Abstract
Enhanced resistance is a key strategy of controlling ‘Verticillium stem striping’ in Brassica napus caused by the soil-borne vascular pathogen Verticillium longisporum. The present study analyses the role of a broad range of components in the phenylpropanoid and salicylic acid (SA) pathways in basal and cultivar-related resistance of B. napus towards V. longisporum. A remarkable increase of susceptibility to V. longisporum in SA-deficient transgenic NahG plants indicated an essential role of SA in basal resistance of B. napus to V. longisporum. Accordingly, elevated SA levels were also found in a resistant and not in a susceptible cultivar during early asymptomatic stages of infection (7 dpi), which was associated with increased expression of PR1 and PR2. In later symptomatic stages (14 or 21 dpi), SA responses did not differ anymore between cultivars varying in resistance. In parallel, starting at 7 dpi, an overall increase in phenylpropanoid syntheses developed in the resistant cultivar, including the activity of some key enzymes, phenylalanine ammonium lyase (PAL), cinnamyl alcohol dehydrogenase (CAD) and peroxidase (POX) and the expression of key genes, PAL4, CCoAMT, CCR, POX. As a consequence, a remarkable increase in the levels of phenolic acids (t-cinnamic acid, p-coumaric acid, caffeic acid, ferulic acid, sinapic acid) occurred associated with cultivar resistance. A principal component analysis including all 27 traits studied indicated that component 1 related to SA synthesis (PR1, PR2, POX, level of free SA) and component 2 related to lignin synthesis (level of free ferulic acid, free p-coumaric acid, conjugated t-cinnamic acid) were the strongest factors to determine cultivar-related resistance. This study provides evidence that both SA and phenolic acid synthesis are important in cultivar-related resistance, however, with differential roles during asymptomatic and symptomatic stages of infection.
1. Introduction
Verticillium longisporum (VL) is a soil-borne vascular fungal pathogen with host specificity to Brassicaceae [1]. The pathogen widely occurs in oilseed rape production regions in Europe and North America [2,3,4,5]. Oilseed rape (Brassica napus) is the most important crop for oil production in Europe and Canada and the prevalent host of V. longisporum [6]. Due to the relatively short crop rotation and increased area of oilseed rape cultivation, incidence of ‘Verticillium stem striping’ is on the rise and threatens oilseed rape production. In the absence of their host, the melanized microsclerotia of V. longisporum remain dormant and viable in soil for several years [6,7,8]. As control of V. longisporum with fungicides has not been successful [9], the most promising measure against this disease is breeding for effective resistance.
Salicylic acid (SA) is an important phytohormone which has been shown to play a role not only in local resistance but also in the activation of systemic acquired resistance (SAR) against pathogens [10]. SA can be synthesized either via the phenylalanine or isochorismate pathways in Arabidopsis [11,12]. Application of SA or its analogues (benzo (1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester, BTH) enhances the resistance of plants against pathogens, while in SA-deficient NahG transformed plants the susceptibility to pathogens was increased [13,14]. However, surprisingly, in B. napus, a positive correlation between the concentration of SA in shoot extracts and the biomass of V. longisporum colonizing the plant was reported [15].
In Arabidopsis, soluble phenylpropanoids are involved in defense responses to V. longisporum [16]. In oilseed rape, genomic loci (QTL) for a number of phenylpropanoids were found to co-localize with the QTL for V. longisporum resistance [17]. Lignin, an important compound among phenylpropanoids, is mainly deposited in secondary cell walls, which may provide strength for preventing direct penetration by the fungus. Besides, several phenolic acids, intermediates in the lignin biosynthetic pathway, were accumulated in vascular tissues after infection of oilseed rape with V. longisporum [18].
Although previous studies have indicated a role of SA and lignin biosynthesis in resistance responses of oilseed rape to V. longisporum, the role of specific components and the interplay between these pathways during infection is not yet understood. Therefore, the aim of this study was to explore the role in resistance of a larger set of metabolites, enzymes and genes involved in the biosynthesis of SA and lignin during infection of B. napus with V. longisporum. More specifically, we followed three hypotheses, (1) that SA is required in basal resistance of B. napus to V. longisporum, (2) that the phenylpropanoid pathways related to lignin synthesis determine cultivar-related resistance to V. longisporum and (3) that biosynthesis of SA and phenolic acids are in a competitive relationship.
2. Results
2.1. V. longisporum Disease Development and Plant Colonization in NahG Transformed Oilseed Rape
Typical symptoms of V. longisporum infection on B. napus under greenhouse or climate chamber conditions were leaf yellowing, vein blacking, premature senescence of leaves and stunting. In the NahG transformants, severe symptoms were observed already two weeks after inoculation, while in the wild type plants, only relative mild symptoms occurred at 21 dpi. In addition, diseased transformant plants showed a severely crippled, deformed shoot growth at 21 dpi. Except for the first week after inoculation, significantly higher levels of disease severity were recorded in V. longisporum infected NahG transformants than in the wild type plants. However, exogenously supplied SA by root dipping prior to inoculation did not effectively reduce disease severity (Table 1). The stunting effect of V. longisporum was significantly stronger on NahG transformants than on wild type plants. No significant effects by V. longisporum on the dry weight of roots and shoots was observed at early time points (7, 14 dpi). However, roots of NahG transformants were comparatively more sensitive to the root application of SA, which induced a reduction of biomass in mock-inoculated plants at 7 dpi. At 21 dpi, a significant reduction of root and shoot biomass was observed in all V. longisporum-inoculated NahG transformants. Except for root biomass at 21 dpi without exogenous application of SA, biomass of wild type plants was not significantly reduced by V. longisporum inoculation. Correspondingly, significantly higher amounts of fungal DNA were found in the hypocotyls of NahG transformants compared to wild type plants (Figure 1A). Exogenous SA treatment did not significantly reduce the amount of fungal DNA (Figure 1B).

Table 1.
Disease severity, plant height and dry weight of mock- and V. longisporum-inoculated oilseed rape. Different letters indicate significant differences within rows for each parameter (LSD test, p < 0.05). SA, salicylic acid; VL, V. longisporum inoculated.
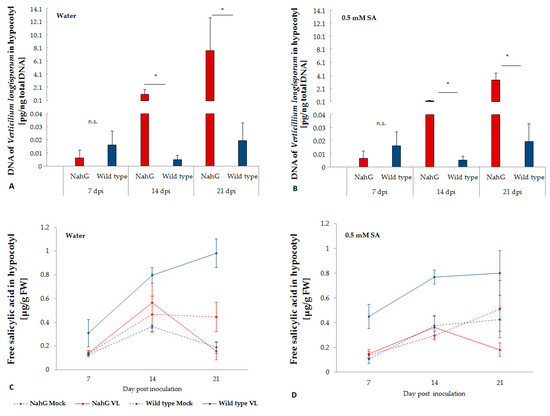
Figure 1.
Colonization of in B. napus cv. Drakkar and its NahG transformant with V. longisporum with (A) or without (B) exogenous application of salicylic acid and the endogenous free salicylic acid content in the hypocotyl after infection (C,D). Bars indicate standard errors. Mean data were obtained from four biological replications. Asterisks on the bars indicate significant differences between two genotypes at the same time point (LSD, p < 0.05).
2.2. Endogenous SA in the Hypocotyl of Wild Type and NahG Transformant Plants
NahG transformants were not able to accumulate free SA upon infection with V. longisporum, while in wild type plants a significant increase of free SA was observed in V. longisporum infected plants, especially two weeks after inoculation (Figure 1C), when V. longisporum biomass in hypocotyls significantly increased in NahG transformants but not in wild type plants. Exogenous application of SA did not affect the level of free SA in plant tissue (Figure 1D). Free SA showed a slight negative correlation with logarithm of V. longisporum DNA (r = −0.24, p = 0.09).
2.3. V. longisporum Disease Development in Resistant and Susceptible Cultivars
No visual symptoms were observed during the first week after inoculation in either cultivar. However, two weeks after inoculation with V. longisporum, the susceptible cultivar Falcon began to display significantly more severe disease symptoms on leaves and a stronger stunting, while the resistant cultivar only exhibited a slight reduction of plant height (Figure 2A,C). A significant accumulation of V. longisporum DNA in the hypocotyl of the susceptible cultivar was detected which was lacking in the resistant cultivar SEM confirming the disease phenotyping data (Figure 2B).
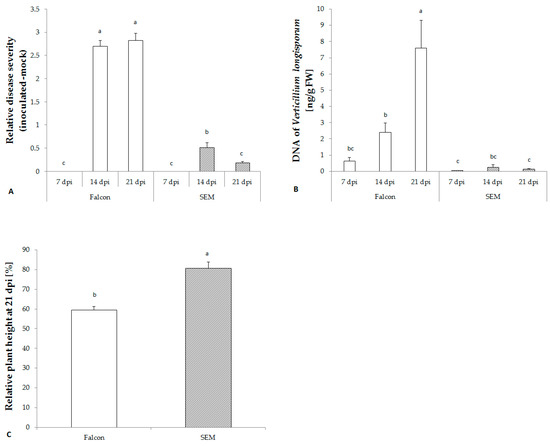
Figure 2.
Development of V. longisporum in B. napus cv. Falcon and SEM determined by disease severity (A), fungal biomass in hypocotyls (B), and plant height at 21 dpi (C). Bars indicate standard errors. Different letters indicate significant differences among the treatments (LSD test, p < 0.05). FW, fresh weight.
2.4. Changes in the SA Biosynthetic Pathway of B. napus Infected with V. longisporum
In the absence of disease symptoms of V. longisporum infection, free benzoic acid (BA) in the resistant cultivar SEM was strongly used for the production of free SA which was evident by fast increase of free SA at the same time (Figure 3A,B). Similar to free SA, the activity of benzoic acid 2-hydroxylase (BA2H) was transiently enhanced at 7 dpi by infection. In general, responses in both metabolite levels and enzyme activities relative to fungal biomass in the susceptible cultivar Falcon were low (Figure 4B). Besides, a stronger increase in expression of SA mediated marker genes PR1 and PR2 relative to the fungal biomass was found in the resistant cultivar (Figure 5A,B), while the jasmonic acid mediated marker gene PDF1.2 was down-regulated (Figure 5C).
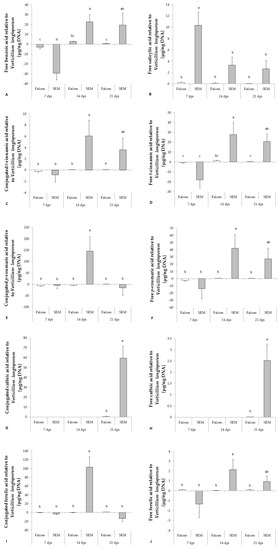
Figure 3.
Response of free benzoic acid (A), salicylic acid (B) and different phenolic acids (C–L) in the hypocotyl of B. napus cv. Falcon and SEM upon infection of V. longisporum. Bars indicate standard errors. Different letters indicate significant differences among the treatments (LSD test, p < 0.05). FW, fresh weight; n.s., no significant difference.
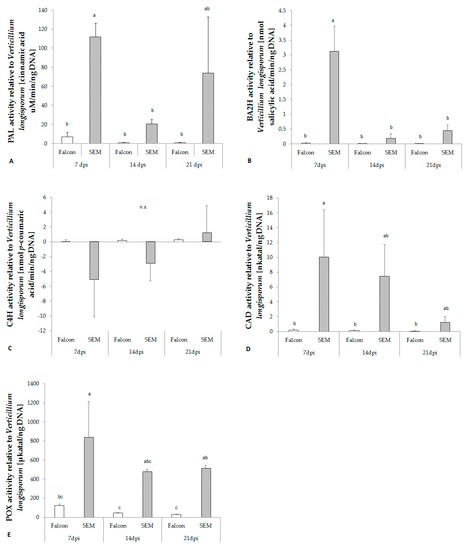
Figure 4.
Enzyme activity in the hypocotyl of B. napus cv. Falcon and SEM inoculated with V. longisporum. (A) PAL, phenylalanine ammonia lyase; (B) BA2H, benzoic acid 2-hydroxylase; (C) C4H, cinnamate 4-hydroxylase; (D) CAD, cinnamyl alcohol dehydrogenase; (E) POX, peroxidase. Bars indicate standard errors. Different letters indicate significant differences among the treatments (LSD test, p < 0.05). n.s. means no significant difference.
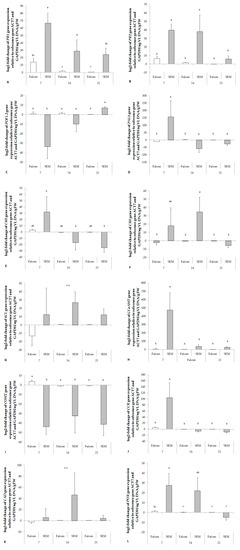
Figure 5.
Fold change of salicylic acid mediated resistance marker genes (A, B), jasmonic acid mediated resistance marker gene (C), and genes of key enzymes involved in lignin synthesis (D–L) in the hypocotyl of B. napus after infection by V. longisporum. The change rates were derived from a comparison of mock-inoculated plants. Target gene expression was normalized to the expression of ACT7 and GAPDH. The log2-fold change was related to the fungal biomass. PR1, pathogenesis-related protein 1; PR2, pathogenesis-related protein 2; PDF1.2, plant defensin 1.2; PAL4, phenylalanine ammonia lyase; C4H, cinnamate 4-hydroxylase; C3H, p-coumarate 3-hydroxylase; 4CL, 4-coumarate:CoA ligase; CCoAMT, caffeoyl-CoA 3-O-methyltransferase; COMT, catechol-O-methyl transferase; CCR, cinnamoyl-CoA reductase; CAD, cinnamyl alcohol dehydrogenase; POX, peroxidase; ACT7, actin 7; GAPDH, Glyceraldehyde-3-phosphate dehydrogenase. Bars indicate standard errors. Different letters indicate significant differences among the treatments (LSD test, p < 0.05). FW, fresh weight; VL, V. longisporum; n.s., no significant difference.
2.5. Changes in Phenolic Acid Levels related to Lignin Biosynthesis in Response to V. longisporum Infection
Phenolic acids are formed in the lignin biosynthetic pathway in the following order: trans-cinnamic acid (tCA), p-coumaric acid (pCA), caffeic acid (CA), ferulic acid (FA), sinapic acid (SiA). In healthy B. napus plants, the contents of all conjugated phenolic acids increased with growth, whereas free phenolic acids were relatively stable in content all over the time of sampling (data not shown). In contrast to SA, no significant regulation of phenolic acids was found in either cultivar one week after inoculation with V. longisporum. Except CA and free SiA, significant increases of tCA, pCA, FA and conjugated SiA toward infection of V. longisporum were detected in the resistant cultivar at 14 dpi (Figure 3). The amount of CA was under detection limit until 14 dpi, but a clear increase was found in the resistant cultivar at 21 dpi (Figure 3G,H).
2.6. Activity of Key Enzymes in Lignin Biosynthetic Pathway
The activity of phenylalanine ammonia lyase (PAL) towards infection of V. longisporum was remarkably increased in the resistant cultivar at 7 dpi, while in the susceptible cultivar less activity was found (Figure 4A). Prior to production of phenolic acids, the activity of cinnamyl alcohol dehydrogenase (CAD) and peroxidase (POX) increased in the resistant cultivar at 7 dpi (Figure 4D,E). However, cinnamate 4-hydroxylase (C4H), which catalyzes conversion of tCA to pCA, did not differ between resistant and susceptible cultivars (Figure 4C).
2.7. Regulation of Genes of Key Enzymes involved in Lignin Synthesis
Similar to enzyme activity, expression of almost all genes involved in lignin synthesis (PAL4, C4H, C3H, CCoAMT, CCR, CAD and POX) was up-regulated relative to fungal biomass in the resistant cultivar already at 7 dpi. In contrast, COMT was strongly down - regulated in the resistant cultivar (Figure 5). No significant responses were found in the expression of 4CL (Figure 5G).
2.8. Principal Component Analysis
Principal component analysis is a powerful statistical tool for multivariate experiments. Using principal components 1 and 2 based on 27 traits, a clear separation of resistant and susceptible cultivars is possible, with the resistant cultivar at 7 dpi being most distinct cluster (Figure 6A). The distributions of cos2 values among principal components indicate that gene expression of PR1, PR2, POX and level of free SA were the strongest contributors to the component 1, while component 2 was mainly determined by the level of free FA, free pCA, conjugated tCA (Figure 6B). These parameters seem to be the best variables to explain the variation in response of both genotypes to fungal infection.
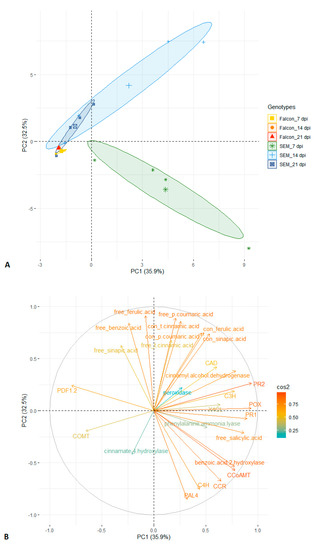
Figure 6.
Principal component analysis of twenty-seven traits (metabolites, enzyme activities and gene expression) recorded in two B. napus genotypes with V. longisporum infection (A) and circles of cos2 value of the variables (B). Labels of traits in capital letters are names of genes. Numbers of clusters were determined by elbow method. PC, principal component; con, conjugated form.
3. Discussion
3.1. SA Plays a Role in Basal Resistance of B. napus to V. longisporum
SA, an important phytohormone involved in disease defense, has been shown to play a role not only in local resistance but also in the activation of systemic acquired resistance (SAR) [10]. Most of the SA synthesized in plants is modified by glucosylation and methylation, which are induced upon pathogen infection [14]. Free SA can be released from this inactive storage form when necessary and maintain SAR over extended periods of time [19]. Methyl SA, a volatile ester, as well as free and conjugated SA in tobacco were induced after infection with avirulent strains of Pseudomonas syringae [20]. However, overexpression of glucosyl and methyl transferases in A. thaliana suppressed the accumulation of SA and SA-glucoside, and the AtSGT1 and OsBSMT1 mutants became more susceptible to disease [21,22]. Like SA and SA-glucoside concentrations in the xylem sap of B. napus [15], the concentrations of free SA in wild type plants measured in the present study were strongly modulated by infection of V. longisporum. V. longisporum penetrates the roots within 60 hpi and starts to colonize the xylem of the shoot three weeks after inoculation [23]. Accordingly, an increase of free SA in the hypocotyl was observed in wild type plants one week after inoculation in the present study. Previous studies showed that the SA-dependent defense pathway was not effective to increase resistance to all pathogens studied [13,14]. SA has been reported to be involved in basal defense and to induce resistance to Oidium neolycopersici in tobacco and to Botrytis cinerea in tomato; however, SA-deficient NahG transformed tobacco and tomato did not enhance the susceptibility to B. cinerea and powdery mildew, respectively [13,14]. As described by Johansson et al. [24], no enhanced susceptibility to V. longisporum was found in a NahG mutant of Arabidopsis. The NahG transformant in the present study showed a remarkable increase in susceptibility to V. longisporum, highlighting that SA probably plays an important role in basal defense of B. napus against V. longisporum. Although no linear correlation exists between SA levels and fungal growth, a threshold level of SA is required for resistance of B. napus to V. longisporum.
However, endogenous free SA was not accumulated by exogenous application of 0.5 mM of SA to the roots, which neither had direct negative effects on V. longisporum nor was it phytoxic to B. napus [25]. In Arabidopsis, SA pretreatment did not show any significant alterations in fresh weight loss or symptoms caused by V. longisporum [24]. Similarly, in our experiment, no clear reduction in V. longisporum biomass and in dry weight losses of roots and stems was observed in SA pretreated NahG transformants and wild type plants.
3.2. Role of SA and Phenolic Acids in Cultivar Resistance of B. napus to V. longisporum
Cultivar-related resistance was associated with a significantly higher increase of SA relative to fungal biomass in the early asymptomatic interaction stage (7 dpi). The resistant cultivar reacted much stronger by shifting more BA to SA. SA can also directly bind to NPR1, the core compound of the SA signaling network, forming a copper-binding transcription-regulator to activate the expression of PR1 [26]. In contrast to A. thaliana [17], B. napus showed a strong enhancement of expression of SA-mediated marker genes PR1 and PR2 upon V. longisporum infection, while JA-dependent PDF1.2 was relatively stable or even down-regulated.
Lignin is an aromatic polymer that is deposited during secondary cell wall thickening providing a physical barrier against initial pathogen penetration and colonization. The biosynthesis of SA from phenylalanine and lignin synthesis both depend on PAL activity, which may lead to a competitive relationship between these two synthesis pathways [27]. The phenylalanine route branches into lignin synthesis from tCA catalyzed by the enzyme C4H, and the synthesized lignin monomers are transported to the cell wall and polymerized by POX [28]. In Arabidopsis, soluble phenylpropanoids were involved in the defense response against V. longisporum infection. Such accumulated soluble phenolic compounds may be toxic to pathogens [16]. Maury et al. [29] demonstrated that tobacco compromised in O-methyltransferase activity produced lower amounts of phenolic bacterial virulence gene inducers and thus had smaller tumors caused by Agrobacterium tumefaciens. Previous studies showed that both conjugated and free phenolic acids were induced by V. longisporum infection, and higher levels were found in hypocotyls of resistant cultivars of B. napus, thus indicating a role in cultivar resistance [18].
In the present study, production of SA declined in the resistant cultivar starting from 14 dpi, which coincided with a strong increase in levels of phenolic acids. However, enzymes involved in lignin synthesis were already activated at 7 dpi, which occurred alongside with an enhanced expression of the related genes. Our study showed consistently higher activity of POX in the resistant cultivar from 7 to 21 dpi and a strong up-regulation of POX until 14 dpi indicating that the resistant cultivar may have accumulated sufficient amounts of lignin by 21 dpi after infection, which is in agreement with previously shown histochemical analyses [18].
V. longisporum is considered a hemibiotroph, which has a biotrophic life phase in the roots and the xylem and a late necrotrophic phase in the stem parenchyma [30]. As described in previous studies, some pathogen effectors, such as VdIsc1 secreted by V. dahliae, can target SA signaling in plants to enhance virulence by preventing SA accumulation [31]. Necrotrophic pathogens, such as B. cinerea and Alternaria solani, have been reported to enhance the SA signaling pathway in order to antagonize jasmonic acid and to promote disease development in tomato [32]. Accordingly, in the early biotrophic stage of infection, V. longisporum may be able to secrete an effector targeting SA synthesis and thus reducing SA levels in susceptible cultivars and allowing higher infection. Since SA is important for basal resistance in oilseed rape, and SA may be induced by infection with biotrophic or hemibiotrophic pathogens [31], avirulent strains of viruses or biotrophic fungi may be efficient biocontrol agents to prevent infection with V. longisporum by inducing enhanced levels of SA.
Until present, responses of B. napus to V. longisporum infection on the metabolomic and transcriptomic level were assessed in relation to plant but not to fungal biomass. This resulted in poor contrasts between responses in resistant and susceptible tissues as their expression was similar related to the same sample volume although responses relative to fungal biomass strongly differed. We believe that the latter is more relevant to accurately describe plant responses to a certain unit of pathogen biomass.
4. Conclusions
In summary, a remarkable increase of susceptibility to V. longisporum was observed on SA-deficient transgenic NahG oilseed rape indicating that SA plays a role in basal resistance of B. napus to V. longisporum. In cultivar-related resistance, a stronger increase of SA relative to fungal growth was observed in the resistant cultivar, indicating an important role of elevated SA in defense during early stages of infection, while at later stages, when SA responses do not differ anymore between cultivars, higher levels of phenolic acids are associated with cultivar resistance.
5. Materials and Methods
5.1. Plant Material and Cultivation
Four cultivars and genotypes, B. napus cv. Drakkar, NahG transformed Drakkar, Falcon (Norddeutsche Pflanzenzucht Hans-Georg Lembke KG, NPZ, Hohenlieth, Germany), SEM 05-500256 (SEM, Syngenta, Germany) were used in the study. Cultivar Falcon is a susceptible German commercial winter oilseed rape cultivar, and SEM is a winter type breeding line resistant to V. longisporum [18]. Seeds of these cultivars were surface sterilized with 70% ethanol for 1 min under constant shaking and subsequently rinsed with sterilized ddH2O and pre-germinated in quartz sand. Plants were kept in a climate chamber with a 16 h photoperiod and a temperature of 22 ± 2 °C for 12 days before root inoculation with V. longisporum.
5.2. Treatments and Experimental Design
To study the basic function of SA and its role in cultivar-related resistance, pot experiments were conducted in a completely randomized block design under climate chamber conditions and repeated twice. The study on the basic function of SA consisted of a combination of three experimental factors, which were genotype (a spring oilseed rape genotype Drakkar and its NahG transformant), disease (mock-inoculated and V. longisporum-inoculated) and root-dip treatment with SA or water. Treatments were arranged with four biological replicates each composed of 10 plants grown independently in separate pots. The study of cultivar-related resistance consisted of a combination of two experimental factors, which were genotype (two winter oilseed rape genotypes Falcon and SEM) and disease (mock-inoculated and V. longisporum-inoculated). Treatments were arranged in a randomized pattern with four biological replicates each composed of 20 plants grown independently in separate pots.
5.3. Production of Transgenic B. napus Expressing the NahG Gene
Seeds of transgenic OSR carrying the NahG gene were kindly provided by Christian Möllers (Division of Plant Breeding, University of Göttingen). The plasmid used for transformation had been constructed by Corinna Thurow and Christiane Gatz (Institute of Plant Biochemistry, University of Göttingen). Briefly, hypocotyl segments of B. napus cv. Drakkar (spring type) were transformed by Agrobacterium mediated transformation [33,34]. A binary plasmid, pCAMBIA2300 (Figure 7) containing the NahG gene of Pseudomonas putida ND6, which encodes salicylate hydroxylase [35] for degradation of SA to catechol, was used for construction of Agrobacterium strain AGLO. A northern blot analysis was performed for expression analysis of transformed plants. Endogenous SA levels in hypocotyl and shoot tissues of transformed plants were measured. One transformant, which had the strongest expression of the NahG gene and low levels of SA, was used for the study.
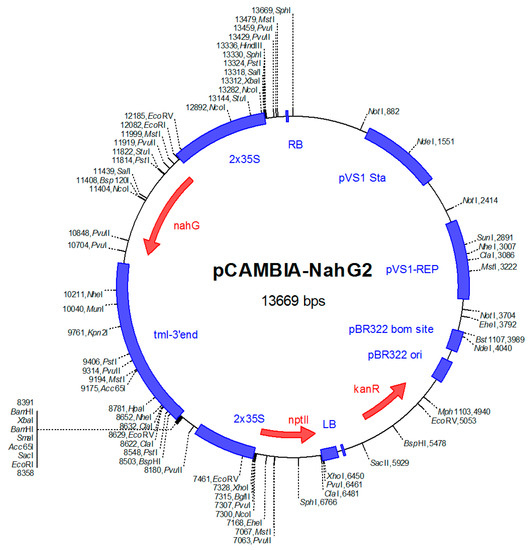
Figure 7.
Structure of binary plasmid pCAMBIA2300 containing the NahG gene. A fragment of double 35S-NahG gene-tml-3´end from pCIB200-NahG was inserted into pCAMBIA2300 by using Xba I restriction enzyme recognition site. Kanamycin-resistance (kanR) marker was the selectable marker used for transformed plant cells. Neomycin phototransferase II (npt II) was the selectable marker for plant transformation.
5.4. Exogenous Application of SA
Prior to inoculation with V. longisporum by the root-dip method, roots of Drakkar and its NahG transformant were dipped in 0.5 mM of SA or sterile tap water for 24 h. After treatment, plants were rinsed with sterile tap water several times and dried on clean filter papers.
5.5. Fungal Culture and Inoculation
V. longisporum isolate VL43 obtained from a diseased B. napus plant [4,36] was used for inoculations. Conidial suspension kept in 25% glycerol at −80 °C was used to initiate fresh cultures. Fungal cultures were prepared from 400 µl stock conidial suspension added to 250 ml of autoclaved (121 °C, 20 min) potato dextrose broth and then incubated on a rotary shaker at 80 rpm at 22 °C for 10 days. The resulting suspension was filtered through sterile gauze to remove mycelia.
Twelve-day-old seedlings with cotyledons completely unfolded were inoculated or mock-inoculated using a root-dip method [18]. Plant roots were rinsed with tap water and dipped in a conidial suspension (1 × 106 cfu/ml) or water for 50 min and replanted in pots (7 × 7 × 8 cm) containing a sterile soil-sand mixture (3:1).
5.6. Disease Assessment
According to the assessment key (Table 2) described by Eynck et al. [37], disease severity (DS) was quantified at 7, 14 and 21 dpi. Plant height was measured at 21 dpi. Dry weight of roots and shoots of Drakkar and NahG transformants were determined at 7, 14 and 21 dpi.

Table 2.
Assessment key for scoring foliar symptoms induced by V. longisporum on Brassica species inoculated with the root dip method.
5.7. Extraction and Quantification of Fungal DNA
Total DNA from hypocotyl samples was extracted using a cetyltrimethylammonium bromide (CTAB) method [38]. About 100 mg of ground fresh plant tissue was suspended in 1 ml CTAB with 2 µl ß-mercaptoethanol and 1 µl 1x proteinase K. The extracted DNA was dissolved in 200 µl TE buffer.
A CFX384 real-time PCR detection system (Bio-Rad Laboratories Inc., Kabelsketal, Germany) was used for the amplification and quantification of V. longisporum DNA using primers OLG70 (5′-CAGCGAAACGCGATATGTAG-3′) and OLG71 (5′-GGCTTGTAGGGGGTTTAGA-3′) [39]. The amplification mix consisted of 1× (NH4)2SO4 buffer, 2.5 mM of MgCl2, 100 µM of dNTPs, 0.02 U/µl of BioTaq DNA polymerase (Bioline, Luckenwalde, Germany), 0.1x SYBR Green I solution (Invitrogen, Karlsruhe, Germany), 0.3 µM each of primers OLG70 and OLG71 and 1 µl of template DNA and filled up to a total volume of 10 µl with ddH2O. PCR conditions were as described in Table 3. PCR for all treatment samples were performed with four biological and three technical replicates and data were analyzed using CFX Manager Software.

Table 3.
PCR program for quantification of DNA of V. longisporum and gene expression assay.
5.8. Quantification of Endogenous SA
Free SA was extracted from hypocotyl tissue according to a method modified from Kamble et al. [40]. About 200 mg of liquid nitrogen ground fresh hypocotyl samples was suspended in 1.5 ml of acetone, shaked vigorously and centrifuged at 5500 rpm at 4 °C for 45 min. The supernatant was transferred and evaporated in a speed vacuum centrifuge at 30 °C. The residue was dissolved in 1 ml demineralized water, and 1 ml ethyl acetate was added subsequently. The upper phase from the mixture was transferred and evaporated to dryness at 35 °C. The residue was dissolved again in 200 µl of HPLC grade methanol.
A dilution series of 100 nM to 20 µM of SA dissolved in HPLC grade methanol was used as internal standard. The peak of SA was identified by comparing retention times of samples and standards and confirmed by addition of standard SA to the samples. Before loading into a HPLC vial, all samples or standards were centrifuged at 5000 rpm for 5 min to precipitate unsolvable particles to prevent injection problems.
A HPLC-fluorescence system consisting of a Varian 410 automatic injector, two Varian 210 pumps with 10 W SS head, a Lichrospher RP-18 column (250 × 4 mm, 5 µm) protected by a Security Guard™ Carbo-H precolumn (4 × 3 mm, 5 µm) kept at 30 °C and a Varian 363 fluorescence detector with excitation wavelength at 315 nm and emission wavelength at 405 nm. Each sample was analyzed for 33 min under a bi-mobile phase with (A) 20 mM sodium acetate, pH 5.0 and (B) methanol with a flow rate of 1 ml/min with the following protocol: initial 10% B for 2 min, linear gradient to 38% B in 13 min, increased to 98% B in 30 s and held for 9 min, equilibrated to initial condition in 30 s and hold for 8 min. The injection volume was 10 µl.
5.9. Quantification of Phenolic Acids in Hypocotyls
Phenolic acids were extracted from hypocotyl following published protocols [18,41,42]. About 200 mg of hypocotyl samples ground in liquid nitrogen were suspended in 2 ml of 80% methanol with 0.2 mg/ml of 2,6-di-tert-butyl-4-methylphenol. The mixture was well mixed and sonicated for 10 s. After incubation for 30 min at room temperature, the mixture was centrifuged at 1000 rpm for 10 min. The procedure was repeated twice.
Free phenolic acids. The supernatant was transferred and evaporated in a speed vacuum centrifuge at 30 °C. The residue was dissolved in 1 ml demineralized water, and 0.5 µl of 37% HCl was added to adjust to pH 2–3. Ethyl acetate (1 ml) was used twice for extraction of free phenolic acids from the crude extract. After thoroughly mixing, the upper phase was transferred and evaporated to dryness at 35 °C. The residue was dissolved again in 200 µl of 80% HPLC grade methanol.
Methanol-insoluble ester bound phenolic acids. The pellet after methanol extraction was hydrolyzed with 1 ml of 2 M NaOH at 95 °C for 1 h and mixed four times in between. For acidification of the mixture, 218 µl of 37% HCl was added. Ester bound phenolic acids were extracted twice with 1 ml ethyl acetate. The supernatant was transferred and evaporated to dryness using speed vacuum centrifugation at 35 °C. The residue was dissolved in 200 µl of 80% HPLC grade methanol.
A standard series mix of CA, pCA, FA, SiA, tCA, BA and SA dissolved in 80% HPLC grade methanol, from 50 ppb to 10 ppm for each compound, was used as internal standards. Peak assignment of each component was made by comparing retention times of samples and standards as well as by comparing the UV absorption spectra (200~500 nm) of analytes to purchased standards.
The HPLC-fluorescence/DAD system consisted of a JASCO AS-2051 Plus intelligent sampler (4 °C), a DG-2080-54 4-line degasser, an LG-2080-04S quaternary gradient unit, a Kinetex EVO C18 column (250 × 4 mm, 5 µm) protected by a Gemini NX C18 guard column (4 × 3.0 mm, 5 µm) kept in a column oven at 40 °C (CO-2060 Plus Intelligent Column Thermostat), an FP-2020 Plus intelligent fluorescence detector with excitation wavelength at 315 nm and emission wavelength at 405 nm and a MD-2015 Plus multi-wavelength detector measuring over the range of 200~500 nm. Each sample was analyzed for 65 min in a bi-mobile phase run with (A) 0.1% phosphoric acid in water and (B) 0.1% phosphoric acid in acetonitrile with a flow rate of 1 ml/min following this protocol: initial 9% B for 5 min, linear gradient to 32% B in 39 min, increased to 98% B in 30 s and hold for 10 min, equilibrated to initial condition in 30 s and hold for 10 min. The sample injection volume was 10 µl.
All analytical data from metabolite assays were recorded as µg per g plant FW and related to fungal biomass measured in the plant tissue as ng fungal DNA per g plant FW.
5.10. Enzyme Assays
The hypocotyls were harvested from experimental plants (Falcon and SEM) at 7, 14, 21 dpi. Eight to twelve fresh plant samples were ground in liquid nitrogen as one pooled sample. Four independent samples were taken from each treatment at each time point. Each sample had three technical replicates.
BA2H was extracted as described by Leon et al. [43]. About 100 mg powder was suspended in 500 µl of extraction buffer (20 mM Hepes, containing 10 mM sorbitol, 1% polyvinylpyrrolidone (PVP), 1 mM phenylmethylsulfonyl fluoride, 12.5 mM ß-mercaptoethanol, adjusted with NaOH to pH 7.0). The suspension was mixed, sonicated for 2 min and mixed again before being centrifuged for 10 min at 9,274 rpm and 4 °C. The supernatant was used as the enzyme extract. The reaction mixture (250 µl) contained 20 mM Hepes buffer, pH 7.0, 1 mM NADPH, 1 mM BA and 100 µl enzyme extract, and was incubated for 30 min at 30 °C. To stop the reaction, 125 µl of 15% (w/v) trichloroacetic acid was added. The mixture was centrifuged for 5 min at 10,000 × g, the supernatant was extracted twice with 250 µl of ethyl acetate:cyclopentane:isopropanol (100:99:1). The upper organic phase was evaporated to dryness for 1 h at 30 °C and the pellet was resuspended in 200 µl of HPLC grade methanol. The BA2H activity was determined as the rate of conversion of BA to SA. SA was quantified by HPLC as described above.
PAL was extracted according to Kamble et al. [44]. About 200 mg homogenized sample was suspended in 1 ml of 5 mM Tris-HCl buffer, pH 8.3 and centrifuged at 10,000 rpm for 15 min at 4 °C. The supernatant was used as a crude enzyme extract. The activity of PAL was determined as the rate of conversion of L-phenylalanine to tCA. The reaction mixture (1 ml) contained 25 mM Tris HCl buffer, pH 8.8, 100 µM L-phenylalanine and 100 μl enzyme extract, and was incubated for 60 min at 30 °C. To terminate the reaction, 400 μl of 2 N HCl was added. For further extraction, 800 μl of toluene was added, and the samples were well mixed and then centrifuged at 1000 rpm for 5 min. The upper toluene layer was measured at 290 nm (HP845 × UV-Visible System) and pure toluene was set as blank. A series of tCA with concentrations of 5 μM to 80 μM in toluene was used as standards. Enzyme activity was expressed as change in tCA in µM/min/g FW.
C4H was extracted as described previously [45,46,47]. About 100 mg powder was suspended in 600 µl of extraction buffer (100 mM Tris-HCl buffer, pH 7.5 with 15 mM ß-mercaptoethanol). The suspension was well mixed, sonicated for 2 min, and mixed again before being centrifuged for 20 min at 15,000 rpm at 4 °C. The supernatant was used as the enzyme extract. The reaction mixture (1.5 ml) contained 100 mM Tris-HCl buffer, pH 7.5, 2 mM NADPH, 1.33 mM tCA and 250 µl enzyme extract, and was incubated for 35 min at 30 °C. To stop the reaction, 50 µl of 37% HCl was added and then adjusted to pH 11 with 2 M NaOH. The mixture was extracted twice with 1 ml of diethyl ether. After short centrifugation, the upper organic phase was evaporated to dryness for 65 min at 30 °C and the pellet was resuspended in 500 µl of 1 M NaOH, and measured at 330 nm (µQuant, Bio-Tek). The C4H activity was determined as the rate of conversion of tCA to pCA.
CAD was extracted as described by Chabannes et al. [48]. About 100 mg powder was suspended on ice in 500 µl of 100 mM Tris-HCl buffer, pH 7.5 with 2% PEG6000, 2% PVP and 5 mM freshly prepared dithiothreitol. The suspension was mixed, sonicated for 2 min, and mixed again before being centrifuged for 10 min at 9,274 rpm at 4 °C. The supernatant was used as the enzyme extract. The reaction mixture (500 µl) contained 100 mM Tris-HCl buffer, pH 8.8, 1 mM NADP, 1 mM coniferyl alcohol and 50 µl enzyme extract, and was incubated for 20 min at 30 °C. The formation of coniferaldehyde was monitored at 400 nm using the molar extinction coefficient of coniferaldehyde (2.1 × 104 M−1 cm−1), and the activity of CAD was expressed as change in absorbance as nkatal.g−1 FW (Sibout et al., 2003; Zhang et al., 2006).
POX measurement was carried out according to Mandal et al. [49]. About 200 mg of homogenized samples were resuspended in 2 ml of 0.1 M phosphate buffer, pH 7.5, containing 0.5 mM of Na-EDTA and 1% of PVP. The mixture was then centrifuged at 9,000 rpm for 30 min at 4 °C. The supernatant was used as enzyme extract. The activity of POX was determined as the rate of conversion of guaiacol to oxidized (dehydrogenated) guaiacol. The reaction mixture (1 ml) contained 81 mM phosphate buffer, pH 7.0, 4.5 mM guaiacol and 10 μl enzyme extract. After 30 μl of 10 mM, H2O2 was added to the reaction mixture, the measurement was taken immediately at 470 nm for 7.5 min. The enzyme activity was calculated using the molar extinction coefficient of dehydrogenated guaiacol (26.6 mM−1 cm−1) and expressed as change in absorbance as μkat/g FW.
Similar to the metabolite assays, enzyme activity data were related to fungal biomass measured in the plant tissue as ng fungal DNA per g plant FW.
5.11. Gene Expression
5.11.1. RNA Extraction and Synthesis of cDNA
About 100 mg of homogenized fresh hypocotyl tissue was suspended in 1 ml TRI-reagent (Invitrogen™, ThermoFisher) and incubated for 15 min at room temperature. Subsequently, 100 µl bromochloropropane was added, and the samples were shaken for 15 s. After incubation for 15 min at room temperature, the mixture was centrifuged for 15 min at 10,159 rpm at 4 °C. For RNA precipitation, 0.5 ml isopropanol was added to the upper aqueous phase (RNA), mixed for 10 s, incubated for 10 min at room temperature, and centrifuged for 10 min at 10,159 rpm at 4 °C. The supernatant was gently discarded, and the pellet was washed with 75% ethanol (freshly prepared in DEPC water). The dried RNA was dissolved in 30 µl DEPC treated ddH2O. To check the quality of extracted total RNA, 4.5 µl of RNA was added to a mixture containing 1 × formaldehyde gel-running buffer, 50% formamide, 17.5% formaldehyde, and then incubated for 15 min at 65 °C and immediately chilled on ice to break down the secondary structure. Treated RNA was run on 1.5% agarose gel using TBE buffer.
The samples with good RNA quality were digested again with DNase to remove contaminant genomic DNA before being used for cDNA synthesis. The quality of the digested RNAs was confirmed again on 1.5% agarose gel. The RNA concentration was determined using a microplate spectrophotometer (Epoch, Bio-Tek) at 260 nm and controlled by the ratio OD260/OD280. For reversing mRNA to cDNA, a First Strand cDNA Synthesis Kit (ThermoFisher) with oligo dT18, M-MLV reverse transcriptase and RiboLock RNase inhibitor (1 U/reaction) was used according to the manufacturer’s protocol.
5.11.2. Reverse Transcription Quantitative PCR (RT-qPCR)
Primers for selected genes were constructed by using online primer tools such as Primer3 (Version 4.0), IDT OligoAnalyzer 3.1 with the help of sequence databases (http://www.ncbi.nlm.nih.gov and http://www.brassocadb.org). The sequences (5´ to 3´) of forward (F) and reverse (R) primers of each candidate gene used for RT-qPCR are listed in Table 4. The efficiency of each primer was tested by using standard cDNA copies. RT-qPCR was performed in a CFX384 real-time PCR detection system to determine relative gene expression levels with actin-7 and GAPDH as endogenous reference genes with four independent biological replicates using SYBR Green for staining. PCR conditions were as described in Table 3. A no-template control was included in each experiment. Expression values considering the primer efficiency were normalized to the endogenous reference genes with the formula following Pfaffl [50], and the log2-fold change was related to fungal biomass measured in the plant tissue as ng fungal DNA per g plant FW.

Table 4.
Primers used for reference and candidate genes in the biosynthetic pathway of salicylic acid and lignin.
5.12. Statistical Analysis
The experimental data were analyzed as completely randomized designs with four replications using STATISTICA 13.2. All data were normal distributed and analyzed using factorial ANOVA. A multiple comparison was analyzed by Fisher LSD test. To analyze the relationship between disease severity and physiological parameters, Pearson’s linear correlation was performed, and correlation coefficients were calculated. The experimental results were presented as means ± standard error at 5% significance level. A principal component analysis was performed using R package factoextra and FactoMineR.
Author Contributions
Conceptualization, A.v.T.; data curation, X.Z.; funding acquisition, A.v.T.; investigation, X.Z.; supervision, B.K. and A.v.T.; validation, B.K.; writing—original draft, X.Z.; writing—review and editing, A.v.T.
Funding
This study was partly funded by an ERA-CAPS grant of the German Research Foundation (DFG; TI 170/13–1).
Acknowledgments
We gratefully acknowledge the support of Corinna Thurow and Christiane Gatz, Division of Plant Molecular Biology and Physiology, University of Göttingen, and Christian Möllers, Division of Plant Breeding, University of Göttingen, for providing the NahG gene construct and performing the transformation of oilseed rape plants, respectively.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Inderbitzin, P.; Subbarao, K.V. Verticillium systematics and evolution: How confusion impedes Verticillium wilt management and how to resolve it. Phytopathology 2014, 104, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Steventon, L.A.; Fahleson, J.; Hu, Q.; Dixelius, C. Identification of the causal agent of Verticillium wilt of winter oilseed rape in Sweden, V. longisporum. Mycol. Res. 2002, 106, 570–578. [Google Scholar] [CrossRef]
- Gladders, P.; Smith, J.A.; Kirkpatrick, L.; Clewes, E.; Grant, C.; Barbara, D.; Barnes, A.V.; Lane, C.R. First record of Verticillium wilt (Verticillium longisporum) in winter oilseed rape in the UK. New Dis. Rep. 2011, 23, 8. [Google Scholar] [CrossRef]
- Zeise, K.; von Tiedemann, A. Host specialization among vegetative compatibility groups of Verticillium dahliae in relation to Verticillium longisporum. J. Phytopathol. 2002, 150, 112–119. [Google Scholar] [CrossRef]
- Canadian Food Inspection Agency. Pest Risk Management Document: Verticillium longisporum (Verticillium stripe). Available online: http://www.inspection.gc.ca/plants/plant-pests-invasive-species/directives/risk-management/rmd-17-01/eng/1487004855251/1487004951480 (accessed on 13 November 2018).
- Depotter, J.R.L.; Deketelaere, S.; Inderbitzin, P.; von Tiedemann, A.; Höfte, M.; Subbarao, K.V.; Wood, T.A.; Thomma, B.P.H.J. Verticillium longisporum, the invisible threat to oilseed rape and other brassicaceous plant hosts. Mol. Plant. Pathol. 2016, 17, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S. Longevity of the Verticillium wilt fungus in the laboratory and field. Phytopathology 1955, 455, 180–181. [Google Scholar]
- Karapapa, V.K.; Bainbridge, B.W.; Heale, J.B. Morphological and molecular characterization of Verticillium longisporum comb, nov., pathogenic to oilseed rape. Mycol. Res. 1997, 101, 1281–1294. [Google Scholar] [CrossRef]
- Klosterman, S.J.; Atallah, Z.K.; Vallad, G.E.; Subbarao, K.V. Diversity, pathogenicity, and management of verticillium species. Annu. Rev. Phytopathol. 2009, 47, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, Y.; Bouazizi, E.; Triki, M. Inductions of defense response in olive plants against Verticillium dahliae through application of salicylic acid as abiotic inducer. J. Adv. Biol. Biotechnol. 2016, 5, 1–9. [Google Scholar] [CrossRef]
- Maruri-López, I.; Aviles-Baltazar, N.Y.; Buchala, A.; Serrano, M. Intra and extracellular journey of the phytohormone salicylic acid. Front. Plant. Sci. 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Torrens-Spence, M.P.; Bobokalonova, A.; Carballo, V.; Glinkerman, C.M.; Pluskal, T.; Shen, A.; Weng, J.-K. PBS3 and EPS1 complete salicylic acid biosynthesis from isochorismate in Arabidopsis. bioRxiv 2019, 601948. [Google Scholar] [CrossRef]
- Achuo, E.A.; Audenaert, K.; Meziane, H.; Hofte, M. The salicylic acid-dependent defence pathway is effective against different pathogens in tomato and tobacco. Plant. Pathol. 2004, 53, 65–72. [Google Scholar] [CrossRef]
- Loake, G.; Grant, M. Salicylic acid in plant defence--the players and protagonists. Curr. Opin. Plant. Biol. 2007, 10, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Ratzinger, A.; Riediger, N.; von Tiedemann, A.; Karlovsky, P. Salicylic acid and salicylic acid glucoside in xylem sap of Brassica napus infected with Verticillium longisporum. J. Plant. Res. 2009, 122, 571–579. [Google Scholar] [CrossRef] [PubMed]
- König, S.; Feussner, K.; Kaever, A.; Landesfeind, M.; Thurow, C.; Karlovsky, P.; Gatz, C.; Polle, A.; Feussner, I. Soluble phenylpropanoids are involved in the defense response of Arabidopsis against Verticillium longisporum. New Phytol. 2014, 202, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, C.; Hossain, M.A.; Snowdon, R.; Knüfer, J.; von Tiedemann, A.; Friedt, W. Genetic analysis of phenylpropanoid metabolites associated with resistance against Verticillium longisporum in Brassica napus. Mol. Breed. 2013, 31, 347–361. [Google Scholar] [CrossRef]
- Eynck, C.; Koopmann, B.; Karlovsky, P.; von Tiedemann, A. Internal resistance in winter oilseed rape inhibits systemic spread of the vascular pathogen Verticillium longisporum. Phytopathology 2009, 99, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Cardoza, Y.J.; Schmelz, E.A.; Raina, R.; Engelberth, J.; Tumlinson, J.H. Differential volatile emissions and salicylic acid levels from tobacco plants in response to different strains of Pseudomonas syringae. Planta 2003, 217, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Song, J.T.; Koo, Y.J.; Seo, H.S.; Kim, M.C.; Choi, Y.D.; Kim, J.H. Overexpression of AtSGT1, an Arabidopsis salicylic acid glucosyltransferase, leads to increased susceptibility to Pseudomonas syringae. Phytochemistry 2008, 69, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.J.; Kim, M.A.; Kim, E.H.; Song, J.T.; Jung, C.; Moon, J.-K.; Kim, J.-H.; Seo, H.S.; Song, S.I.; Kim, J.-K.; et al. Overexpression of salicylic acid carboxyl methyltransferase reduces salicylic acid-mediated pathogen resistance in Arabidopsis thaliana. Plant. Mol. Biol. 2007, 64, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Eynck, C.; Koopmann, B.; Grunewaldt-Stoecker, G.; Karlovsky, P.; von Tiedemann, A. Differential interactions of Verticillium longisporum and V. dahliae with Brassica napus detected with molecular and histological techniques. Eur. J. Plant. Pathol. 2007, 118, 259–274. [Google Scholar] [CrossRef]
- Johansson, A.; Staal, J.; Dixelius, C. Early responses in the Arabidopsis-Verticillium longisporum pathosystem are dependent on NDR1, JA-and ET-associated signals via cytosolic NPR1 and RFO1. Mol. Plant. Microbe. Interact. 2006, 19, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X. Verticillium longisporum on Oilseed Rape (Brassica napus L.): Differential Roles of Salicylic Acid, Seed Transmission and Plant Colonization in Greenhouse and Field Conditions. Ph.D. Thesis, Georg-August University Göttingen, Göttingen, Germany, December 2018. [Google Scholar]
- Kuai, X.; MacLeod, B.J.; Després, C. Integrating data on the Arabidopsis NPR1/NPR3/NPR4 salicylic acid receptors; a differentiating argument. Front. Plant. Sci. 2015, 6, 235. [Google Scholar] [CrossRef] [PubMed]
- Camm, E.L.; Towers, G.H.N. Phenylalanine ammonia lyase. Phytochemistry 1973, 12, 961–973. [Google Scholar] [CrossRef]
- Miedes, E.; Vanholme, R.; Boerjan, W.; Molina, A. The role of the secondary cell wall in plant resistance to pathogens. Front. Plant. Sci. 2014, 5, 358. [Google Scholar] [CrossRef] [PubMed]
- Maury, S.; Delaunay, A.; Mesnard, F.; Crônier, D.; Chabbert, B.; Geoffroy, P.; Legrand, M. O-methyltransferase(s)-suppressed plants produce lower amounts of phenolic vir inducers and are less susceptible to Agrobacterium tumefaciens infection. Planta 2010, 232, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Reusche, M.; Thole, K.; Janz, D.; Truskina, J.; Rindfleisch, S.; Drübert, C.; Polle, A.; Lipka, V.; Teichmann, T. Verticillium infection triggers VASCULAR-RELATED NAC DOMAIN7-dependent de novo xylem formation and enhances drought tolerance in Arabidopsis. Plant. Cell 2012, 24, 3823–3837. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Song, T.; Zhang, X.; Yuan, H.; Su, L.; Li, W.; Xu, J.; Liu, S.; Chen, L.; Chen, T.; et al. Unconventionally secreted effectors of two filamentous pathogens target plant salicylate biosynthesis. Nat. Commun. 2014, 5, 4686. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.A.E.; Oirdi, M.E.; Gonzalez-Lamothe, R.; Bouarab, K. Necrotrophic pathogens use the salicylic acid signaling pathway to promote disease development in tomato. Mol. Plant. Microbe. Interact. 2012, 25, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Weier, D.; Hanke, C.; Eickelkamp, A.; Lühs, W.; Dettendorfer, J.; Schaffert, E.; Möllers, C.; Friedt, W.; Wolter, F.P.; Frentzen, M. Trierucoylglycerol biosynthesis in transgenic plants of rapeseed (Brassica napus L.). Lipid 1997, 99, 160–165. [Google Scholar] [CrossRef]
- Hüsken, A.; Baumert, A.; Strack, D.; Becker, H.C.; Möllers, C.; Milkowski, C. Reduction of sinapate ester content in transgenic oilseed rape (Brassica napus) by dsRNAi-based Suppression of BnSGT1 Gene Expression. Mol. Breed. 2005, 16, 127–138. [Google Scholar] [CrossRef]
- Chung, Y.S.; Lee, N.R.; Cheon, C.L.; Song, E.S.; Lee, M.S.; Kim, Y.; Min, K.H. Molecular cloning of the nahG gene encoding salicylate hydroxylase from Pseudomonas fluorescens. Mol. Cells 2001, 11, 105–109. [Google Scholar] [PubMed]
- Zeise, K.; von Tiedemann, A. Morphological and physiological differentiation among vegetative compatibility groups of Verticillium dahliae in relation to V. longisporum. J. Phytopathol. 2001, 149, 469–475. [Google Scholar] [CrossRef]
- Eynck, C.; Koopmann, B.; von Tiedemann, A. Identification of Brassica accessions with enhanced resistance to Verticillium longisporum under controlled and field conditions. J. Plant. Dis. Protect. 2009, 116, 63–72. [Google Scholar] [CrossRef]
- Zheng, X.; Pfordt, A.; Khatri, L.; Eseola, A.B.; Wilch, A.; Koopmann, B.; Tiedemann, A. von. Contrasting patterns of colonization with Verticillium longisporum in winter and spring type oilseed rape (Brassica napus L.) in the field and greenhouse and the role of soil temperature. Plant. Dis. 2019, 103, 2090–2099. [Google Scholar] [CrossRef] [PubMed]
- Lopisso, D.T.; Knüfer, J.; Koopmann, B.; von Tiedemann, A. The vascular pathogen Verticillium longisporum does not affect water relations and plant responses to drought stress of its host, Brassica napus. Phytopathology 2017, 107, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Kamble, A.; Bhargava, S. ß-aminobutyric acid-induced resistance in Brassica juncea against the necrotrophic pathogen Alternaria brassicae. J. Phytopathol. 2007, 155, 152–158. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Hayırlıoglu-Ayaz, S.; Alpay-Karaoglu, S.; Grúz, J.; Valentová, K.; Ulrichová, J.; Strnad, M. Phenolic acid contents of kale (Brassica oleraceae L. var. acephala DC.) extracts and their antioxidant and antibacterial activities. Food Chem. 2008, 107, 19–25. [Google Scholar] [CrossRef]
- Mattila, P.; Kumpulainen, J. Determination of free and total phenolic acids in plant-derived foods by HPLC with diode-array detection. J. Agric. Food Chem. 2002, 50, 3660–3667. [Google Scholar] [CrossRef] [PubMed]
- Leon, J.; Yalpani, N.; Raskin, I.; Lawton, M.A. Induction of benzoic acid 2-hydroxylase in virus-inoculated tobacco. Plant. Physiol. 1993, 103, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Kamble, A.; Koopmann, B.; von Tiedemann, A. Induced resistance to Verticillium longisporum in Brassica napus by β-aminobutyric acid. Plant. Pathol. 2013, 62, 552–561. [Google Scholar] [CrossRef]
- Salvador, V.H.; Lima, R.B.; dos Santos, W.D.; Soares, A.R.; Böhm, P.A.F.; Marchiosi, R.; Ferrarese, M.d.L.L.; Ferrarese-Filho, O. Cinnamic acid increases lignin production and inhibits soybean root growth. PLoS ONE 2013, 8, e69105. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Alejo, N.; Gómez-Peralta, J.E. Activity of enzymes involved in capsaicin biosynthesis in callus tissue and fruits of chili pepper (Capsicum annuum L.). J. Plant. Physiol. 1993, 141, 147–152. [Google Scholar] [CrossRef]
- Phimchan, P.; Chanthai, S.; Bosland, P.W.; Techawongstien, S. Enzymatic changes in phenylalanine ammonia-lyase, cinnamic-4-hydroxylase, capsaicin synthase, and peroxidase activities in capsicum under drought stress. J. Agric. Food Chem. 2014, 62, 7057–7062. [Google Scholar] [CrossRef] [PubMed]
- Chabannes, M.; Barakate, A.; Lapierre, C.; Marita, J.M.; Ralph, J.; Pean, M.; Danoun, S.; Halpin, C.; Grima-Pettenati, J.; Boudet, A.M. Strong decrease in lignin content without significant alteration of plant development is induced by simultaneous down-regulation of cinnamoyl CoA reductase (CCR) and cinnamyl alcohol dehydrogenase (CAD) in tobacco plants. Plant. J. 2001, 28, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Mitra, A. Reinforcement of cell wall in roots of Lycopersicon esculentum through induction of phenolic compounds and lignin by elicitors. Physiol. Mol. Plant. Pathol. 2007, 71, 201–209. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 45. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).