafeghi, A.; Dadpour, M.R.; Naghiloo, S.; Ranjbar, M.; Prenner, G. Comparative study of floral development in Onobrychis melanotricha, Hedysarum varium and Alhagi persarum (Leguminosae: Papilionoideae: Hedysareae). Flora 2014, 209, 23–33. [Google Scholar] [CrossRef]
- Payer, J.B. Traité Dòrganogénie Comparée de la Fleur; Libraire de Victor Masson: Paris, France, 1857. [Google Scholar]
- Braun, A. über den Bluthenbau der Gattung Delphinium. Jahrb. Wiss. Bot. 1858, 1, 307–370. [Google Scholar]
- Eichler, A.W. Blütendiagramme, Parts 2; Engelmann: Leipzig, Germany, 1878. [Google Scholar]
- Benzing, L. Die Sporentwicklung der Blüte von Delphinium (Ranunculaceae). Preslia 1970, 42, 249–255. [Google Scholar]
- Mair, O. Zur Entwicklungsgeschichte monosymmetrischer Dicotylen-Blüten. Diss. Bot. 1977, 38, 1–90, 260–274. [Google Scholar]
- Tucker, S.C. Evolutionary lability of symmetry in early floral development. Int. J. Plant Sci. 1999, 160, S25–S39. [Google Scholar] [CrossRef]
- Feng, M.; Fu, D.Z.; Liang, H.X.; Lu, A.M. Floral morphogenesis of Aquilegia L. (Ranunculaceae). Acta Bot. Sin. 1995, 37, 791–794. [Google Scholar]
- Chang, H.L.; Ren, Y.; Lu, A.M. Floral morphogenesis of Anemone rivularis Buch.-Ham. ex DC. var. flore-minore Maxim. (Ranunculaceae) with special emphasis on androecium developmental sequence. J. Integr. Plant Biol. 2005, 47, 257–263. [Google Scholar] [CrossRef]
- Ren, Y.; Chang, H.L.; Endress, P.K. Floral development in Anemoneae (Ranunculaceae). Bot. J. Linn. Soc. 2010, 162, 77–100. [Google Scholar] [CrossRef]
- Kitazawa, M.; Fujimoto, K. Spiral phyllotaxis underlies constrained variation in Anemone (Ranunculaceae) tepal arrangement. J. Plant Res. 2018, 131, 459–468. [Google Scholar] [CrossRef]
- Tucker, S.C.; Hodges, S.C. Floral ontogeny of Aquiegia, Semiaquilegia, and Enemion (Ranunculaceae). Int. J. Plant Sci. 2005, 166, 557–574. [Google Scholar] [CrossRef]
- Kosuge, K. Petal evolution in Ranunculaceae. Plant Syst. Evol. 1994, 8, 185–191. [Google Scholar]
- Endress, P.K. Angiosperm floral evolution: Morphological developmental framework. Adv. Bot. Res. 2006, 44, 1–61. [Google Scholar]
- Tepfer, S.C. Floral Anatomy and Ontogeny in Aquilegia Formosa var. Truncata and Ranunculus Repens; University of California Publicaton in Botany: Berkeley, CA, USA, 1953; Volume 25, pp. 513–648. [Google Scholar]
- Lehmann, N.; Sattler, L.R. Floral development and homeosis in Actaea Rubra (Ranunculaceae). Int. J. Plant Sci. 1994, 155, 658–671. [Google Scholar] [CrossRef]
- Ronse De Craene, L.P. Homology and evolution of petals in the core eudicots. Syst. Bot. 2008, 33, 301–325. [Google Scholar] [CrossRef]
- Endress, P.K. Diversity and Evolutionary Biology of Tropical Flowers; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Reilly, S.M.; Wiley, E.O.; Meinhardt, D.J. An integrative approach to heterochrony: The distinction between interspecific and intraspecific phenomena. Biol. J. Linn. Soc. 1997, 60, 119–143. [Google Scholar] [CrossRef]
- Ronse De Craene, L.P. Are petals sterile stamens or bracts? The origin and evolution of petals in the core eudicots. Ann. Bot. 2007, 100, 621–663. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, W.; Ren, Y.; Bachelier, J.B. Floral development in Asteropyrum (Ranunculaceae): Implications for its systematic position. Ann. Bot. Fenn. 2012, 49, 31–42. [Google Scholar] [CrossRef]
- Gu, T.Q.; Ren, Y. Floral morphogenesis of Coptis (Ranunculaceae). Chin. Bull. Bot. 2007, 24, 80–86. [Google Scholar]
- Huth, E. Monographie der Gattung Delphinium. Bot. Jahrb. Syst. 1895, 20, 322–499. [Google Scholar]
- Endress, P.K. Floral phyllotaxis and floral evolution. Bot. Jahrb. Für Syst. 1987, 108, 417–438. [Google Scholar]
- Ren, Y.; Li, Z.-J.; Chang, H.-L.; Lei, Y.-J.; Lu, A.-M. Floral Development of Kingdonia (Ranunculaceae s. l., Ranunculales). Plant Syst. Evol. 2004, 247, 145–153. [Google Scholar] [CrossRef]
- Jensen, U. Serological legumin data and the phylogny of the Ranunculaceae. Plant Syst. Evol. 1995, 9, 217–227. [Google Scholar]
- Endress, P.K. Symmetry in flowers: Diversity and evolution. Int. J. Plant Sci. 1999, 160, S3–S23. [Google Scholar] [CrossRef]
- Stebbins, G.L. Flowering Plants: Evolution above the Species Level; Belknap: Cambridge, MA, USA, 1974. [Google Scholar]
- Walker-Larsen, J.; Harder, L.D. The evolution of staminodes in angiosperms: Patterns of stamen reduction, loss, and functional re-invention. Am. J. Bot. 2000, 87, 1367–1384. [Google Scholar] [CrossRef]
- Rudall, P.J.; Bateman, R.M. Evolution of disymmetry in monocot flowers: Iterative patterns and developmental constraints. New Phytol. 2004, 162, 25–44. [Google Scholar] [CrossRef]
- Novikoff, A.V.; Jabbour, F. Floral anatomy of Delphinieae (Ranunculaceae): Comparing flower organization and vascular patterns. Mod. Phytomorphol. 2014, 5, 35–44. [Google Scholar]
- Ashman, T.L.; Majetic, C.J. Genetic constraints on floral evolution: A review and evaluation of patterns. Heredity 2006, 96, 343–352. [Google Scholar] [CrossRef] [PubMed]




| Floral Organ and Trait | Aconitum taipeicum | Aconitum napellus1 | Delphinium giraldii | Delphinium grandiflorum1 | Consolida ajacis | Nigella damascena2 |
|---|---|---|---|---|---|---|
| Sepal | ||||||
| Initiation pattern | spiral | spiral | spiral | spiral | spiral | spiral |
| No. of sepals | 5 | 5 | 5 | 5 | 5 | 5 |
| The largest sepal | sepal 2 | N/A | sepal 2 | N/A | sepal 2 | sepal 1 |
| Appendant on sepal 2 | helmet | helmet | spur | spur | spur | N/A |
| Spurred petal | ||||||
| Initiation pattern | spiral | spiral | spiral | spiral | spiral | spiral |
| No. of spurred petals | 2 | 2 | 2 | 2 | 1 | 5–8 |
| Status at anthesis | separate | separate | separate | separate | connate | separate |
| Development of spur | ||||||
| Primordia growing into spurred petals | petals 2 and 5 | petals 2 and 5 | petals 2 and 5 | petals 1 and 4 | petals 2 and 5 | N/A |
| Stalk | present | present | absent | absent | absent | present |
| No. of bulges | 2 | N/A | 1 | N/A | 0 | 11 |
| Depression | present | present | present | N/A | absent | present |
| Reduced petal | ||||||
| No. of reduced petals | 6 | 6 | 4 | 4 | 6 | 0 |
| Status at anthesis | present | present | present | present | absent | N/A |
| Plate petal (No.) | absent | absent | Present (2) | Present (2) | absent | absent |
| Stamen | ||||||
| Initiation pattern | spiral | spiral | spiral | spiral | spiral | spiral |
| No. of anthers | 35–45 | 503 | 18–30 | N/A | 13–20 | 30–45 |
| Development sequence | centripetal | centripetal | centripetal | centripetal | centripetal | centripetal |
| Anther maturation pattern | centripetal | N/A | centripetal | N/A | centripetal | centripetal |
| Carpel | ||||||
| Initiation pattern | spiral | spiral | spiral | spiral | spiral | spiral |
| No. of carpels | 5 | 3 | 3 | 3 | 1 | 3–5 |
| Arrangement | whorled | whorled | whorled | whorled | N/A | whorled |
| Floral organ arrangement | parastichy | parastichy | parastichy | parastichy | orthostichy | parastichy |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.; Downie, S.R.; Peng, H.; Sun, F. Floral Organogenesis in Three Members of the Tribe Delphinieae (Ranunculaceae). Plants 2019, 8, 493. https://doi.org/10.3390/plants8110493
Chang H, Downie SR, Peng H, Sun F. Floral Organogenesis in Three Members of the Tribe Delphinieae (Ranunculaceae). Plants. 2019; 8(11):493. https://doi.org/10.3390/plants8110493
Chicago/Turabian StyleChang, Hongli, Stephen R. Downie, Hongli Peng, and Fengjie Sun. 2019. "Floral Organogenesis in Three Members of the Tribe Delphinieae (Ranunculaceae)" Plants 8, no. 11: 493. https://doi.org/10.3390/plants8110493
APA StyleChang, H., Downie, S. R., Peng, H., & Sun, F. (2019). Floral Organogenesis in Three Members of the Tribe Delphinieae (Ranunculaceae). Plants, 8(11), 493. https://doi.org/10.3390/plants8110493





