Nitric Oxide Is Involved in the Regulation of the Ascorbate–Glutathione Cycle Induced by the Appropriate Ammonium: Nitrate to Mitigate Low Light Stress in Brassica pekinensis
Abstract
:1. Introduction
2. Results
2.1. Effects of NO and NH4+:NO3− Ratio on Membrane Lipidation Damage in Leaf under Low Light Stress
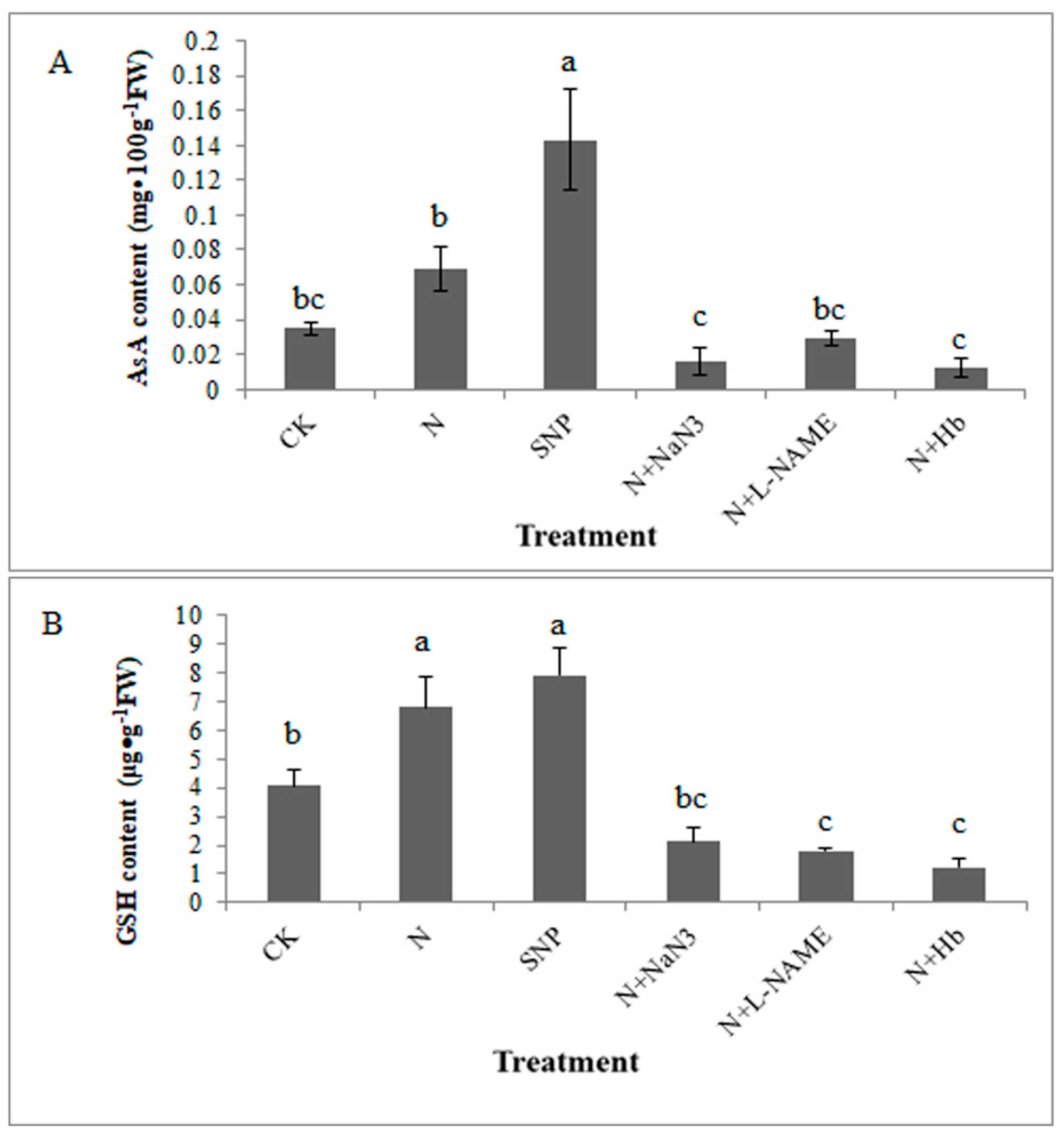
2.2. Effects of NO and NH4+:NO3− Ratio on the Contents of Redox State of Ascorbate and Glutathione in Leaf under Low Light Stress
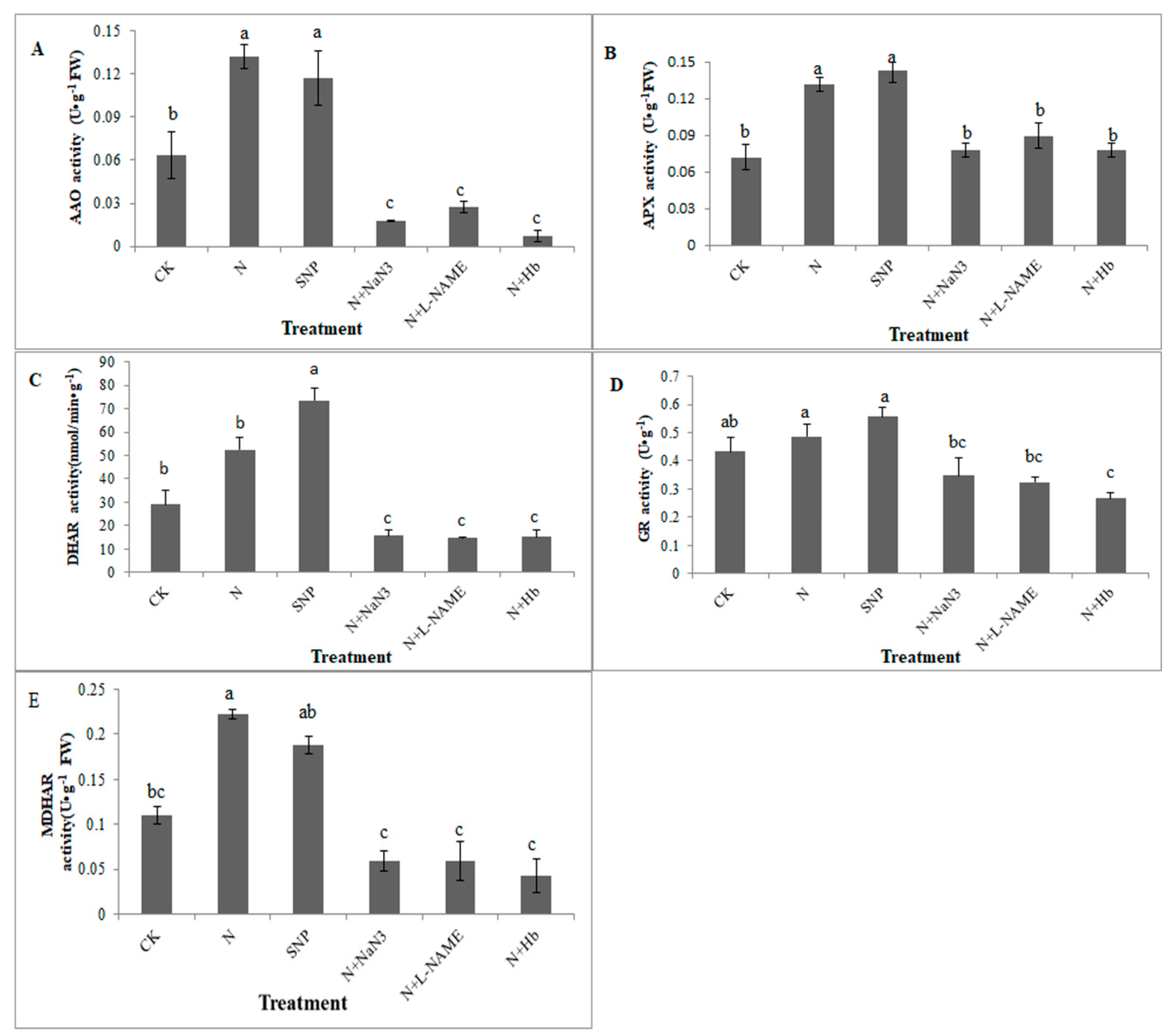
2.3. Effects of NO and NH4+:NO3− Ratio on the Activities of Key Enzymes Involved in the AsA-GSH Cycle in Leaf under Low Light Stress
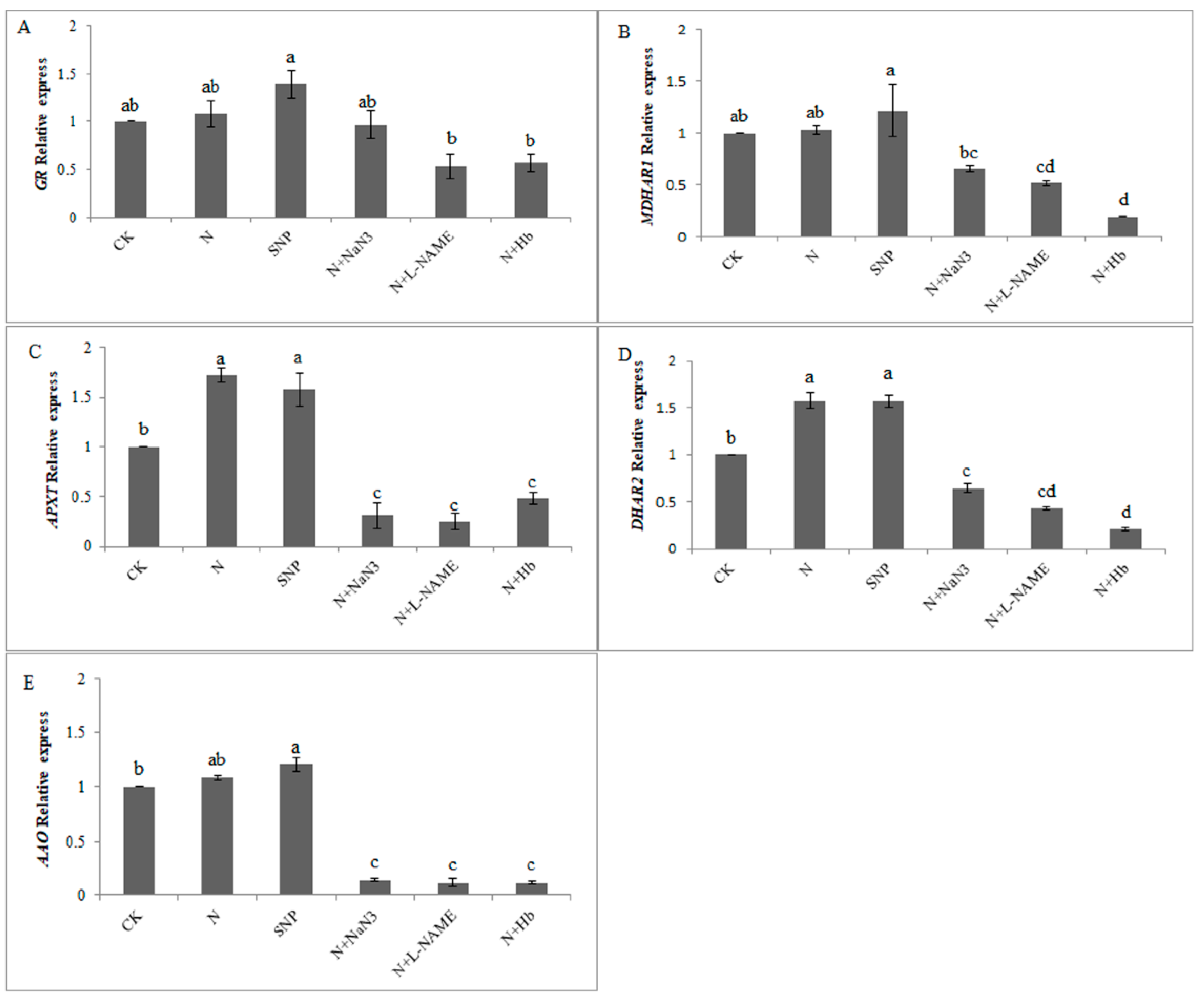
2.4. Effects of NO and NH4+:NO3− Ratio on the Gene Expression Level Related to Antioxidative Enzymes in the AsA-GSH Cycle under Low Light Stress
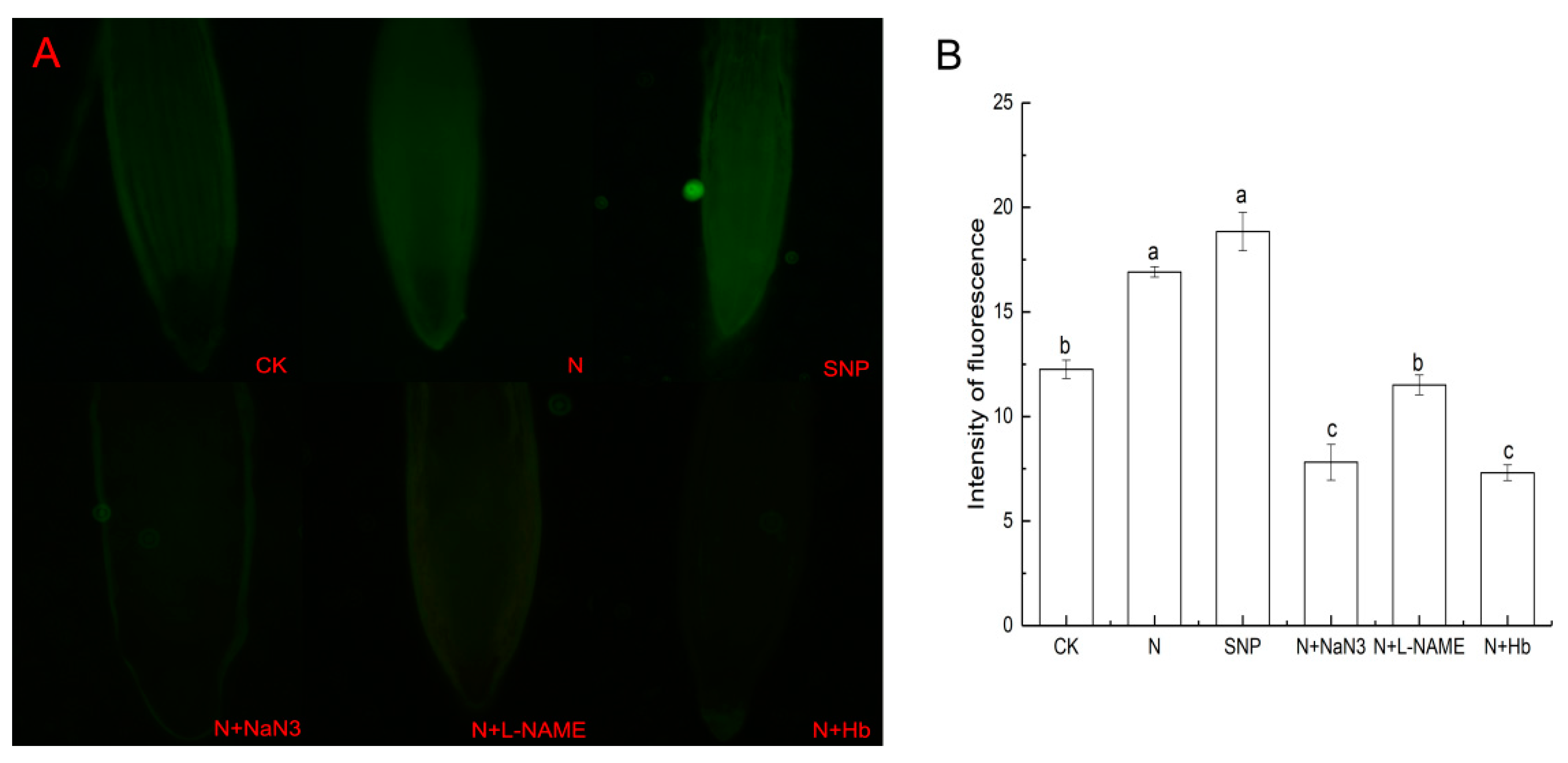
2.5. Effects of NO and NH4+:NO3− Ratio on the NO Level in Root Tissues under Low Light Stress
3. Discussions
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Treatments and Experimental Design
4.3. Determination of the Contents of Hydrogen Peroxide (H2O2), Superoxide Anion Free radical (O2−), and Malondialdehyde (MDA)
4.4. Determination of Glutathione (GSH) and Ascorbate (AsA)
4.5. Determination of Activities of Enzymes of AsA-GSH Cycle
4.6. Transcript Level Estimation with RT-PCR
4.7. Detection of NO Level in Root Tissues with Fluorescence Probe 4,5-Diaminofluorescein Diacetate (DAF-2DA)
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Yin, Z.; Wang, H.; Guo, W. Climatic change features of fog and haze in winter over North China and Huang-Huai Area. Sci. China Earth Sci. 2015, 58, 1370–1376. [Google Scholar] [CrossRef]
- Yan, S.; Zhu, B.; Kang, H. Long-Term Fog Variation and Its Impact Factors over Polluted Regions of East China. J. Geophys. Res. Atmos. 2019, 124, 1741–1754. [Google Scholar] [CrossRef]
- Hussain, S.; Iqbal, N.; Rahman, T.; Liu, T.; Brestic, M.; Safdar, M.E.; Asghar, M.A.; Farooq, M.U.; Shafiq, I.; Ali, A.; et al. Shade effect on carbohydrates dynamics and stem strength of soybean genotypes. Environ. Exp. Bot. 2019, 162, 374–382. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Shang, Q.M. Photosynthetic Characteristics of Pepper Leaves Under Low Temperature, Weak Light and Salt Stress. Sci. Agric. Sin. 2010, 43, 123–131. [Google Scholar]
- Bi, H.; Dong, X.; Wu, G.; Wang, M.; Ai, X. Decreased TK activity alters growth, yield and tolerance to low temperature and low light intensity in transgenic cucumber plants. Plant Cell Rep. 2015, 34, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Wen, S.; Lu, W.; Lu, D. Effects of Weak-light Stress After Pollination on Grain Filling and Leaf Senescence in Sweet Maize. J. Nucl. Agric. Sci. 2017, 31, 964–971. [Google Scholar]
- Hojati, M.; Modarressanavy, S.A.M.; Karimi, M.; Ghanati, F. Responses of growth and antioxidant systems in Carthamus tinctorius L. under water deficit stress. Acta Physiol. Plant. 2011, 33, 105–112. [Google Scholar] [CrossRef]
- Li, Y.L.; Liu, Y.F.; Zhang, J.G. Advances in the research on the AsA-GSH cycle in horticultural crops. Front. Agric. Chin. 2010, 4, 84–90. [Google Scholar] [CrossRef]
- Shan, C.; Zhou, Y.; Liu, M. Nitric oxide participates in the regulation of the ascorbate-glutathione cycle by exogenous jasmonic acid in the leaves of wheat seedlings under drought stress. Protoplasma 2015, 252, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Asadi, K.E.; Maresca, V.; Sorbo, S.; Keramat, B.; Basile, A. Effects of triacontanol on ascorbate-glutathione cycle in Brassica napus L. exposed to cadmium-induced oxidative stress. Ecotoxicol. Environ. Saf. 2017, 144, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Sanz, L.; Albertos, P.; Mateos, I.; Sánchez-Vicente, I.; Lechón, T.; Fernández-Marcos, M.; Lorenzo, O. Nitric oxide (NO) and phytohormones crosstalk during early plant development. J. Exp. Bot. 2015, 66, 2857–2868. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liao, W.; Wang, M.; Niu, L.; Xu, Q.; Jin, X. Nitric oxide is required for hydrogen gas-induced adventitious root formation in cucumber. J. Plant Physiol. 2016, 195, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, J.; Zhang, J.; Ye, N.; Zhang, H.; Tan, M.; Jiang, M. Nitric oxide mediates brassinosteroid-induced ABA biosynthesis involved in oxidative stress tolerance in maize leaves. Plant Cell Physiol. 2011, 52, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H.; Cohen, M.F. NO signal at the crossroads: Polyamine-induced nitric oxide synthesis in plants? Trends Plant Sci. 2006, 11, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fu, J.; Chu, X.; Sun, Y.; He, Z.; Hu, T. Nitric oxide mediates abscisic acid induced light-tolerance in leaves of tall fescue under high-light stress. Sci. Hortic. 2013, 162, 1–10. [Google Scholar] [CrossRef]
- Shan, C.; He, F.; Xu, G.; Han, R.; Liang, Z. Nitric oxide is involved in the regulation of ascorbate and glutathione metabolism in Agropyron cristatum leaves under water stress. Biol. Plant. 2012, 56, 187–191. [Google Scholar] [CrossRef]
- Hu, L.; Yu, J.; Liao, W.; Zhang, G.; Xie, J.; Lv, J.; Xiao, X.; Yang, B.; Zhou, R.; Bu, R. Moderate ammonium: Nitrate alleviates low light intensity stress in mini Chinese cabbage seedling by regulating root architecture and photosynthesis. Sci. Hortic. 2015, 186, 143–153. [Google Scholar] [CrossRef]
- Astier, J.; Jeandroz, S.; Wendehenne, D. Nitric oxide synthase in plants: The surprise from algae. Plant Sci. 2018, 268, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Besson-Bard, A.; Pugin, A.; Wendehenne, D. New Insights into Nitric Oxide Signaling in Plants. Annu. Rev. Plant Biol. 2008, 59, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Fernie, A.R.; Kaiser, W.M.; van Dongen, J.T. On the origins of nitric oxide. Trends Plant Sci. 2011, 16, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Liao, W.; Dawuda, M.M.; Yu, J.; Lv, J. Appropriate NH4(+): NO3(-) ratio improves low light tolerance of mini Chinese cabbage seedlings. BMC Plant Biol. 2017, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Wang, Z.; Fang, Y.; Hu, Z.; Yong, H.; Deng, M.; Zhang, Z. Effects of 24-epibrassinolide on antioxidation defense and osmoregulation systems of young grapevines (V. vinifera L.) under chilling stress. Plant Growth Regul. 2013, 71, 57–65. [Google Scholar] [CrossRef]
- Yu, W.; Cao, F.; Wang, G. Relationship between Active Oxygen Metabolism of Ginkgo Leaf and Cell Membrane Injury under Low-temperature Stress. J. Northeast For. Univ. 2010, 38, 46–48. [Google Scholar]
- Yuan, L.I.; Jin-Juan, L.I.; Wei, X.H. Responses of antioxidative capability in horsebean seedling to NO and H2O2 under Cd stress. Acta Prataculturae Sin. 2009, 18, 186–191. [Google Scholar]
- Tabatabaei, S.J.; Yusefi, M.; Hajiloo, J. Effects of shading and NO3:NH4 ratio on the yield, quality and N metabolism in strawberry. Sci. Hortic. 2008, 116, 264–272. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.R.; Yamamoto, Y.; Matsumoto, H. An intracellular mechanism of aluminum tolerance associated with high antioxidant status in cultured tobacco cells. J. Inorg. Biochem. 2003, 97, 59–68. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Nagalakshmi, N.; Prasad, M.N. Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci. 2001, 160, 291–299. [Google Scholar] [CrossRef]
- Aravind, P.; Prasad, M.N.V. Modulation of cadmium-induced oxidative stress in Ceratophyllum demersum by zinc involves ascorbate–glutathione cycle and glutathione metabolism. Plant Physiol. Biochem. 2005, 43, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hu, L.; Liao, W.; Mujitaba Dawuda, M.; Lyu, J.; Xie, J.; Feng, Z.; Calderón-Urrea, A.; Yu, J. Foliar application of 5-aminolevulinic acid (ALA) alleviates NaCl stress in cucumber (Cucumis sativus L.) seedlings through the enhancement of ascorbate-glutathione cycle. Sci. Hortic. 2019, 257, 108761. [Google Scholar] [CrossRef]
- Zhao, H.; Ye, L.; Wang, Y.; Zhou, X.; Yang, J.; Wang, J.; Cao, K.; Zou, Z. Melatonin Increases the Chilling Tolerance of Chloroplast in Cucumber Seedlings by Regulating Photosynthetic Electron Flux and the Ascorbate-Glutathione Cycle. Front. Plant Sci. 2016, 7, 1814. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Exogenous Silicon Attenuates Cadmium-Induced Oxidative Stress in Brassica napus L. by Modulating AsA-GSH Pathway and Glyoxalase System. Front. Plant Sci. 2017, 8, 1061. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hu, X.; Zhang, J.; Zhang, J.; Du, Q.; Li, J. H2O2 mediates ALA-induced glutathione and ascorbate accumulation in the perception and resistance to oxidative stress in Solanum lycopersicum at low temperatures. BMC Plant Biol. 2018, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, M.K.; Bhardwaj, R. Arsenic induced modulation of antioxidative defense system and brassinosteroids in Brassica juncea L. Ecotoxicol. Environ. Saf. 2015, 115, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Conklin, P.L.; Barth, C. Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ. 2010, 27, 959–970. [Google Scholar] [CrossRef]
- Haihua, R.; Shen, W.; Liu, K.; Xu, L. Effects of exogenous NO donor on glutathione-dependent antioxidative systems in wheat seedling leaf under salt stress. Acta Agron. Sin. 2005, 31, 1144–1149. [Google Scholar]
- Li, X.Y.; Wang, X.F.; Lu, L.F.; Yin, B.; Zhang, M.; Cui, X.M. Effects of exogenous nitric oxide on ascorbate-glutathione cycle in tomato seedlings roots under copper stress. Ying Yong Sheng Tai Xue Bao 2013, 24, 1023–1030. [Google Scholar] [PubMed]
- Sun, C.; Liu, L.; Yu, Y.; Liu, W.; Lu, L.; Jin, C.; Lin, X. Nitric oxide alleviates aluminum-induced oxidative damage through regulating the ascorbate glutathione cycle in roots of wheat. J. Integr. Plant Biol. 2015, 57, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Begara-Morales, J.C.; Sánchez-Calvo, B.; Chaki, M.; Valderrama, R.; Mata-Pérez, C.; Padilla, M.N.; Corpas, F.J.; Barroso, J.B. Modulation of the Ascorbate–Glutathione Cycle Antioxidant Capacity by Posttranslational Modifications Mediated by Nitric Oxide in Abiotic Stress Situations. In Reactive Oxygen Species and Oxidative Damage in Plants under Stress; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 305–320. [Google Scholar]
- Chang, H.S.; Hong, J.K. Sodium nitroprusside mediates seedling development and attenuation of oxidative stresses in Chinese cabbage. Plant Biotechnol. Rep. 2010, 4, 243–251. [Google Scholar]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2010, 24, 1337–1344. [Google Scholar] [CrossRef]
- Nakajima, A.; Tahara, M.; Yoshimura, Y.; Nakazawa, H. Determination of free radicals generated from light exposed ketoprofen. J. Photochem. Photobiol. A Chem. 2005, 174, 89–97. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Genes | Accession Number | Primer Name | Primer Sequence |
|---|---|---|---|
| AAO | Bra002355 | Primer F | TGATGCTACCGCCGGAGACAC |
| Primer R | TGCCGTGCCAATGGATGACAAC | ||
| MDHAR1 | Bra006954 | Primer F | GGCGGTGGCTCCTTATGAACG |
| Primer R | TCCACCACTACCAACACAGCAATG | ||
| DHAR2 | Bra008188 | Primer F | TCCTCCTGAGTTCGCCTCTGTTG |
| Primer R | GCCTTGTCGGAACCGTCACTG | ||
| APXT | Bra015668 | Primer F | TCGCCTCCTCCTCCTCCTCTC |
| Primer R | ACCACCGTGTTACTAGAGCCTCTG | ||
| GR | Bra001931 | Primer F | GCTGGAGCTGTGAAGGTTGATGAG |
| Primer R | CCATTAAGGCAACAGGCGTGAGG | ||
| Actin | JN120480.1 | Primer F | CCAGGAATCGCTGACCGTAT |
| Primer R | CTGTTGGAAAGTGCTGAGGGA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Li, Y.; Wu, Y.; Lv, J.; Dawuda, M.M.; Tang, Z.; Liao, W.; Calderón-Urrea, A.; Xie, J.; Yu, J. Nitric Oxide Is Involved in the Regulation of the Ascorbate–Glutathione Cycle Induced by the Appropriate Ammonium: Nitrate to Mitigate Low Light Stress in Brassica pekinensis. Plants 2019, 8, 489. https://doi.org/10.3390/plants8110489
Hu L, Li Y, Wu Y, Lv J, Dawuda MM, Tang Z, Liao W, Calderón-Urrea A, Xie J, Yu J. Nitric Oxide Is Involved in the Regulation of the Ascorbate–Glutathione Cycle Induced by the Appropriate Ammonium: Nitrate to Mitigate Low Light Stress in Brassica pekinensis. Plants. 2019; 8(11):489. https://doi.org/10.3390/plants8110489
Chicago/Turabian StyleHu, Linli, Yutong Li, Yue Wu, Jian Lv, Mohammed Mujitaba Dawuda, Zhongqi Tang, Weibiao Liao, Alejandro Calderón-Urrea, Jianming Xie, and Jihua Yu. 2019. "Nitric Oxide Is Involved in the Regulation of the Ascorbate–Glutathione Cycle Induced by the Appropriate Ammonium: Nitrate to Mitigate Low Light Stress in Brassica pekinensis" Plants 8, no. 11: 489. https://doi.org/10.3390/plants8110489
APA StyleHu, L., Li, Y., Wu, Y., Lv, J., Dawuda, M. M., Tang, Z., Liao, W., Calderón-Urrea, A., Xie, J., & Yu, J. (2019). Nitric Oxide Is Involved in the Regulation of the Ascorbate–Glutathione Cycle Induced by the Appropriate Ammonium: Nitrate to Mitigate Low Light Stress in Brassica pekinensis. Plants, 8(11), 489. https://doi.org/10.3390/plants8110489






